Abstract
We report constitutive production of glucose isomerase (GI) under submerged growth of Aspergillus sp. in glucose phosphate broth (GPB). The fungus produced significant quantities of extracellular GI in GPB without supplementing the inducer (xylose). The maximum biomass (872 mg) and highest level of GI (1126 U) were obtained in 42 h at 30 °C and 120 rpm. Equal level of biomass and enzyme were produced in GPB with glucose and xylose, but the amount of biomass and enzyme was drastically reduced when the fungus was grown on other carbon sources. Optimum biomass, enzyme units and enzyme activity were obtained with 40 and 1 g/l of glucose, respectively. Growth of Aspergillus sp. and enzyme synthesis even at high glucose concentration (60 g/l) indicated the osmophillic nature of the fungus. Increasing the glucose concentration above 1 and 40 g/l did not support the growth and enzyme activity. Among various organic and inorganic nitrogen sources used, yeast extract, peptone and NH4SO4 gave the best biomass and enzyme yields. Addition of Mg2+ and Mn2+ in GPB significantly enhanced the enzyme production. Under optimized conditions in modified GPB, the yield of biomass and synthesis and activity of GI were significantly enhanced.
Keyword: Glucose isomerase, Aspergillus sp., Constitutive production
Introduction
Because of the ever increasing demand for sugar and its rising price, considerable efforts have been made to find alternative sweeteners. The producers of starch syrup have attempted to enhance the sweetness of the syrup so that it can be used as substitute for sugar. The use of glucose isomerase (GI) to convert glucose in corn syrup to fructose has been in practice for some time. Pseudomonas hydrophila was the first organism to be reported to convert D-glucose to D-fructose (Marshall and Kooi 1957). After this a large number of microorganisms that are capable of producing GI have been reported (da Silva et al. 2006; Dhungel et al. 2007; Tumturk et al. 2008).
D-Glucose/xylose isomerase (D-xylose ketol isomerase; EC. 5.3.1.5), commonly referred to as glucose isomerase (GI), is one of the three highest tonnage value enzymes (Bhosale et al. 1990). It catalyzes the equilibrium reversible isomerization of D-glucose and D-xylose to D-fructose and D-xylulose (Crueger and Crueger 1992). Isomerization of glucose to fructose catalyzed by GI is commercially important for the production of High Fructose Corn Syrup [HFCS] (Crueger and Crueger 1992; Gaikwad and Deshpande 1992). Application of GI for the production of fructose and HFCS has long been a goal of corn refining industry (Frost and Moss 1987), since this process converts glucose into a mixture, which is as sweet as sucrose (Lee et al. 1972).
GI produced by various microbial genera is intracellular or cell bound enzyme (Koyal et al. 1992) except Streptomyces, where it is extracellular (Joseph et al. 1977). Most of the GI producing organisms have obligate requirement of D-xylose to induce enzyme production. However, xylose is expensive for use on commercial scale (Crueger and Crueger 1992). Extracellular production of GI from Aspergillus sp. has not been reported so far. Therefore, an organism capable of producing extracellular GI constitutively shall have great commercial value. Considering the industrial significance of GI, we examined Aspergillus sp. for the presence of extracellular GI independent of xylose.
Materials and methods
Source of culture
The fungus used in the present study was isolated from molasses sample collected from Shri SSTPSK sugar industry, Shahada, Nandurbar (Dist.), Maharashtra, India. The culture was identified as Aspergillus sp. based on its characteristic white colonies, which turned black upon maturity and septate mycelium bearing single celled conidia in chain borne at the end of sterigmata, produced from conidiophores. The culture was routinely maintained on potato dextrose agar at 4 °C.
Production of GI
A loopful growth of Aspergillus sp. from Czepek-Dox agar slant was inoculated in GPB containing (g/l), peptone—5, K2HPO4—5 and glucose—5 and pH set to 7 with 1 N HCl and incubated at 28 °C for 42 h for sporulation. GI activity was monitored in screening medium containing (g/l), peptone—0.1, yeast extract—0.1, MnCl2—0.001, FeSO4⋅7H2O—0.001, CoCl2⋅6H2O—0.002, MgSO4⋅7H2O—0.1, glucose—0.1 and corn steep liquor—0.2%. The pH was adjusted to 7. The fungal culture was raised from the initial population of 7 × 105 spores/ml and incubated at 28 °C for 42 h at 120 rpm (Joseph et al. 1977).
