Abstract
1-Methylcyclopropene (1-MCP) at a dose of 0.3 μl l−1 for 24 h delayed the ripening of tomato (Solanum lycopersicum L.) at higher storage temperature (30.5 ± 1 °C). The dose was effective at green mature (stored at 30.5 ± 1 °C) and breaker (stored at 25 ± 1 °C) stages. There was a significant reduction in % ripening index and % red tomatoes due to this treatment on green mature tomatoes (stored at 25 ± 1 °C). Depending on the variety, the rate of respiration was either reduced or remained unaffected by the treatment. Post-harvest life was enhanced in all the varieties due to the delay in red colour development and reduced rottage. The results imply prospects for the use of 1-MCP (0.3 μl 1−1 for 24 h) for storage of tomato fruits at higher ambient temperatures of tropical and sub-tropical regions.
Keywords: 1-Methylcyclopropene, Tomato, Solanum lycopersicum, High temperature storage, Post-harvest life, Respiration, Ripening, Varietal variability
Enhancing the post-harvest life of tomato (Solanum lycopersicum L.) is an important aspect in view of its large post-harvest losses. The onset of ripening in tomato is governed by an increase in ethylene production and it is highly dependent on continuous presence of ethylene and ethylene-mediated actions (Hoeberichts et al. 2002). 1-Methylcyclopropene (1-MCP) is an effective gaseous inhibitor of ethylene action in the plant tissues. It binds to the ethylene receptor irreversibly and thereby stops the ethylene-mediated signal transduction cascade (Sisler and Blankenship 1996; Sisler and Serek 1997; Sisler et al. 2006). 1-MCP extends the storability in flowers, vegetables and fruits including tomato (Blankenship and Dole 2003; Watkins and Miller 2003). In tomato, 7 nl l−1 of 1-MCP blocked the green to red colour change for 8 days (Sisler et al. 1996). Higher concentration of 1-MCP (0.1–1.0 μl l−1) for a short duration (2 h) was effective on green tomatoes (Wills and Ku 2002) but for ripe tomatoes at least 20 μl l−1 were required for enhancing the post-harvest life. Ripening was delayed in tomatoes by 5–10 days with single application of 1-MCP (Sisler et al. 1996; Hoeberichts et al. 2002; Wills and Ku 2002). In general, 1-MCP delays only the onset of ripening related changes and did not alter significantly the firmness, colour and content of lycopene and chlorophyll (Mostofi et al. 2003).
The EPA (2002) refers 1-MCP as a reduced-risk product due to its highly favourable safety profiles. However, there are some constraints, which limited the commercialisation of 1-MCP. These include modification of its response by its concentration, exposure duration, temperature (during treatment and storage), development stage/maturity of the fruit, variety and interaction among these factors (Sisler and Serek 1997; Jiang et al. 1999; Jeong et al. 2002; Wills and Ku 2002; Blankenship and Dole 2003; Sisler et al. 2006). So far, most of the studies on tomato with 1-MCP have been conducted at lower storage temperatures of 15 to 20 °C (Wills and Ku 2002; Hoeberichts et al. 2002; Mir et al. 2004; de Wild et al. 2005; Guillen et al. 2006; Guillen et al. 2007). Delay in ripening of tomato by 1-MCP was found to be inversely related to the storage temperature (Mostofi et al. 2003; Blankenship and Dole 2003; Jiang et al. 2004). In tomato fruits, only a single study is available at 25 °C storage (Mostofi et al. 2003) and none at higher storage temperature.
The present study was therefore taken up firstly, to find out an effective dose of 1-MCP that could delay ripening in tomato stored at higher temperature (30–31 °C). Secondly, to test optimised dose of 1-MCP at green mature (stored at about 30 °C) and breaker stages (stored at 25 °C) in selected tomato varieties and also for fast and slow ripening varieties at green mature stage (when stored at 25 °C).
Materials and methods
Seeds of fast (‘Pusa Ruby’, ‘Pusa Early Dwarf’, ‘Pusa Sheetal’, ‘Pusa Uphar’) and slow (‘Pusa Gaurav’, ‘Pusa 120’, ‘Pusa Selection 8’ and ‘Pusa Rohini’) ripening tomatoes were obtained from Division of Vegetable Crops of the institute. Tomato was grown during the seasons of 2005–2006 and 2006–2007 by following recommended cultural practices. Healthy tomatoes (35–55 g each) were harvested manually at required ripening stages UFFVA (1975). Fruits were then washed in tap water and air dried. Experiments were carried out in different batches of 3 or 4 lots with 10–15 fruits in each lot at different times during 2005–2007.
1-MCP treatment
Tomato fruits were treated with 1-MCP 1 day after harvest. This 1-day rest was given for the fruit to adapt and normalize from the immediate and subsequent effects of injuries, stress or induced respiration due to their plucking from the plants. Treatment of 1-MCP was given in airtight containers of 5.65 l capacity. Desired concentration of 1-MCP, as gas, was released from the required weight of 1-MCP formulation (‘SmartFresh’, Rohm and Haas, USA with a.i. of 0.14%) as per the technical bulletin. Initially, the exposure was given for different concentrations of 1-MCP (0.3 and 0.5 μl l−1) for 6, 12 or 24 h.
