Abstract
Effect of drying on protein, trypsin inhibitor (TI), nitrogen solubility, water absorption, colour and electrophoretic patterns of flours prepared from germinated soybean varieties ‘JS 9305’ and ‘MAUS 47’ was studied. Drying included sun drying (35–40°C), steaming followed by hot air oven drying at 60°C, hot air oven drying at 100°C and microwave heating at 400 W and 630 W. Sun drying did not reduce the TI to the required 80% and steaming followed by drying and microwave heating affected colour, nitrogen solubility and electrophoretic pattern adversely. Drying at 100°C reduced TI to safe limits and did not adversely affect the nitrogen solubility, colour and electrophoretic patterns and can be recommended for commercial scale drying of germinated soybean to prepare good quality soy flour for product development. Drying at 35–40°C minimally affected the colour, nitrogen solubility and electrophoretic pattern and can be used to prepare soy flour for bakery purposes.
Keywords: Drying, Sprouted soybean, Nutritional properties, Functional properties, Electrophoretic patterns
Recently increased interest has been shown for germinated soybean as a potential protective human food. The proteins in germinated soybean are of very high quality akin to animal proteins. Development of food products from germinated soybean may increase the versatility and utility of soybean for both developing and industrialized countries as germination modifies certain specific biologically active components, palatability and nutritive value. The thermolabile anti-nutritional factors in germinated seeds are easier to inhibit by heat treatment than those of dry beans. The germinated seeds also require less cooking time for the same degree of palatability (Bau et al. 1997). Flours from germinated soybean may have better bread making properties than flours made from mature soybean. Thermal treatment of germinated soybean is necessary as the process of germination brings down the trypsin inhibitor (TI) content by about 60%, whereas 80% inactivation has been recommended as being safe for consumption (Leontowicz et al. 1998). The levels of TI should not be below 20%, as this level of inhibitor has been known to be beneficial because of its biological activities in pharmacological and medical applications (Scarafoni et al. 2007). However, overheating will cause a loss in nutritional value by reducing the protein efficiency ratio (Salvage et al. 1995). This is due to destruction or rendering of several essential amino acids, such as cystine and lysine unavailable (De Valle 1981). In addition, overheating adversely affects the functionality of soy in a food system. An optimal thermal process inactivates deleterious enzymes, microbes and biologically active components, while maximizing retention of nutrients and other quality attributes such as flavour, colour, texture and functionality. Temperature, time and the method of drying of germinated soybean may greatly affect the quality of flour produced in terms of its appearance, nutritional quality and functional quality. Therefore, an attempt has been made to study the effects of different drying temperatures and methods to produce best quality sprouted soy flour.
Materials and methods
Two varieties of soybean ‘JS9305’ and ‘MAUS 47’ were procured from Agharkar Research Institute, Pune and National Research Centre on Soybean, Indore respectively to represent the major soybean growing states of Madhya Pradesh and Maharashtra. These cultivars were harvested in November 2006 and stored at 20°C for further studies. Soybean seeds were cleaned thoroughly to make free from dust, dirt, stubbles and foreign matter. Spoiled or immature/ broken seeds were discarded. The seeds were surface sterilized with 0.1% (w/v) potassium permanganate solution and rinsed thoroughly with distilled water to remove any traces of potassium permanganate. About 100 g of cleaned and sterilized seeds were soaked in 300 ml distilled water for 4 h followed by drainage of excess water and rinsing with distilled water. The seeds were kept on filter paper in a single layer in sterile Petridishes and placed in the seed germinator (Indosaw, Ambala, India) at the 30°C and 90% RH for 72 h.
Drying of sprouts
The sprouts obtained after germination were subjected to various modes of drying to bring down the moisture content between 6 and 8%, a level recommended for the production of soyflour (Gandhi 2008). The drying treatments were:
T1: Unprocessed soybean (negative control), T2: Sun drying (35–40°C), T3: Hot air oven drying (100°C), T4: Steaming for 10 min followed by hot air oven drying (60°C), T5: Microwave heating at 400 W for 15 min, T6: Microwave heating at 630 W for 9 min and T7: Soybean boiled and dried in hot air oven (60°C) (positive control).
Sun drying was done in summer where the maximum temperature was around 45°C. The average temperature in the day from sunrise to sunset was around 35°C. In Indian villages where electricity supply is uncertain, this is the method followed for drying most agricultural produce.