GI assay
Cell free filtrate obtained by filtration (filter membrane system) was assayed for enzyme activity. GI activity was measured in 2.4 ml reaction mixture containing 0.5 ml of filtrate (crude enzyme), 0.5 m/l 0.05 M phosphate buffer (pH 7.2), 0.5 ml 0.01 M CaCl2, 0.1 ml 0.5 M glucose, 0.2 ml 0.1 M MgSO4.7H2O and 0.6 ml distilled water. The mixture was incubated at 60 °C for 1 h and fructose formed was estimated following Selwinoff’s method (Gupta and Bhargav 1985), in which 1 ml of reaction mixture and 3 ml of Selwinoff reagent were heated to boiling in a boiling water bath, cooled to room temperature (28 °C) and absorbance was recorded at 540 nm with fructose as an internal standard. Residual glucose was spectrophotmetrically estimated with dinitrosalysilic acid (DNS) reagent using maltose as an internal standard (Miller 1972), the reaction mixture comprised of 1 ml of supernatant and 0.5 ml DNS reagent, was incubated in a boiling water bath for 10 min, cooled to room temperature and residual sugar was estimated at 540 nm. One ml supernatant and 5.5 ml alkaline CuSO4 were incubated at room temperature for 10 min followed by the addition of 0.5 ml Folin Ciocalteau reagent (freshly prepared and 1:1 diluted) and incubated at room temperature for 30 min. Protein content of the mixture was estimated by measuring the absorbance at 650 nm (Lowry et al. 1951), using UV-visible spectrophotometer (Systronic, India) with bovine serum albumin as internal standard. One unit (U) of GI was defined as the amount of enzyme that produced 1 μM of fructose per min per mg (Joseph et al. 1977).
Influence of incubation period
Influence of incubation period on biomass and GI synthesis and activity was studied by growing Aspergillus sp. in GPB at 30 °C for 72 h at 120 rpm. Samples withdrawn after 6 h intervals up to 72 h were observed for the estimation of biomass (dry weight of mycelium) and GI activity.
Influence of carbon sources
To examine the effect of various carbon sources on biomass and GI synthesis and activity, Aspergillus sp. was separately grown in 100 ml GPB supplemented individually with 1 g/l each of glucose, sucrose, maltose, mannitol, arabinose, xylose and starch hydrolysate. Following 42 h incubation at 30 °C and 120 rpm, each set was subjected for the estimation of biomass and GI activity.
Influence of substrate concentration
In order to optimize the substrate concentration for optimum biomass and GI synthesis and activity, Aspergillus sp. was grown in 100 ml GPB separately prepared with glucose concentrations of 1–60 g/l. The broth was incubated at 30 °C for 42 h at 120 rpm. Following the incubation, pellets of biomass were dried at 60 °C to a constant weight. Cell free filtrate obtained by membrane filtration was used for the estimation of GI units and GI activity.
Influence of nitrogen sources
For studying the effect of various nitrogen sources, Aspergillus sp. was separately grown in 100 ml GPB externally supplemented individually with 1 g/l each of yeast extract, peptone, ammonium sulphate (NH4)2SO4, ammonium nitrate (NH4NO3), dibasic ammonium phosphate ((NH4)3PO4), and ammonium chloride (NH4Cl). After 42 h incubation at 30 °C and 120 rpm, each set was analyzed for biomass and GI activity.
Influence of mineral salts
For detecting the influence of mineral salts each of 100 ml of GPB was individually supplemented with 25 μM of CoCl2, MoCl2, MgCl2, MnCl2 and ZnCl2. The flasks were incubated at 30 °C and 120 rpm for 42 h followed by the estimation of biomass and GI activity.
Production of GI under optimized conditions
Modified GPB with all optimized parameters containing (g/l), yeast extract—1, K2HPO4-5, glucose—1, 25 μM of MgCl2, pH set to 7 with 1 N HCl was inoculated with Aspergillus sp. (7 × 105 spores/ml) and incubated at 28 °C for 42 h at 120 rpm. Following the incubation, biomass and GI activity was measured as described earlier.
Statistical analysis
All experiments were carried out in triplicate and the results were the mean of three values. The results were statistically analyzed for mean standard deviation and Spearman rank correlation coefficient (r2) to ensure the significance of effect of different parameters on biomass and GI.