Storage of tomatoes
Each lot (replication) of 10 or 15 fruits control (without 1-MCP) as well as treated fruits was stored in well-ventilated plastic baskets either at room conditions (30.6 ± 1/31.0 ± 1/30.5 ± 1 °C and 37.0 ± 10/45.0 ± 10/47.0 ± 10% RH) or in cold room (25 ± 1 °C and 80 ± 5% RH).
Ripening index (RI) indicates the extent of ripening in a given lot of tomato fruits. This was calculated as per the formula described by Wang and Morris (1993).
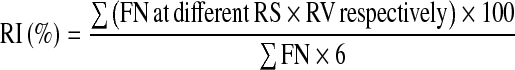 |
where, RI: Ripening index, FN: Fruit number, RS: Ripening stage, RV: Representative value (0 = Green mature, 1 = Breaker, 2 = Turning, 3 = Pink, 4 = Light red, 5 = Red, 6 = Red ripe).
Red tomatoes (RT) expressed as % also indicates the extent of ripening and was calculated as per the method of Wills and Ku (2002). It refers to the number of tomato fruits that have attained the red stage or beyond the red stage (UFFVA 1975) out of the total number of fruits in a lot after a given day after storage (DAT).
Physiological loss in weight (PLW) was determined by weighing the fruits at the start of experiment and later at required DAT during storage (Wills and Ku 2002).
Respirometer, based on infrared gas analyzer (Model EGM-4 CO2 Analyser PP Systems, Hertfordshire, UK), was used for measuring the respiration rate and expressed as μmole CO2 g−1 fresh weight h−1.
Out of the total number of tomato fruits in a lot, fruits either showing the sign of infection, decay/rottage or already decayed/rotted (as observed on the day of observation) irrespective of physiological or pathological causes, provided rottage (%) as per Wills and Ku (2002).
Post-harvest life (shelf-life) was calculated as per the method of Wills and Ku (2002). The end of post-harvest life at a given DAT was considered as the time (day) when fruit showed decay/rottage, a moderate level of shrinkage or appearance of spot/s that could make it unacceptable to the consumers. The fruits that completed their post-harvest life were expressed in term of % at different DAT.
Statistical analysis
Each treatment was replicated 3 or 4 times. The data were transformed to arc sin or square root values for analysis. Statistical analysis was done using either 1 or 2 factor/s complete randomized design or by t-test. Wherever required, mean values were ranked by using Duncan’s multiple range test. MSTAT-C software was used for all the statistical analysis. Statistical procedures were followed as per Gomez and Gomez (1984).
Results and discussion
Optimizing the dose of 1-MCP
Treatment with 0.3 μl l−1 of 1-MCP for 24 h (T3) caused maximum delay in ripening of ‘Pusa Ruby’ (fast ripening variety) at higher storage temperature of 30.6 °C (Table 1). In comparison with control at 14 DAT, 0.3 μl l−1 1-MCP caused a reduction of 34% and 67% in the RI and RT, respectively. When compared with control, PLW also showed significant reduction (1.4%) for this dose of 1-MCP at 7 DAT. However, at 14 DAT no significant difference was observed.
Table 1.
Effect of 1-MCP on ripening and physiological loss in weight (PLW) of tomatoes represented by equal proportions of fruits at green mature, breaker and turning stages in variety ‘Pusa Ruby’ stored at 30.6 ± 1 °C and 37 ± 10% RH
| RI,% | RT,% | PLW,% | ||||
|---|---|---|---|---|---|---|
| 7 | 14 | 7 | 14 | 7 | 14 | |
| C (Control) | 76a | 91a | 42a | 78a | 8.8a | 14.2NS |
| T1 (0.3 μl l−1, 12 h) | 57b | 78b | 0d | 33c | 7.1b | 12.0 |
| T2 (0.3 μl l−1, 24 h) | 46c | 57c | 0d | 11d | 7.4b | 11.8 |
| T3 (0.5 μl l−1, 6 h) | 75a | 85a | 25b | 56b | 7.8ab | 12.8 |
| T4(0.5 μl l−1, 12 h) | 54b | 89a | 8c | 33c | 7.7ab | 12.6 |
| T5 (0.5 μl l−1, 24 h | 57b | 75b | 8c | 50b | 7.2b | 12.4 |
Values in each column followed by different superscript/s are significant over one another at p ≤ 0.05. NS: not significant (n = 4 lots with 15 fruits in each lot)
DAT days after treatment, RI ripening index, RT red tomatoes, 7 and 14: DAT
In comparison to control, treatment T2 in ‘Pusa Gaurav’ (slow ripening variety) when stored at 31.0 °C also showed a significant reduction of 22 and 21% in RI and RT, respectively at 14 DAT (Table 2). The values were 21 and 28% at 18 DAT. The 1-MCP treatment of fruits at green mature stage in the above 2 varieties (stored at 30.5 °C) showed significant reduction in ripening. In ‘Pusa Ruby’ the reduction in RI at 10 and 14 DAT was 38 and 20%, respectively whereas the values were 18 and 21% in ‘Pusa Gaurav’ (Table 3). Further the same dosage (T2) at breaker stage in ‘Pusa Ruby’ at 25 °C (Table 4) indicated its effectiveness beyond the green mature stage also.
Table 2.