Determination of nutrient and anti-nutrient contents: The dried samples were ground to a fine powder and defatted using cold extraction with n-hexane. The samples were sealed and stored in vacuum desiccators until used for further analysis. The moisture content and crude protein (defatted) contents were estimated by AOAC (1995) methods. TI activity was determined by the procedure of Kakade et al. (1974) as modified by Hammerstrand et al. (1981). Nitrogen solubility index (NSI) at pH 7 (Daun and DeClercq 1994), water absorption index (WAI) (Smith and Circle 1972) (which gives the amount of water that can be absorbed by the soy flour) and water holding capacity (WHC) (Heywood et al. 2002) (which gives the amount of absorbed water that can be held by the flour) were determined. The Hunter colour was measured using Lab Scan XE spectrocolorimeter ( LX16244, Hunter Associate Laboratory, Virginia) in terms of CIE ‘L’ (lightness), ‘a’ (redness and greenness) and ‘b’ (yellowness and blueness). L, a and b scale is recommended by the Commission International de 1 Eclairage (CIE) (Anon 1998). Sensor was standardized with a white and black tile to measure the colour. Flour colour at 4 different orientations was measured by placing the flour over the 10 mm aperture of sample measurement port of the colorimeter. Average L, a, b, Whitness index (Wi) and Yellowness index (Yi) values of samples were reported.
Electrophoresis
Dry sprouted soybean flour (100 mg) was suspended in 2 ml of prechilled Tris buffer (50 mM Tris HCl), pH 7.6; 1 mM DDT, 150 mM NaCl and 1 ml EDTA) for 2 h (20°C) in an orbital shaker and centrifuged for 10 min, at 35,500 x g and 20°C (JA-20 Beckman Rotor, Mumbai, India). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (1-DE SDS-PAGE) was performed on a vertical slab gel (160 × 200 × 1 mm; Model Protean xi Cell, Bio Rad, Hercules, USA) according to Laemmli (1970) with a 12% separating gel (acrylamide to bis acrylamide ratio 29:1) and a 4% (w/v) stacking gel. Aliquots containing 50–100 μg of protein were applied onto each of the 7 wells used for samples and one outside well was used for standard. The standard marker was a Broad band Protein Molecular Weight Marker, which is a mixture of purified proteins, supplied, pre-diluted with sample loading buffer for direct loading to an SDS PAGE by Ms Bangalore Genie, Bangalore, India. The marker contained myosin rabbit muscle (2,05,000 KDa), phosphorylase b (97,400 KDa), bovine serum albumin (66,000 KDa), ovalbumin (43,000 KDa), carbonic anhydrase (29,000 KDa), soybean TI (20,100 KDa), lysozyme (14,300 KDa), aprotinin (6,500 KDa) and insulin (α and β chains) 3,000 (2300–3400 KDa). 1-DE SDS-PAGE was performed at constant current first at 15 mA for 1 h followed by 25 mA. At the end of the run, the gels were stained with Brilliant (Coomassie) Blue R-250 (0.05%w/v) in methanol-acetic acid-water (25:10:65 v/v/v), destained overnight with methanol-acetic acid-water (25:7:68 v/v/v) and washed with deionized water. Detection of protein bands was performed using Biovis/ D gel analysis software.
The experiments were conducted in triplicate and the average values were computed. The data were statistically analysed using statistical packages SYSSTAT 7.0 for Windows.
Results and discussion
The moisture content of soybean seeds ranged from 5.2 in the raw unprocessed seeds to 7.1 in germinated seeds that were sun dried (Table 1). The moisture content of the germinated seeds was high as the process of germination involved soaking in water (Agrahar-Murugkar and Jha 2009). There was no significant difference (p < 0.05) in the protein contents under different treatments. However, the raw seeds of both varieties contained higher protein contents as compared to sprouted counterparts. Sprouting caused a decline in the levels of protein as has been reported by previous researchers (Bates et al. 1977). The decrease may be due to increases in proteolytic activity during the initial stages of germination and also because of loss of some soluble proteins due to leaching as well as degradation of proteins like TI and lipoxygenase (Adjei-Twum et al. 1976). The boiled and dried samples showed the highest decline in protein content probably due to loss of water-soluble proteins during soaking and boiling.