Results and discussion
GI production
The conversion of glucose to fructose by adding cell free filtrate (crude enzyme) of Aspergillus sp. to the glucose containing reaction mixture indicated the presence of extracellular GI in the cell free filtrate. So far, the extracellular GI has been reported only in Streptomyces sp (Joseph et al. 1977). This is the first report on the presence of extracellular GI from Aspergillus sp. The production of GI in GPB without xylose (inducer) supplementation indicated the constitutive synthesis of GI, which has been reported from other organisms like Actinoplanes missouriensis, an organism used for the commercial production of GI (Anheuser Bosch Inc. 1974). Constitutive synthesis of GI has significant potential to improve the existing process for HFCS production. It shortens the reaction time and minimizes the process economics (Bhosale et al. 1990). For the production of GI, most organisms require xylose as an inducer. However, purified xylose is an expensive substrate for commercial production of GI. Takasaki (1966) described the production of xylanase by S. albus in the presence of xylan, and the main product from xylan was xylobiose. Natake and Yoshimura (1964) have reported xylanase production from S. bikiniensis in xylan containing medium. These organisms were able to produce GI on xylan containing materials, thus reducing the production cost, because xylan-containing materials are cheaper than purified xylose. Our isolate produced GI in the medium without xylose (inducer) supplementation. These characteristics are especially important in the economical production of GI, because xylose is expensive and its use in production medium increases the cost of GI production.
Influence of incubation period
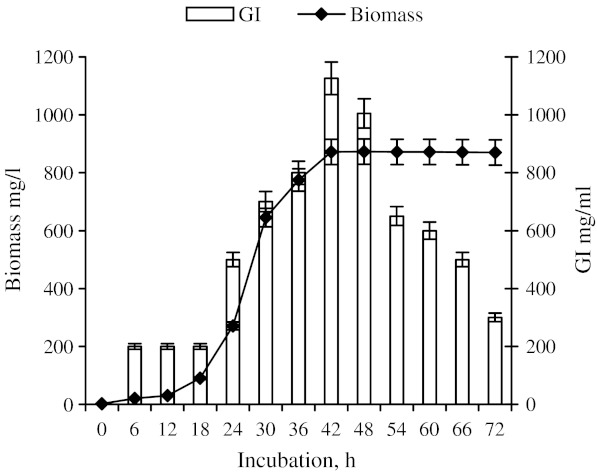
The fungus showed a lag phase of about 12 h, it reached the log phase in 30–42 h and then attained stationary phase which continued up to 72 h. Enzyme synthesis started after 6 h and increased up to 42 h. Maximum biomass (874 mg/l) and optimum enzyme units (1126 U) were produced at 42 h incubation (Fig. 1). After 42 h incubation the biomass yield remained constant while the enzyme synthesis drastically decreased. No further increase in biomass and enzyme units after 42 h may be due the entry of organism into stationary phase, when the growth and metabolic activity ceases.
Fig. 1.
Influence of incubation period on biomass and activity of glucose isomerase (GI) produced by Aspergillus sp. (n = 3)
Influence of carbon source
Table 1 shows the level of GI produced by Aspergillus sp. grown on various carbon sources. The organism produced a maximum of 1126 and 996 U of GI with glucose and xylose, respectively in 42 h (Table 1). GI activity reported in this study is higher than earlier report (Takasaki 1966). The level of GI produced on other carbon sources was significantly lower when compared to glucose. GI activity has been detected in some organisms grown on a medium containing glucose or xylose (Natake and Yoshimura 1964; Bravo et al. 1998). The Aspergillus sp. produced good quantity of the enzyme in a medium containing glucose but lacking xylose (inducer). Thus, it has a great economic advantage over the previous processes, because glucose is less expensive than xylose or xylan.
Table 1.