Effect of (T2) 1-MCP (0.3 μl l−1, 24 h) on ripening status of tomatoes represented by equal proportions of fruits at green mature, breaker and turning stages in variety ‘Pusa Gaurav’ stored at 31 ± 1 °C and 45 ± 10% RH
| RI,% | RT,% | |||
|---|---|---|---|---|
| 14 | 18 | 14 | 18 | |
| C | 72** | 77** | 21** | 28** |
| T2 | 50 | 54 | 0 | 0 |
**Significantly higher value (for each column) based on the comparison of 2 means by t-test (p ≤ 0.01) (n = 4 lots with 15 fruits in each lot)
DAT, RI, RT, C: As in Table 1. 14 and 18: DAT
Table 3.
Effect of (T2) 1-MCP (0.3 μl l−1, 24 h) on RI (%) of green mature tomato fruits stored at 30.5 ± 1 °C and 47 ± 10% RH
| ‘Pusa Ruby’ | ‘Pusa Gaurav’ | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 | 8 | 10 | 14 | 5 | 8 | 10 | 14 | |
| C | 33** | 56** | 65** | 70* | 8** | 15** | 21** | 30** |
| T2 | 0 | 25 | 27 | 50 | 0 | 0 | 3 | 9 |
*p ≤ 0.05, **p ≤ 0.01 for each column based on the comparison of 2 means by t-test (n = 4 lots with 15 fruits in each lot)
RI, DAT, C: As in Table 1. 5, 8, 10, 14: DAT
Table 4.
Effect of (T2) 1-MCP (0.3 μl l−1, 24 h) on ripening status of tomato fruits in variety ‘Pusa Ruby’ harvested at breaker stage and stored at 25 ± 1 °C and 80 ± 10% RH
| RI,% | RT,% | |||||
|---|---|---|---|---|---|---|
| 10 | 14 | 18 | 10 | 14 | 18 | |
| C | 94** | 98** | 100* | 100** | 100** | 100** |
| T2 | 67 | 75 | 92 | 0 | 50 | 83 |
*p ≤ 0.05, **p ≤ 0.01 for each column based on the comparison of 2 means by t-test (n = 4 lots with 15 fruits in each lot)
RI, RT, DAT, C: As in Table 1. 10, 14, 18: DAT
Thus, 1 MCP treatment at 0.3 μl l−1 for 24 h was effective in delaying the ripening of fruits at different ripening stages when stored at 30.5–31.0 °C (Tables 1, 2 and 3) and 25 °C (Table 4). Our results on the 1-MCP treated fruits (at green mature stage) stored at 25 °C (Table 3) were comparable to those of Mostofi et al. (2003).
Interrupting ripening at early stages inhibits the ripening more strongly as evident from RI% of ‘Pusa Ruby’ at 14 DAT. But, treating the fruits at early stage of ripening with ethylene biosynthetic or action inhibitors showed negative effect on final organoleptic characteristics in tomato (Hayase et al. 1984; Hobson 1989; Guillen et al. 2006). Hence interfering the ripening process at later stages of ripening (breaker and turning) would be more appropriate for attaining an acceptable quality of treated fruits. It has also been observed that slow ripening variety ‘Pusa Gaurav’, the fruits at green mature stage have not reached to red ripe stage even after 14 DAT in comparison with fruits of fast ripening variety like ‘Pusa Ruby’ (Table 5). This was evident both in 1-MCP treated and control fruits. Therefore, slow ripening varieties should be treated with 1-MCP either at breaker or turning stages so that the fruit could finally achieve the acceptable organoleptic characteristics. It was reported by Huber (2008) that recovery of ripening characteristics and attainment of optimum quality for climacteric fruits are best achieved if 1-MCP is applied after the initiation of ripening. This was in fact found to be true when tomato fruits were treated with 1-MCP either at breaker-turning stage (Choi and Huber 2008) or pink stage (Cliff et al. 2009). Besides delaying ripening, 1-MCP treatment at advanced ripening stage delayed the negative effect on the integrity of cell wall but with no adverse effect on flavour of tomato fruit (Cliff et al. 2009).
Table 5.
Effect of (T2) 1-MCP (0.3 μl l−1, 24 h) on RI (%) and RT (%) of green mature tomato fruits stored at 25 ± 1 °C and 80 ± 10% RH
| Fast ripening | Slow ripening | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Pusa Ruby’ | ‘Pusa Early Dwarf’ | ‘Pusa Sheetal’ | ‘Pusa Uphar’ | ‘Pusa Gaurav’ | ‘Pusa 120’ | ‘Pusa Selection 8’ | ‘Pusa Rohini’ | |||||||||
| 10 | 14 | 10 | 14 | 10 | 14 | 10 | 14 | 10 | 14 | 10 | 14 | 10 | 14 | 10 | 14 | |
| RI,% | ||||||||||||||||
| C | 65 | 87 | 65 | 85 | 67 | 92 | 60 | 80 | 38 | 52 | 47 | 69 | 45 | 62 | 50 | 60 |
| T2 | 35 | 60 | 23 | 42 | 42 | 65 | 27 | 60 | 5 | 10 | 7 | 17 | 6 | 40 | 5 | 15 |
| RT,% | ||||||||||||||||
| C | 60 | 82 | 50 | 77 | 45 | 75 | 55 | 81 | 6 | 9 | 32 | 61 | 37 | 48 | 16 | 38 |
| T2 | 14 | 24 | 0 | 0 | 0 | 25 | 0 | 40 | 0 | 0 | 0 | 0 | 3 | 8 | 0 | 0 |
All the control values were found to be significantly higher over the treatment values based on the comparison of 2 means by t-test (p ≤ 0.01). (n = 3 lots with 15 fruits in each lot)
DAT, RI, RT, C: As in Table 1. 10 and 14: DAT
Testing optimum 1-MCP dose on tomato varieties
The evaluation of 1-MCP dose (T2) in 8 varieties of tomato fruits with contrasting ripening behaviour when harvested at green mature stage and stored at 25.0 °C indicated significant reduction in RI and RT in all the varieties compared to control (Table 5). This indicated the efficacy of the selected dose of 1-MCP in delaying the ripening irrespective of varieties.