Table 1.
Effect of different treatments on quality of soybean
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | |
|---|---|---|---|---|---|---|---|
| – | 360 min | 120 min | 180 min | 15 min | 9 min | 180 min | |
| ‘JS9305’ | |||||||
| Crude protein, % (defatted) | 49.6 ± 0.73 | 45.7 ± 2.9 | 44.8 ± 0.23 | 46.0 ± 0.83 | 45.6 ± 2.9 | 45.5 ± 0.00 | 44.2 ± 0.01 |
| TI mg/100 g | 46.8g ± 1.05 (0) | 18.4f ± 0.21 (60.7) | 12.0e ± 0.47 (75.0) | 4.8a ± 0.32 (81.9) | 4.7a ± 0.11 (89.9) | 4.4a ± 0.21 (90.6) | 4.2a ± 0.63 (91.0) |
| NSI | 68.3d ± 0.84 | 73.1e ± 0.46 | 55.4c ± 2.01 | 46.5b ± 0.04 | 63.0d ± 2.42 | 54.6c ± 0.05 | 37.6a ± 0.82 |
| WAI | 167.2a ± 8.28 | 167.7a ± 2.50 | 181.5d ± 2.88 | 180.7d ± 3.09 | 172.1bc ± 3.23 | 168.0a ± 5.62 | 272.6g ± 2.29 |
| WHC | 1.6a ± 0.04 | 1.6a ± 0.03 | 1.9bc ± 0.02 | 2.0c ± 0.02 | 2.0c ± 0.02 | 2.0c ± 0.31 | 2.4d ± 0.04 |
| Hunter colour | |||||||
| L | 81.6c ± 2.10 | 82.3cd ± 1.98 | 77.6b ± 2.03 | 74.1a ± 1.76 | 77.1b ± 1.45 | 75.0a ± 1.77 | 80.5c ± 1.69 |
| a | 0.50a ± 0.00 | 1.26b ± 0.02 | 1.64c ± 0.01 | 2.00d ± 0.36 | 2.69e ± 0.22 | 1.60c ± 0.11 | 2.12d ± 0.33 |
| b | 24.4b ± 1.00 | 23.4ab ± 0.56 | 27.2c ± 0.78 | 28.8d ± 1.55 | 29.1d ± 1.25 | 25.9b ± 1.36 | 25.3b ± 1.00 |
| Yi | 43.7b ± 0.89 | 45.0c ± 1.02 | 54.4f ± 1.36 | 57.1g ± 1.32 | 56.4g ± 2.36 | 54.9f ± 3.3 | 49.3e ± 1.56 |
| Wi | −53.6b ± 1.12 | −59.5c ± 1.22 | −90.7e ± 1.71 | −111.7f ± 2.89 | −101.9e ± 5.02 | −92.9e ± 1.99 | −74.2d ± 5.41 |
| ‘MAUS47’ | |||||||
| Crude protein, % (defatted) | 47.7 ± 0.17 | 46.3 ± 0.95 | 45.3 ± 0.01 | 45.3 ± 0.00 | 45.2 ± 1.3 | 45.9 ± 0.65 | 44.5 ± 2.6 |
| TI mg/100 g | 46.6g ± 0.26 (0) | 18.6f ± 0.42 (60.0) | 9.1d ± 0.63 (80.6) | 5.6b ± 0.47 (84.6) | 7.0c ± 0.63 (82.7) | 5.4ab ± 0.32 (88.9) | 4.5a ± 0.89 (90.7) |
| NSI | 70.4de ± 0.29 | 78.4e ± 1.23 | 62.0cd ± 0.90 | 45.1b ± 0.79 | 52.6bc ± 2.32 | 50.0b ± 1.67 | 43.8b ± 3.38 |
| WAI | 170.3b ± 1.58 | 170.8b ± 0.92 | 187.5e ± 4.67 | 181.0d ± 2.51 | 179.3d ± 5.68 | 173.2c ± 5.86 | 216.5f ± 1.48 |
| WHC | 1.8b ± 0.17 | 1.8b ± 0.05 | 1.9bc ± 0.06 | 2.2cd ± 0.01 | 1.9bc ± 0.01 | 2.0c ± 0.09 | 2.4d ± 0.02 |
| Hunter colour | |||||||
| L | 83.5d ± 2.65 | 80.0c ± 1.88 | 78.0b ± 1.22 | 78.4b ± 2.58 | 77.2b ± 2.54 | 78.5b ± 3.01 | 81.5c ± 2.89 |
| a | 0.66a ± 0.03 | 1.14b ± 0.02 | 1.56c ± 0.05 | 1.21b ± 0.08 | 1.98d ± 0.56 | 1.26b ± 0.05 | 1.67c ± 0.03 |
| b | 22.2a ± 1.02 | 21.7a ± 1.14 | 23.6ab ± 1.74 | 25.9b ± 2.41 | 24.8b ± 1.58 | 24.0ab ± 1.12 | 22.0a ± 1.05 |
| Yi | 41.4a ± 2.36 | 42.4a ± 2.22 | 47.6d ± 1.56 | 50.2e ± 2.03 | 48.8d ± 1.59 | 47.1d ± 2.36 | 42.5a ± 2.55 |
| Wi | −48.6a ± 1.12 | −56.6bc ± 3.26 | −71.6d ± 1.99 | −86.3d ± 3.24 | −76.5d ± 3.02 | −76.6d ± 3.35 | −52.3b ± 3.01 |
| Moisture, % | 5.2 | 7.1 | 6.5 | 6.4 | 6.8 | 6.8 | 6.7 |
T1—Unprocessed (Negative control), T2—Sprouted and sundried, T3—Hot air oven drying (100°C), T4—Steaming for 10 min and hot air oven drying (60°C),T5—Microwave drying at 400 W, T6—Microwave drying at 630 W, T7—Boiled and dried at 60°C (Positive control).NSI—Nitrogen solubility index, WAI—Water absorption index, WHC—Water hydration capacity, min-Drying time in min, values in parenthesis in TI indicates % inhibition Means having different superscripts are significantly (p < 0.05) different (n = 3)