Influence of carbon and nitrogen source on biomass and activity of glucose isomerase (GI) produced by Aspergillus sp.
| Biomass mg/l | GI unitsm mg/ml | GI activity, U/mg | |
|---|---|---|---|
| Carbon sources, 1 g/l, | |||
| Glucose | 872 ± 1.21 | 1126 ± 1.0 | 1.2 ± 1.01 |
| Sucrose | 752 ± 0.15 | 112 ± 0.07 | 1.0 ± 0.03 |
| Maltose | 726 ± 0.13 | 102 ± 0.06 | 1.0 ± 0.03 |
| Mannitol | 640 ± 0.12 | 90 ± 0.04 | 0.9 ± 0.01 |
| Arabionse | 560 ± 0.11 | 86 ± 0.03 | 0.8 ± 0.01 |
| Xylose | 841 ± 0.85 | 996 ± 0.90 | 1.2 ± 0.80 |
| Starch hydrolysate | 811 ± 0.17 | 52 ± 0.01 | 0.7 ± 0.02 |
| Nitrogen sources, 1 g/l | |||
| Organic nitrogen source | |||
| Yeast extract | 872 ± 1.02 | 1120 ± 1.01 | 1.2 ± 1.10 |
| Peptone | 790 ± 0.15 | 1011 ± 0.90 | 1.1 ± 0.80 |
| Inorganic nitrogen source | |||
| NH4SO4 | 861 ± 0.85 | 1116 ± 0.78 | 1.0 ± 0.81 |
| NH4NO3 | 740 ± 0.13 | 838 ± 0.15 | 0.9 ± 0.06 |
| NH4(PO)4 | 796 ± 0.15 | 876 ± 0.17 | 0.8 ± 0.21 |
| NH4Cl | 702 ± 0.05 | 795 ± 0.22 | 0.6 ± 0.31 |
r2 for effect of carbon source = 0.320, r2 for effect of nitrogen source = 0.629, (n = 3)
Influence of substrate concentration
Increase in biomass was reported with increasing concentration of glucose, 40 g/l being optimum for maximum growth (Table 2). Further increase in the concentration of glucose inhibited the growth of organism. Enzyme synthesis continued at all concentrations of glucose; however optimum (1126 U) enzyme synthesis and activity (10.5 U/mg) occurred at 1 g/l concentration of glucose. These values are comparable with the earlier report (Koyal et al. 1992). Increase in the concentration of glucose did not significantly enhance enzyme activity. Inhibition of GI activity at high substrate concentration has been documented by Bravo et al. (1998). A positive control mechanism by catabolite repression of xyl operons exerted by glucose repression has been reported in Salmonella typhimurium and E. coli (Marcel et al. 1987). Glucose repression at the level of transcription has also been reported (Jacob et al. 1991). It is also known that glucose shows additional inducer exclusion type of repression of xyl, which is dependent on the concentration of glucose (Bravo et al. 1998). Bhosale et al. (1990) have reported that exposure of GI to high sugar concentration led to non-enzymatic glycation of lysine residues and subsequent inactivation of the enzyme.
Table 2.
Influence of substrate concentration on biomass and activity of glucose isomerase (GI) produced by Aspergillus sp.
| Glucose content, g/l | Biomass, mg | Glucose left, mg/ml | Fructose, mg/ml | Proteins, mg/ml | GI units, mg/ml | GI activity, U/mg |
|---|---|---|---|---|---|---|
| 1 | 752 ± 1.12 | 640 ± 1.01 | 162 ± 1.11 | 1530 ± 1.04 | 1126 ± 1.02 | 10.5 ± 1.13 |
| 5 | 872 ± 1.01 | 970 ± 0.93 | 132 ± 0.70 | 1500 ± 0.67 | 732 ± 0.56 | 8.8 ± 0.61 |
| 10 | 603 ± 0.91 | 790 ± 0.81 | 128 ± 0.31 | 1475 ± 0.60 | 710 ± 0.41 | 8.6 ± 0.51 |
| 20 | 197 ± 0.64 | 1000 ± 0.61 | 132 ± 0.32 | 1440 ± 0.56 | 732 ± 0.37 | 9.1 ± 0.42 |
| 30 | 1315 ± 0.44 | 1000 ± 0.44 | 137 ± 0.27 | 1410 ± 0.47 | 760 ± 0.25 | 9.7 ± 0.32 |
| 40 | 1640 ± 0.31 | 1032 ± 0.32 | 135 ± 0.11 | 1370 ± 0.21 | 749 ± 0.19 | 9.8 ± 0.24 |
| 50 | 1082 ± 0.22 | 1032 ± 0.25 | 135 ± 0.12 | 1220 ± 0.10 | 749 ± 0.11 | 1.1 ± 0.11 |
| 60 | 1075 ± 0.12 | 1014 ± 0.16 | 140 ± 0.11 | 1123 ± 0.11 | 777 ± 0.10 | 1.2 ± 0.10 |
r2 = 0.016, (n = 3)
Influence of nitrogen sources
Among the organic nitrogen sources, yeast extract produced the highest level of GI activity (1120 U) yeast extract and peptone also produced substantial quantity of GI (Table 1). However, these nitrogen sources are too expensive for practical use. Inorganic nitrogen sources were ineffective except ammonium sulphate, which yielded 1116 U (mg/ml) of GI (Table 1). As a nitrogen source, peptone, yeast extract, beef extract, and malt extract have been used for GI production by Streptomyces, Lactobacillus, and Paracolobactrum (Chou et al. 1976; Vaher and Kauppinen 1977), whereas inorganic nitrogen sources, including dibasic ammonium phosphate and ammonium chloride were used for Aerobacter, Escherichia and Bacillus (Natake and Yoshimura 1964; Strandberg and Smiley 1971).