Measurement of respiration rate at 5 DAT in 4 fast ripening (‘Pusa Ruby’, ‘Pusa Early Dwarf’, ‘Pusa Sheetal’ and ‘Pusa Uphar’) and 2 slow ripening (‘Pusa Gaurav’ and ‘Pusa 120’) varieties showed significant reduction in respiration rate due to the treatment T2 (Table 6). When control fruits were compared, a higher rate of respiration was observed in fast ripening than in slow ripening varieties.
Table 6.
Effect of (T2) 1-MCP (0.3 μl l−1, 24 h) on rate of respiration (μmole CO2 g−1 fresh weight h−1) of green mature tomato fruits stored at 25 ± 1 °C and 80 ± 10% RH (5 DAT)
| Fast ripening | Slow ripening | ||||||
|---|---|---|---|---|---|---|---|
| ‘Pusa Ruby’ | ‘Pusa Early Dwarf’ | ‘Pusa Sheetal’ | ‘Pusa Uphar’ | ‘Pusa Gaurav’ | ‘Pusa 120’ | Mean (T) | |
| C | 16.18a | 10.14b | 15.52a | 8.23bc | 5.29cd | 7.38bcd | 10.45a |
| T2 | 5.68cd | 7.18bcd | 6.74bcd | 3.36d | 3.33d | 8.16bc | 5.74b |
| Mean (V) | 10.93a | 8.60ab | 11.13a | 5.79bc | 4.31c | 7.77b | |
| Variety (V) = **, Treatment (T) = **, V × T = ** | |||||||
Values followed by different superscript/s are significant over one another at **p ≤ 0.01 (n = 4 lots with 10 fruits in each lot)
DAT, C: As in Table 1
1-MCP significantly reduced rottage at 14, 18, 22 and 26 DAT (Table 7). At 10 DAT, there was no rottage in any of the varieties. At 14 DAT, rottage varied from 0% to 30% in control fruits depending upon variety whereas treated fruits, showed only 0–5% rottage indicating the role of 1-MCP in lowering rottage. Interaction data and varietal mean indicated relatively higher rottage in ‘Pusa Ruby’ and ‘Pusa Early Dwarf’ than in ‘Pusa Selection 8’ and ‘Pusa Rohini’.
Table 7.
Effect of (T2) 1-MCP (0.3 μl l−1, 24 h) on rottage (%) of green mature tomato fruits stored at 25 ± 1 °C and 80 ± 10% RH for different days
| Fast ripening | Slow ripening | Mean (T) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ‘Pusa Ruby’ | ‘Pusa Early Dwarf’ | ‘Pusa Sheetal’ | ‘Pusa Uphar’ | ‘Pusa Gaurav’ | ‘Pusa 120’ | ‘Pusa Selection 8’ | ‘Pusa Rohini’ | ||
| DAT 14 | |||||||||
| C | 15b | 30a | 0d | 0d | 1d | 8bc | 0d | 0d | 6.7a |
| T2 | 0d | 5cd | 0d | 0d | 0d | 0d | 0d | 0d | 0.6b |
| Mean (V) | 7.5b | 17.5a | 0.0d | 0.0d | 0.5cd | 4.0bc | 0.0d | 0.0d | |
| Variety (V) = **, Treatment (T) = **, V × T = ** | |||||||||
| DAT 18 | |||||||||
| C | 22ab | 40a | 0d | 0d | 15abc | 18abc | 0d | 0d | 11.9a |
| T2 | 6bcd | 10bcd | 10bcd | 0d | 3cd | 0d | 0d | 0d | 3.6b |
| Mean (V) | 4.0ab | 25.0a | 5.0bc | 0.0c | 9.0b | 9.0bc | 0.0c | 0.0c | |
| V = **, T = **, V × T = ** | |||||||||
| DAT 22 | |||||||||
| C | 25a–d# | 52a | 15a–d | 18ab | 16b–e | 23abc | 5de | 7cde | 20.1a |
| T2 | 11b–e | 18b–e | 18b–e | 0e | 5de | 0e | 0e | 8cde | 7.5b |
| Mean (V) | 18.0ab | 35.0a | 16.5ab | 9.0ab | 10.5bc | 11.5bc | 2.5c | 7.5bc | |
| V = **, T = **, V × T = ** | |||||||||
| DAT 26 | |||||||||
| C | 26 | 60 | 23 | 47 | 17 | 39 | 5 | 8 | 28.1a |
| T2 | 26 | 30 | 22 | 10 | 7 | 13 | 2 | 8 | 14.7b |
| Mean (V) | 26.0ab | 45.0a | 22.5ab | 28.5ab | 12.0b | 26.0ab | 3.5b | 8.0b | |
| V = **, T = **, V × T = NS | |||||||||
Values followed by different superscript/s are significant over one another at ** p ≤ 0.01. #: abcd; NS: not significant (n = 3 lots with 15 fruits in each lot)
DAT, C: As in Table 1
In general, fast ripening varieties showed shorter post-harvest life than slow ripening varieties (Table 8). However, among the fast ripening varieties, ‘Pusa Early Dwarf’ showed relatively longer post-harvest life. Likewise, among slow ripening varieties, ‘Pusa 120’ showed relatively shorter post-harvest life (Table 8). Comparison of control fruits of different varieties indicated that ‘Pusa Sheetal’ and ‘Pusa Ruby’ had poor post-harvest life while ‘Pusa Gaurav’ and ‘Pusa Selection 8’ had longer post-harvest life.