Drying reduced (p < 0.05) the levels of TI from 46.8 and 46.6 mg/100 g in unprocessed samples to 4.2 and 4.5 mg/100 g in ‘JS9305’ and ‘MAUS’ in boiled and dried samples, respectively (Table 1). The reduction in TI in sprouted and sun dried samples to 18.4 and 18.6 mg/100 g in ‘JS9305’ and ‘MAUS 47’, respectively was due to the process of soaking and germination as mild heat treatment was involved. The decrease in TI activity before germination could be attributed to the leaching of soluble fractions during soaking in which TIs are a part (Mostafa et al. 1987). Germination can degrade both Kunitz soybean TI and the major Bowman- Birk soybean TI (Collins and Sand 1976). The decrease in TI may also be due to the proteolytic activity of enzymes, which are activated during germination (Chauhan and Chauhan 2007). The reduction in TI further increased significantly as the severity of the heat treatments increased from T3 to T7. Since TI is thermolabile and completely destroyed at 120°C, drying at temperatures from 50 to 90°C did not bring down the levels of TI to the required safe levels in both varieties. Drying at 100°C resulted in percentage inhibition of 75.0 in ‘JS9305’ and 80.6 in ‘MAUS 47’. More severe heat treatments reduced the TI content to more than the required 80%.
Solubility profile is a good indicator of functionality (Mattil 1971) and directly relates to many other important functional properties such as emulsifying capacity and stability (Volkert and Klien 1979), foaming, gelation and viscosity. The NSI was highest in the samples that were sprouted and sun dried, which were subjected to minimal heat treatment (Table 1). It has also been reported (Pomeranz et al. 1977) where increased levels of NSI could be due to a compensatory increase in free amino acids and peptides (Adjei-Twum et al. 1976) and increase in non-protein nitrogenous constituents during germination. The NSI decreased progressively (p < 0.05) from 73 to 37 in ‘JS 9305’ and 78 to 44 in ‘MAUS 47’ as the severity of heat treatment increased. The lowest NSI was in samples that had been boiled and dried. The intensity of heat treatment, therefore, serves as an important index as far as NSI is concerned. Results from other studies also showed that functional properties like NSI was reduced as heating time was increased (McWatters and Holmes 1979). In microwave heated samples of both varieties however, even though the intensity of heat was high due to a much shorter duration for drying, the NSI did not fall to a great extent. The levels of NSI that are recommended for use range from 90 (in baked goods) to 40–60 (in products involving dry cooking or roasting (BIS 1975). Wheat flour enriched with flour from germinated soybean could be the possible answer in producing low-priced, nutritionally improved, protein-enriched bread (Pomeranz et al. 1977). For use in bakery products, flour from germinated and sun dried soybean is ideal due to its improved functional value.