Influence of mineral salts
Addition of Mg2+ and Mn2+ significantly increased the biomass; however, none of the metals under study enhanced GI activity (Table 3). GI producing organisms generally require Co2+ to stimulate the formation of GI (Giovenco and Morisi 1973). Our study indicated that GI production by Aspergillus sp is independent of Co2+ supplementation. The elimination of Co2+ in the culture medium is important in commercial production of the enzyme because Co2+ is an environmental pollutant (Bucke 1977; Kraus et al. 1994).
Table 3.
Influence of minerals (concentration = 25 μm) on biomass and activity of glucose isomerase (GI) produced by Aspergillus sp.
| Mineral salt | Biomass mg/l | GI units, mg/ml | GI activity, U/mg |
|---|---|---|---|
| Control | 872 ± 1.00 | 1126 ± 1.05 | 1.2 ± 1.03 |
| CoCl2 | 995 ± 0.71 | 732 ± 0.30 | 0.4 ± 0.41 |
| MoCl2 | 792 ± 0.12 | 710 ± 0.22 | 0.6 ± 0.17 |
| MgCl2 | 1425 ± 0.11 | 1124 ± 0.05 | 1.2 ± 0.10 |
| MnCl2 | 1422 ± 0.05 | 1099 ± 0.02 | 1.1 ± 0.01 |
| ZnCl2 | 1370 ± 0.02 | 728 ± 0.01 | 0.4 ± 0.02 |
r2 = 0.049, (n = 3)
Production of GI under optimized conditions
Under optimized conditions in modified GPB Aspergillus sp yielded more biomass (1650 mg), synthesized more (1232 U) GI with increased GI activity of 12.9 U/mg.
Statistical analysis of data showed a positive correlation between the effect of different carbon (r2 = 0.320) and nitrogen source (r2 = 0.629), substrate concentration (r2 = 0.016) and metal ions (r2 = 0.049) on biomass and GI.
Conclusion
Aspergillus sp. produced significant quantities of extracellular GI in GPB without xylose (inducer). Under optimized conditions in modified GPB significant increase in biomass (1.9 fold), enzyme synthesis (1.1 fold) and activity (1.23 fold) of GI was observed. Constitutive production of extracellular GI from Aspergillus sp. can be of great commercial value because GI produced by most of the microbial genera is intracellular and GI producing organisms require D-xylose (inducer) which is expensive for use on commercial scale.