Table 8.
Effect of (T2) 1-MCP (0.3 μl l−1, 24 h) on completion of post-harvest life (%) of green mature tomato fruits stored at 25 ± 1 °C and 80 ± 10% RH for different days
| Fast ripening | Slow ripening | Mean (T) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ‘Pusa Ruby’ | ‘Pusa Early Dwarf’ | ‘Pusa Sheetal’ | ‘Pusa Uphar’ | ‘Pusa Gaurav’ | ‘Pusa 120’ | ‘Pusa Selection 8’ | ‘Pusa Rohini’ | ||
| DAT 14 | |||||||||
| C | 55ab | 40bc | 68a | 40bc | 11de | 31bcd | 9e | 32bcd | 35.7a |
| T2 | 0f | 16cde | 0f | 7e | 0f | 3f | 0f | 0f | 3.2b |
| Mean (V) | 27.5ab | 28.0a | 34.0ab | 23.5ab | 5.5c | 17.0bc | 4.5c | 16.0bc | |
| Variety (V) = **, Treatment (T) = **, V × T = ** | |||||||||
| DAT 18 | |||||||||
| C | 57 | 51 | 89 | 47 | 13 | 48 | 18 | 32 | 44.4a |
| T2 | 36 | 21 | 55 | 23 | 2 | 5 | 0 | 0 | 17.7b |
| Mean (V) | 46.5b | 36.0bc | 72.0a | 35.0bc | 7.5d | 26.5cd | 9.0d | 16.0d | |
| V = **, T = **, V × T = NS | |||||||||
| DAT 22 | |||||||||
| C | 79 | 62 | 100 | 53 | 17 | 55 | 22 | 45 | 54.1a |
| T2 | 48 | 27 | 68 | 29 | 5 | 28 | 1 | 5 | 26.4b |
| Mean (V) | 63.5b | 44.5bc | 84.0a | 41.0bc | 11.0d | 41.5bc | 11.5d | 25.0cd | |
| V = **, T = **, V × T = NS | |||||||||
| DAT 26 | |||||||||
| C | 100 | 76 | 100 | 77 | 20 | 73 | 32 | 60 | 67.2a |
| T2 | 69 | 38 | 72 | 59 | 5 | 46 | 11 | 29 | 41.1b |
| Mean (V) | 84.5a | 57.0bc | 86.0a | 68.0b | 12.5d | 59.5bc | 21.5d | 44.5c | |
| V = **, T = **, V × T = NS | |||||||||
Values followed by different superscript/s are significant over one another at **p ≤ 0.01. NS: not significant (n = 3 lots with 15 fruits in each lot)
DAT, C: As in Table 1
1-MCP treatment reduces the respiration rate in climacteric fruits (Blankenship and Dole 2003; Guillen et al. 2007; Choi and Huber 2008). It inhibits autocatalytic ethylene biosynthesis and thereby climacteric rise in tomato (Guillen et al. 2006) and banana (Jiang et al. 1999). Further, being a response inhibitor of ethylene, 1-MCP not only blocks the response of basal level of ethylene but also the autocatalytic production of ethylene (Grichko et al. 2006; Barry and Giovannoni 2007). Accordingly, the reduction in respiration rate in fast ripening varieties (Table 6) in the present study might be due to the inhibition of autocatalytic ethylene production. But the respiration rate was not reduced significantly in slow ripening varieties. This might be because by 5 DAT these varieties have not entered into the phase of autocatalytic production of ethylene. There is an inverse relationship between respiration rate and post-harvest life for number of commodities including tomato (Kader 1987; Kader and Saltveit 2003b; Varoquaux and Ozdemir 2005). Thereby, the observed inherent differences in the rate of respiration of control fruits in different varieties (Table 6) could have contributed for the varietal differences in the rate of ripening (Table 5). Such a relationship was already reported by us in 2 varieties of tomato in view of the possible differences in the internal gaseous environment of the fruits due to the morphological and anatomical differences (Paul and Srivastava 2006). Further, efficacy of 1-MCP in delaying the ripening could also be correlated with the rate of respiration as varieties with lower rate of respiration (‘Pusa Gaurav’ and ‘Pusa 120’) showed more delay in ripening when compared with varieties having higher rate of respiration (‘Pusa Ruby’, ‘Pusa Early Dwarf’, ‘Pusa Sheetal’ and ‘Pusa Uphar’) (Tables 5 and 6).