The WAI was affected (p < 0.05) by the intensity of heat treatment (Table 1). It ranged from 167 in raw samples to 272 in blanched and dried samples of ‘JS 9305’ and from 170 in raw samples to 217 in blanched and dried samples of ‘MAUS 47’. In treatments T5 to T6, where the microwave heat treatment was for a short duration the WAI did not increase as with other heat treatments. Wu and Inglett (1974) found similar results and reported increased water absorption capacity of soyflour due to heat processing. High molecular weight proteins, namely, 7S and 11S constitute nearly 70% of soy protein (Naismith 1955). These proteins consist of sub unit structures and the proteins are disassociated on heating (Catsimpoolas et al. 1970), which could be why heat treatment of soy flour increased the water absorption capacity. Carbohydrates may also play a role in water absorption. During heating gelatinisation of carbohydrates and swelling of crude fibre may occur and could also lead to an increase in water absorption (Narayana and Narasingha Rao 1982).
The colour parameters were all affected (p < 0.05) by the drying treatments. The quality of soybean is assessed by yellowness index. The loss in intensity of yellowness indicates the deterioration in the quality. The result showed that b value of all the treatments were positive integer and positive b* is an indicator of yellowness. L values of all the samples were ~80. Based on the values of L and b it is evident that all the samples were light yellow in colour. However, the Yi values were higher (p < 0.05) than 45 in some samples where the heat intensity was high especially in ‘JS9305’ variety where the highest value was observed in steamed and dried sample (57.1) showing browning. The Yi values of ‘MAUS 47’ were nearer to the range of 45 as compared to ‘JS9305’. Positive a value indicates redness of the sample. The value of a ranged from 0.50 to 3.64 in ‘JS9305’ and 0.66 to 2.56 in ‘MAUS 47’. Most values were almost at the centre of axis a indicating that it is neither red nor green. Over heated samples gave high positive a values as seen in samples that were microwaved at 400 W in ‘JS9305’ (2.69). McNaughton (1981) reported that colour could predict the over processing of soybean meal. In terms of colour, therefore, steaming followed by drying may not be the ideal methods of drying.
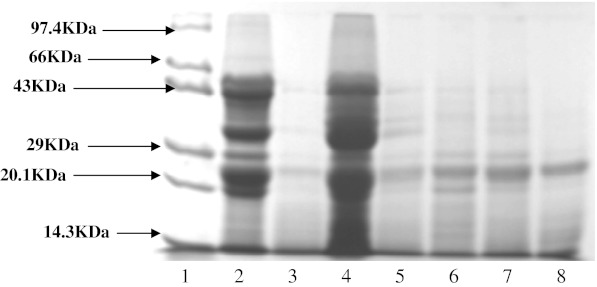
Electrophoretic patterns in unprocessed soybean ‘JS9305’ (Fig. 1) a total of 7 major protein bands having molecular weights 54.06, 48.18, 41.20, 33.95, 28.57, 23.33 and 20.46 KDa were observed .In the variety ‘MAUS 47’ (Fig. 2) two additional bands having molecular weights 64.7 and 45.70 KDa were observed along with 7 bands observed in ‘JS9305’. On germination there was a reduction in the protein band at 20.46 KDa, corresponding to Kunitz inhibitor. Vineet Kumar et al. (2006) have reported similar results, where about 80% of soybean TI activity was reduced due to the Kunitz inhibitor and this band progressively decreased in intensity as germination progressed. Sprouting of soybean ‘JS 9305’ (Lane 4), ‘MAUS 47’ (Lane 3) showed the appearance of 2 new bands having molecular weight 93.94 and 30.89 KDa. Notable variations in protein profiles were observed when the seeds were subjected to different heat treatments. This could be due to heat denaturation of proteins and consequent dissociation of both 7S and 11S globulins (Utsumi and Kinsella 1985). However, the protein with molecular weight 23.3 KDa was fairly heat stable and persisted in all forms of heat treatment. Low molecular weight proteins as a result of heat stability were not observed in these samples, which may be due to moisture content or some other factors. The heat treatment also altered the protein profiles of ‘MAUS 47’ variety though to a lower extent as compared to ‘JS9305’.