References
- Anheuser-Bosch Inc (1974) Method of making glucose isomerase and using same to convert glucose to fructose. UK Patent 1 399 408, Sec D 50: 113-123
- Bhosale SH, Rao MB, Deshpande VV. Molecular and industrial aspects of glucose isomerase. Microbiol Rev. 1990;60:2870–3000. doi: 10.1128/mr.60.2.280-300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo V, Juradoi E, Luzoni E, Cruz N. Kinetics of fructose glucose isomerisation with sweetzyme type A. Can J Chem Eng. 1998;76:778–793. doi: 10.1002/cjce.5450760413. [DOI] [Google Scholar]
- Bucke C. Industrial glucose isomerase. In: Wiseman A, editor. Topics in enzyme and fermentation biotechnology. Chichester: Ellis Horwood Ltd; 1977. pp. 147–171. [Google Scholar]
- Chou CC, Ladisch MR, Tsao GT. Studies on glucose isomerase from a Streptomyces species. Appl Environ Microbiol. 1976;32:489–493. doi: 10.1128/aem.32.4.489-493.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crueger A, Crueger W. Glucose transforming enzymes. In: Fogarty WM, Kelly CT, editors. Microbial enzymes biotechnology. London: Elsevier Science; 1992. pp. 177–226. [Google Scholar]
- da Silva EAB, de Souza AAU, Rodrigues AE, Guelli SMA. Glucose isomerization in simulated moving bed reactor by glucose isomerase. Braz Arch Biol Biotechnol. 2006;49:491–502. [Google Scholar]
- Dhungel B, Subedi M, Tiwari KB, Thapa SU, Pokhrel S, Agrawal VP. Thermostable glucose isomerase from psychrotolerant Streptomyces species. Int J Life Sci. 2007;1:6–10. [Google Scholar]
- Frost GM, Moss DA. Xylose (glucose) isomerase. In: Rehm HJ, Reid G, editors. Biotechnology. Weinheim: VCH Publ; 1987. pp. 172–180. [Google Scholar]
- Gaikwad SM, Deshpande VV. Immobilization of glucose isomerase on indion 48-R. Enzyme Microb Technol. 1992;14:8555–8558. [Google Scholar]
- Giovenco S, Morisi PP. Properties of free and immobilized glucose isomerase. FEBS Lett. 1973;36:57–60. doi: 10.1016/0014-5793(73)80336-9. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Bhargav S. Carbohydrates. New Delhi: Practical Biochemistry, Himalya Publ House; 1985. pp. 15–16. [Google Scholar]
- Jacob S, Allmansberger R, Gartner D, Hillen W. Catabolite repression of the operon for xylose utilization from Bacillus subtilis W23 is mediated at the level of transcription and depends on a cis site in the xyl A reading frame. Mol Gen Genet. 1991;229:189–196. doi: 10.1007/BF00272155. [DOI] [PubMed] [Google Scholar]
- Joseph R, Shanthamma MS, Murthy VS. Isolation of Streptomyces spp. having high glucose isomerase activity and assessment of their efficiency in the production of fructose syrups. J Food Sci Technol. 1977;14:73–77. [Google Scholar]
- Koyal SN, Mukhoupadhyaya AK, Mukhertji RN. Isolation and purification of glucose isomerase and glucosidase. In: Mukherji RN, editor. Downstream processing in biotechnology. New York: Pregmon; 1992. pp. 21–25. [Google Scholar]
- Kraus A, Hueck C, Gartner D, Hilen W. Catabolite repression of the Bacillus subtilis xyl operon involves a cis element functional in the context of an unaltered sequence and glucose exerts additional xylR-dependent repression. J Bacteriol. 1994;176:1738–1745. doi: 10.1128/jb.176.6.1738-1745.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Hayes LE, Long ME (1972) Process of preparing glucose isomerase, United States Patent US 3645848
- Lowry OH, Rosenbrough NH, Farr AL, Randall RJ. Protein measurement with the Folin Phenol Reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marcel T, Drocourt D, Tiraby G. Genetics and regulation of D-xylose utilization in Salmonella typhimurium LTZ. J Bacteriol. 1987;139:71–79. doi: 10.1128/jb.139.1.71-79.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RO, Kooi ER. Enzymatic conversion of D-glucose to D-fructose. Sci. 1957;125:648–649. doi: 10.1126/science.125.3249.648. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalysilic acid for determination of reducing sugars. Anal Chem. 1972;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Natake M, Yoshimura S. Studies on glucose isomerase of bacteria. II. The glucose isomerizing activity of Escherichia intermedia, strain HN-500. Agric Biol Chem. 1964;28:505–509. [Google Scholar]
- Strandberg GW, Smiley KL. Free and immobilized glucose isomerase from Streptomyces phaeochromogenes. Appl Microbiol. 1971;21:588–593. doi: 10.1128/am.21.4.588-593.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki Y. Studies on sugar-isomerizing enzyme: production and utilization of glucose isomerase from Streptomyces sp. Agric Biol Chem. 1966;30:1247–1253. [Google Scholar]
- Tumturk GD, Haydar ASAK, Nesrin H. Immobilization of glucose isomerase in surface-modified alginate gel beads. J Food Biochem. 2008;32:234–246. doi: 10.1111/j.1745-4514.2008.00171.x. [DOI] [Google Scholar]
- Vaher M, Kauppinen V. Improved microbial glucose isomerase production. Process Biochem. 1977;12:5–8. [Google Scholar]