Guillen et al. (2006) reported lower rottage of tomatoes due to 1-MCP treatment. Sensitivity of tomatoes to rot was however found to be variable (13–40%) depending upon the cultivar and ripening stage at 28 days of harvest at storage temperature of 10 °C. We obtained comparable results with 5 to 60% rottage in control in contrast with 2 to 30% in the treatment at 26 DAT even at 25 °C storage temperature (Table 7).
The slower completion of post-harvest life due to 1-MCP treatment (Table 8) was primarily due to delay in the onset of elevated ethylene production and its action under the influence of 1-MCP. 1-MCP delays or reduces ethylene-induced effects by suppressing the ripening and ripening-related changes in many fruits (Blankenship and Dole 2003; Watkins and Miller 2005; Watkins 2006).
The observed variability in the post-harvest life of control fruits among different varieties (Table 8) could be the result of inherent varietal differences in fruits (including tomato) for the levels of ethylene production, rate of respiration, gaseous environment inside the fruits and also sensitivity of fruits toward decay/rottage during storage (Kader 1987; Varoquaux and Ozdemir 2005; Kader and Saltveit 2003a, b; Pruky 2003; Bargel and Neinhuis 2005; Paul and Srivastava 2006; Guillen et al. 2006; Paul et al. 2007).
The results of Table 9 indicated effectiveness of 1-MCP in delaying the ripening of different varieties in the order ‘Pusa Gaurav’ > ‘Pusa 120’ = ‘Pusa Rohini’ > ‘Pusa Early Dwarf’ > ‘Pusa Selection 8’ > ‘Pusa Ruby’ > ‘Pusa Sheetal’ > ‘Pusa Uphar’.
Table 9.
Effect of (T2) 1-MCP (0.3 μl l−1, 24 h) on % reduction (over the respective control value) in RI and RT of green mature tomato fruits stored at 25 ± 1 °C and 80 ± 10% RH (14 DAT)
| Fast ripening | Slow ripening | |||||||
|---|---|---|---|---|---|---|---|---|
| ‘Pusa Ruby’ | ‘Pusa Early Dwarf’ | ‘Pusa Sheetal’ | ‘Pusa Uphar’ | ‘Pusa Gaurav’ | ‘Pusa 120’ | ‘Pusa Selection 8’ | ‘Pusa Rohini’ | |
| RI (% reduction) | 32b | 51ab | 29b | 25b | 81a | 76a | 36b | 76a |
| RT (% reduction) | 71bc | 100a | 67bc | 52c | 100a | 100a | 84b | 100a |
Values (for each row) followed by different superscript/s are significant over one another at **p ≤ 0.01 (n = 3 lots with 15 fruits in each lot) DAT, RI, RT: As in Table 1
Differential absorption capacity of 1-MCP by different commodities depending on their lipid content and absorption to other cellular constituents has also been demonstrated (Dauny et al. 2003; Nanthachai et al. 2007). 1-MCP can be slowly desorbed during storage to become available to a newly synthesized or regenerated ethylene sites (Golding et al. 1998; Dauny and Joyce 2002) and this thereby can determine the efficacy of 1-MCP treatment. Further, differences in the ethylene receptor activation sensitivity were observed (Sisler and Lallu 1994; Blankenship and Dole 2003). Once the ethylene receptor sites were blocked by 1-MCP, the availability of fresh sites was found to be crop dependent. Some crops can regenerate sites fairly quickly (flowers, tomatoes) while others (banana) take more time (Blankenship and Dole 2003). Recently, it has been demonstrated that extent of 1-MCP mediated delay in ripening depends on the endogenous level of ethylene in tomato fruits (Zhang et al. 2009).
Conclusion
An effective dose of 1-MCP (0.3 μl l−1 for 24 h) was obtained from this study which can delay the ripening of tomato fruits stored at higher temperatures of about 30 and 25 °C in all the varieties. This dose delayed ripening not only at specific ripening stage (green mature or breaker) of tomato fruits but also for a mixture of fruits at different ripening stages (green mature, breaker and turning). Storability of tomato in tropical and sub-tropical regions can be benefited from this study as the post-harvest life of all the tomato varieties was enhanced by this dose of 1-MCP treatment.
Acknowledgement
Authors are thankful to Warner HL, Rohm and Haas Company, Research Laboratories, 727, Norristown Road, USA for providing the sample of ‘SmartFresh’ (formulation of 1-MCP) along with its technical bulletin for the research purpose.