Fig. 1.
Effect of different drying treatments on the electrophoretic pattern of proteins of ‘JS 9305’ variety. 1. Marker, 2. Raw soybean (Negative control), 3.Boiled and dried (Positive control), 4. Sprouted and sun dried, 5. Sprouted and oven dried at 100°C, 6. Sprouted steamed and dried at 60°C, 7. Sprouted and microwave heated at 400W for 15 min, 8. Sprouted and microwave heated at 630W for 9 min
Fig. 2.
Effect of different drying treatments on the electrophoretic pattern of proteins of ‘MAUS 47’ variety. 1. Marker, 2. Raw soybean (Negative control), 3. Sprouted and sun dried, 4. Sprouted and oven dried at 100°C, 5. Sprouted steamed and dried at 60°C, 6. Sprouted and microwave heated at 400W for 15 min, 7. Sprouted and microwave heated at 630W for 9 min, 8. Boiled and dried (Positive control)
Conclusion
Heat treatment affected the quality of the soyflour. The variety ‘MAUS 47’ of soybean was more resistant to change in nutritional, functional and electrophoretic pattern upon drying. For bread making, sprouting followed by drying at low temperatures like sun drying can be used. For making other products, flour from sprouted seeds dried at 100°C where nutritional, functional and electrophoretic patterns are minimally affected can be used.
References
- Adjei-Twum DC, Splittstoessor WE, Vandemack JS. Use of soybeans as sprouts. Hort Sci. 1976;11:235–236. [Google Scholar]
- Agrahar-Murugkar MD, Jha K. Effect of sprouting on nutritional and functional characteristics of soybean. J Food Sci Technol. 2009;46:240–243. [Google Scholar]
- Anon (1998) Soya blue book plus. Soy Tech Inc., Me-04609, US: 312
- Official methods of analysis. 16. Washington DC: Association of Official Analytical Chemists; 1995. [Google Scholar]
- Bates RP, Knapp FW, Araujo PE. Protein quality of green mature, dry mature and sprouted soybeans. J Food Sci. 1977;42:271–272. doi: 10.1111/j.1365-2621.1977.tb01269.x. [DOI] [Google Scholar]
- Bau HM, Villaume C, Nicolas JP, Mejan L. Effect of germination on chemical composition, biochemical constituents and antinutritional factors of soybean seeds. J Sci Food Agric. 1997;73:1–9. doi: 10.1002/(SICI)1097-0010(199701)73:1<1::AID-JSFA694>3.0.CO;2-B. [DOI] [Google Scholar]
- IS: 7837, Specifications for edible grade full fat soyflour. New Delhi: Indian Standards Institution; 1975. [Google Scholar]
- Catsimpoolas N, Funk SK, Meyer EW. Thermal aggregation of glycinin sub units. Cereal Chem. 1970;47:331–344. [Google Scholar]
- Chauhan OP, Chauhan GS. Development of anti-nutrients free soy beverage using germinated soybean. J Food Sci Technol. 2007;44:62–65. [Google Scholar]
- Collins JL, Sand GG. Changes in trypsininhibitory activity in some soybean varieties during maturation and germination. J Food Sci. 1976;41:168–172. doi: 10.1111/j.1365-2621.1976.tb01127.x. [DOI] [Google Scholar]
- Daun J, DeClercq D. Comparison of combustion and Kjeldahl methods for determination of nitrogen in oilseeds. J Am Oil Chem Soc. 1994;71:1069–1072. doi: 10.1007/BF02675898. [DOI] [Google Scholar]
- De Valle FR. Nutritional qualities of soy as affected by processing. J Am Oil Chem Soc. 1981;58:419–429. doi: 10.1007/BF02582392. [DOI] [Google Scholar]
- Gandhi AP. Development of HACCP procedures for production of full fat soy flour. Int Food Res J. 2008;15:141–154. [Google Scholar]
- Hammerstrand GE, Black LT, Glover JD. Trypsin inhibitor in soy products: modification of standard analytical procedures. Cereal Chem. 1981;15:215–218. [Google Scholar]
- Heywood AA, Meyers DJ, Bailey TB, Johnson LA. Functional properties of LFSF produced by an extrusion expelling system. J Am Oil Chem Soc. 2002;79:1249–1253. doi: 10.1007/s11746-002-0635-y. [DOI] [Google Scholar]
- Kakade ML, Simons N, Liner IE. An evaluation of natural versus synthetic substrates for measuring the antitryptic activity of soybean samples. Cereal Chem. 1974;46:518–526. [Google Scholar]
- Kumar V, Rani A, Vimal P, Chauhan GS. Changes in lipoxygenase isozymes and trypsin inhibitor activity in soybean during germination at different temperatures. Food Chem. 2006;99:563–568. doi: 10.1016/j.foodchem.2005.08.024. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leontowicz H, Kostyra H, Leontowicz M, Kulasek GW. The inactivation of legume seed haemagglutinin and trypsin inhibitor boiling. In: Jansman AJM, Hill GD, Poel V, editors. Recent advances of research in antinutritonal factors in legume seeds and rape seeds. Wageningen: The Netherlands; 1998. pp. 429–432. [Google Scholar]
- Mattil KF. The functional requirements of proteins in food. J Am Oil Chem Soc. 1971;48:477–480. doi: 10.1007/BF02544664. [DOI] [PubMed] [Google Scholar]
- McNaughton JL. Colour, trypsin inhibitor and urease activity as it affects growth of broilers. JAOCS. 1981;58:321–324. doi: 10.1007/BF02582367. [DOI] [Google Scholar]
- McWatters KH, Holmes MR. Influence of moist heat on solubility and emulsification properties of soy and peanut flours. J Food Sci. 1979;44:774–776. doi: 10.1111/j.1365-2621.1979.tb08498.x. [DOI] [Google Scholar]
- Mostafa MM, Rahma EH, Rady AH. Biochemical and nutritional changes in soybean during germination. J Food Biochem. 1987;23:257–275. doi: 10.1016/0308-8146(87)90113-0. [DOI] [Google Scholar]
- Naismith WEF. Ultracentrifuge studies on soybean protein. Biochem Biophys Acta. 1955;16:203–210. doi: 10.1016/0006-3002(55)90205-5. [DOI] [PubMed] [Google Scholar]
- Narayana K, Narasingha Rao MS. Functional properties of raw and heat processed winged bean (Psophocarpus tetragonolobus) flour. J Food Sci. 1982;47:1534–1538. doi: 10.1111/j.1365-2621.1982.tb04976.x. [DOI] [Google Scholar]
- Pomeranz Y, Shogren MD, Finney KF. Flour from germinated soybeans in high-protein bread. J Food Sci. 1977;42:824–827. doi: 10.1111/j.1365-2621.1977.tb12613.x. [DOI] [Google Scholar]
- Salvage WD, Wei LS, Sutherland JW, Schmidt SJ. Biologically active components inactivation and protein insolubilization during heat processing of soybeans. J Food Sci. 1995;60:164–180. doi: 10.1111/j.1365-2621.1995.tb05630.x. [DOI] [Google Scholar]
- Scarafoni A, Magni C, Duranti M. Molecular nutraceutics as a mean to investigate the positive effects of legume seed proteins on human health. Tr Food Sci Technol. 2007;18:454–463. doi: 10.1016/j.tifs.2007.04.002. [DOI] [Google Scholar]
- Smith AK, Circle SJ. Soybeans: Chemistry and technology. West Port: The Avi Publ Co; 1972. [Google Scholar]
- Utsumi S, Kinsella JE. Structure-function relationships in food proteins: subunit interaction in heat-induced gelation of 7 S, 11 S and soy isolate proteins. J Agric Food Chem. 1985;33:297–303. doi: 10.1021/jf00062a035. [DOI] [Google Scholar]
- Volkert MA, Klien BP. Protein dispersibility and emulsion characteristics of four soy products. J Agric Food Chem. 1979;44:93–96. [Google Scholar]
- Wu YV, Inglett GE. Denaturation of plant proteins related to functionality and food applications. J Food Sci. 1974;39:218–225. doi: 10.1111/j.1365-2621.1974.tb02861.x. [DOI] [Google Scholar]