References
- Bargel H, Neinhuis C. Tomato (Lycopersicon esculentum Mill.) fruit growth and ripening as related to the biomechanical properties of fruit skin and isolated cuticle. J Exp Bot. 2005;56:1049–1060. doi: 10.1093/jxb/eri098. [DOI] [PubMed] [Google Scholar]
- Barry CS, Giovannoni JJ. Ethylene and fruit ripening. J Plant Growth Regul. 2007;26:143–159. doi: 10.1007/s00344-007-9002-y. [DOI] [Google Scholar]
- Blankenship SM, Dole JM. 1-Methylcyclopropene: a review. Postharvest Biol Technol. 2003;28:1–25. doi: 10.1016/S0925-5214(02)00246-6. [DOI] [Google Scholar]
- Choi ST, Huber DJ. Influence of aqueous 1-methylcyclopropene concentration, immersion duration, and solution longevity on the postharvest ripening of breaker-turning tomato (Solanum lycopersicum L.) fruit. Postharvest Biol Technol. 2008;49:147–154. doi: 10.1016/j.postharvbio.2008.01.003. [DOI] [Google Scholar]
- Cliff M, Lok S, Lu C, Toivonen PMA. Effect of 1-methylcyclopropene on sensory, visual and analytical quality of greenhouse tomatoes. Postharvest Biol Technol. 2009;53:11–15. doi: 10.1016/j.postharvbio.2009.02.003. [DOI] [Google Scholar]
- Dauny PT, Joyce DC. 1-Methylcyclopropene (1-MCP) improves storability of ‘Queen Cox’ and ‘Bramley’ apple fruit. HortSci. 2002;37:1082–1085. [Google Scholar]
- Dauny PT, Joyce DC, Gamby C. 1-Methylcyclopropene influx and efflux in ‘Cox’ apple and ‘Hass’ avocado fruit. Postharvest Biol Technol. 2003;29:101–105. doi: 10.1016/S0925-5214(03)00042-5. [DOI] [Google Scholar]
- de Wild HPJ, Balk PA, Fernandes ECA, Peppelenbos HW. The action site of carbon dioxide in relation to inhibition of ethylene production in tomato fruit. Postharvest Biol Technol. 2005;36:273–280. doi: 10.1016/j.postharvbio.2005.02.004. [DOI] [Google Scholar]
- EPA (2002) Federal Register. Environmental Protection Agency. July 28. 67(144):48796–48800
- Golding JB, Shearer D, Wyllie SG, McGlasson WB. Application of 1-MCP and propylene to identify ethylene dependent ripening processes in mature banana fruit. Postharvest Biol Technol. 1998;14:87–98. doi: 10.1016/S0925-5214(98)00032-5. [DOI] [Google Scholar]
- Gomez KA, Gomez AA. Statistical procedures for agricultural research. 2. Singapore: Wiley; 1984. [Google Scholar]
- Grichko V, Serek M, Watkins CB, Yang SF. Father of 1-MCP. Biotechnol Adv. 2006;24:355–356. doi: 10.1016/j.biotechadv.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Guillen F, Castillo S, Zapata PJ, Martinez-Romero D, Vallero D, Serrano M. Efficacy of 1-MCP treatment in tomato fruit. 2. Effect of cultivar and ripening stage at harvest. Postharvest Biol Technol. 2006;42:235–242. doi: 10.1016/j.postharvbio.2006.07.005. [DOI] [Google Scholar]
- Guillen F, Castillo S, Zapata PJ, Martinez-Romero D, Serrano M, Vallero D. Efficacy of 1-MCP treatment in tomato fruit. 1. Duration and concentration of 1-MCP treatment to gain an effective delay of postharvest ripening. Postharvest Biol Technol. 2007;43:23–27. doi: 10.1016/j.postharvbio.2006.07.004. [DOI] [Google Scholar]
- Hayase J, Chung T-Y, Kato H. Changes in volatile components of tomato fruit during ripening. Food Chem. 1984;14:113–124. doi: 10.1016/0308-8146(84)90050-5. [DOI] [Google Scholar]
- Hobson GE. Manipulating the ripening of tomato fruit—low and high technology. Acta Hortic. 1989;258:593–600. [Google Scholar]
- Hoeberichts FA, Van Der Plas LHW, Woltering EJ. Ethylene perception is required for the expression of tomato ripening related genes and associated physiological changes even at advanced stages of ripening. Postharvest Biol Technol. 2002;26:125–133. doi: 10.1016/S0925-5214(02)00012-1. [DOI] [Google Scholar]
- Huber DJ. Suppression of ethylene responses through application of 1-MCP: a powerful tool for elucidating ripening and senescence mechanisms in climacteric and non-climacteric fruits and vegetables. HortSci. 2008;43:106–111. [Google Scholar]
- Jeang J, Huber DL, Sargent SA. Influence of 1-methylcyclopropene (1-MCP) on ripening and cell wall matrix polysaccharides of avocado (Persea americana) fruit. Postharvest Biol Technol. 2002;25:241–254. doi: 10.1016/S0925-5214(01)00184-3. [DOI] [Google Scholar]
- Jiang Y, Joyce DC, Macnish AJ. Extension of the shelf life of banana fruit by 1-methylcyclopropene in combination with polyethylene bags. Postharvest Biol Technol. 1999;16:187–193. doi: 10.1016/S0925-5214(99)00009-5. [DOI] [Google Scholar]
- Jiang W, Zhang M, He J, Zhou L. Regulation of 1-MCP treated banana fruit quality by exogenous ethylene and temperature. Food Sci Technol Int. 2004;10:15–20. doi: 10.1177/1082013204042189. [DOI] [Google Scholar]
- Kader AA. Respiration and gas exchange of vegetable. In: Weichmann J, editor. Postharvest physiology of vegetables. New York: Marcel Dekker; 1987. pp. 25–43. [Google Scholar]
- Kader AA, Saltveit ME. Atmosphere modification. In: Bartz JA, Brecht JK, editors. Postharvest physiology and pathology of vegetables. New York: Marcel Dekker; 2003. pp. 229–246. [Google Scholar]
- Kader AA, Saltveit ME. Respiration and gas exchange. In: Bartz JA, Brecht JK, editors. Postharvest physiology and pathology of vegetables. New York: Marcel Dekker; 2003. pp. 7–29. [Google Scholar]
- Mir N, Canoles M, Beaudry R, Baldwin E, Mehla CP. Inhibiting tomato ripening with 1-methylcyclopropene. J Am Soc Hortic Sci. 2004;129:112–120. [Google Scholar]
- Mostofi Y, Toivonen PMA, Lessani H, Babalar M, Lu C. Effects of 1-methylcyclopropene on ripening of green house tomatoes at three storage temperatures. Postharvest Biol Technol. 2003;27:285–292. doi: 10.1016/S0925-5214(02)00113-8. [DOI] [Google Scholar]
- Nanthachai N, Ratanachinakorn B, Kosittrakun M, Beaudry RM. Absorption of 1-MCP by fresh produce. Postharvest Biol Technol. 2007;43:291–297. doi: 10.1016/j.postharvbio.2006.10.003. [DOI] [Google Scholar]
- Paul V, Srivastava GC. Role of surface morphology in determining the ripening behaviour of tomato (Lycopersicon esculentum Mill.) fruits. Sci Hortic. 2006;110:84–92. doi: 10.1016/j.scienta.2006.06.023. [DOI] [Google Scholar]
- Paul V, Malik SK, Srivastava GC. Intervareital differences in the surface morphology and anatomy of mango (Mangifera indica L.) fruits. Phytomorphol. 2007;57:211–220. [Google Scholar]
- Pruky D. Mechanisms of resistance of fruits and vegetable to postharvest diseases. In: Bartz JA, Brecht JK, editors. Postharvest physiology and pathology of vegetables. New York: Marcel Dekker; 2003. pp. 581–598. [Google Scholar]
- Sisler EC, Blankenship SM (1996) Method for counteracting an ethylene response in plants. United States Patent US 5518988
- Sisler EC, Lallu N. Effect of diazocyclopentadiene (DACP) on tomato fruit harvested at different ripening stages. Postharvest Biol Technol. 1994;4:245–254. doi: 10.1016/0925-5214(94)90034-5. [DOI] [Google Scholar]
- Sisler EC, Serek M. Inhibitors of ethylene responses in plants at receptor level: recent developments. Physiol Plant. 1997;100:577–582. doi: 10.1111/j.1399-3054.1997.tb03063.x. [DOI] [Google Scholar]
- Sisler EC, Serek M, Dupille E. Comparison of cyclopropane, 1-methylcyclopropene and 3, 3-dimewthylcyclopropene as ethylene antagonists in plants. Plant Growth Regul. 1996;18:164–174. [Google Scholar]
- Sisler EC, Grichko VP, Serek M. Interaction of ethylene and other compounds with the ethylene receptor: agonists and antagonists. In: Khan NA, editor. Ethylene action in plants. Berlin: Springer Verlag; 2006. pp. 1–34. [Google Scholar]
- UFFVA (1975) United Fresh Fruit and Vegetable Association, Colour classification requirements in tomatoes. USDA Visual Aid TM-L-1. The John Henry Co., Lansing, Michigan
- Varoquaux P, Ozdemir IS. Packaging and produce degradation. In: Lamikanra O, Imam S, Ukuku D, editors. Produce degradation pathways and prevention. Boca Raton: CRC Press (Taylor & Francis Group; 2005. pp. 117–153. [Google Scholar]
- Wang S, Morris SC. Effect of borax and quazatine on ripening and postharvest diseases of tomato (cv. Flora Dade) Acta Hortic. 1993;343:331–333. [Google Scholar]
- Watkins CB. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol Adv. 2006;24:389–409. doi: 10.1016/j.biotechadv.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Watkins CB, Miller WB. Implications of 1-methylcyclopropene registration for use on horticultural products. In: Vendrell M, Klee H, Pech JC, Romojaro F, editors. Biology and biotechnology of the plant hormone ethylene. Amsterdam: IOS Press and Kluwer Academic; 2003. pp. 385–390. [Google Scholar]
- Watkins CB, Miller WM 2005. A summary of physiological processes or disorders in fruits, vegetables and ornamental products that are delayed or decreased, increased or unaffected by application of 1-methylcyclopropene (1-MCP): http://www.hort.cornell.edu/mcp/ethylene.pdf. (Accessed on 6 January 2005)
- Wills RBH, Ku VVV. Use of 1-MCP to extend the time to ripen the green mature tomatoes and postharvest life of ripe tomatoes. Postharvest Biol Technol. 2002;26:86–90. doi: 10.1016/S0925-5214(01)00201-0. [DOI] [Google Scholar]
- Zhang Z, Huber DJ, Hurr BM, Rao J. Delay of tomato fruit ripening in response to 1-methylcyclopropene is influenced by internal ethylene levels. Postharvest Biol Technol. 2009;54:1–8. doi: 10.1016/j.postharvbio.2009.06.003. [DOI] [Google Scholar]


