Abstract
The relationship between ripening behaviour and stem scar region of fruit in different tomato varieties (stored at 30.5 ± 1°C or 25 ± 1°C) was studied by blocking the stem scar region either completely or to different extent with high vacuum silicone grease. In ‘Pusa Ruby’ (a fast ripening variety) and ‘Pusa Gaurav’ (slow ripening variety), complete blockage of stem scar region of fruits at green mature stage showed 3-fold reduction in ripening index at 14 days after treatment (DAT) but with increased rottage. It was demonstrated in ‘Pusa Ruby’ and ‘Pusa 120’ that the extent of blockage of stem scar region has dependence on the rate of respiration and ripening suppression. The extent of climacteric rise was reduced significantly in the treated fruits. These suppressive effects of treatment were found to diminish with the advancement in the ripening stage of tomato fruit.
Keywords: Climacteric rise, Respiration, Ripening, Ripening stages, Tomato fruit, Stem scar
Diffusion of gases across the fruit’s boundary occurs through aqueous and waxy layers of epidermis, gaseous pores (stomates and lenticels) and from stem scar region (Solomos 1987, Ben-Yehoshua and Rodov 2003). There is morphological and anatomical basis of gaseous exchange in fruits (Kader and Saltveit 2003a, b) including tomato (Wilson and Sterling 1976, Cameron and Yang 1982). The gaseous exchange determines the relative O2 and CO2 levels inside the fruits (Nuevo et al 1984) and therefore the inside composition of fruit is always different from the external atmosphere in which the fruits are kept (Dadzie et al 1993). Tomato fruits have a relatively thick waxy cuticle without any network of pores, channels or polar pathway (Wilson and Sterling 1976, Das and Barringer 1999, Thompson 2003). Therefore, it may not contribute significantly to the overall gaseous exchange across the fruit. Instead of stomates, trichomes have been noticed on the epidermis and they get transformed into lenticels with maturity of tomato fruit (Clendenning 1941, Blanke 1986, Paul and Srivastava 2006). Earlier studies by Burg and Burg (1965), Cameron and Yang (1982) and de Varies et al (1995) have demonstrated 60, >97 and 85–90% of ethylene exchange through the stem scar region of tomato fruit, respectively. Yang and Shewfelt (1999) found that sealing of stem scar portion of tomato fruit in cultivars ‘Kada Gigante’ and ‘Floradade’ greatly reduced the ripening rate and extended the storage life at 20–24°C. No study was available where the stem scar region was blocked to different extents and fruits were stored at relatively higher temperatures (30 and 25°C) prevailing in tropical and sub-tropical parts of world where tomato is one of the important crops.
The present study, therefore examines the ripening response of different tomato cultivars whose stem scar region was blocked either completely or to different extents at different ripening stages. The fruits were stored at 30–30.5 ± 1°C or 25 ± 1°C.
Materials and methods
Seeds of different tomato cultivars (‘Pusa ruby’, ‘Pusa Gaurav’, ‘Pusa Early Dwarf’, ‘Pusa Sheetal’ and ‘Pusa 120’) were obtained from Division of Vegetable Crops of the institute. Tomato crop was grown in the field during 2005–2006 and 2006-2007 as per the recommended cultural practices. Healthy tomato fruits of 40–50 g each were harvested manually at required ripening stages (UFFVA 1975). Fruits were washed in tap water and air-dried.
Blocking the stem scar region
The stem scar region of tomato fruits was blocked as described by Yang and Shewfelt (1999). The blocking was done either completely or to different extent by placing the minimum quantity of silicone (high vacuum silicone grease) (HiMedia Laboratories Pvt. Ltd., Mumbai, India) at required ripening stage/s on the stem scar region. Silicone was not applied to the control fruits. The silicone was applied one day after the harvest. This one-day rest was given for the fruits to adapt and normalize from the immediate and subsequent effects of injuries, stress or induced respiration due to plucking from plants.
Storage of tomato fruits
Each replication lot of control as well as treated fruits was stored in well-ventilated plastic baskets either at room conditions (30.0 ± 1°C or 30.5 ± 1°C and 35 ± 6% or 38 ± 6% RH) or in cold room (25 ± 1°C and 70 ± 10% RH) up to required days after treatment (DAT).
Ripening index (RI), red tomatoes (RT), rottage and rate of respiration were determined and results analyzed statistically as mentioned in Part I of this paper (Paul et al 2010).
Results and discussion
Complete blockage treatment of green mature tomato fruits in 2 varieties led to a drastic reduction in RI (about 3- fold) at 14 DAT (Table 1). ‘Pusa Ruby’ being a fast ripening variety in comparison with ‘Pusa Gaurav’ showed lower RT. Rottage %, increased significantly due to complete blockage. In treated fruits, rottage was 20.5 and 23.8% in ‘Pusa Ruby’ and ‘Pusa Gaurav’, respectively, as compared 7% and 1.5%, respectively in control (Table 1). Fruits harvested and treated at breaker stage (Table 2) showed trend comparable to fruits at green mature stage (Table 1). However, the treatment at breaker stage was less effective in delaying the ripening especially in ‘Pusa Ruby’ and it also led to more rottage (Table 2) as compared to fruits at green mature stage (Table 1).
Table 1.
Effect of complete blockage of stem scar region of tomato fruits at green mature stage stored at 30 ± 1°C and 35 ± 6% RH on ripening index (RI), red tomatoes (RT) and rottage at 14 days after treatment (DAT)
| ‘Pusa Ruby’ | ‘Pusa Gaurav’ | |||||
|---|---|---|---|---|---|---|
| RI, % | RT, % | Rottage, % | RI, % | RT, % | Rottage, % | |
| C (Control) | 78.9** | 31.0* | 7.0 | 45.3* | 0.0 NS | 1.5 |
| T (Treatment) | 26.2 | 5.0 | 20.5** | 15.1 | 0.0 | 23.8** |
(n = 3 lots with 15 fruits in each lot)
*p ≤ 0.05, **p ≤ 0.01 for each column based on the comparison of 2 means by t-test. NS: Not significant
Table 2.
Effect of complete blockage of stem scar region of tomato fruits at breaker stage stored at 30 ± 1°C and 35 ± 6% RH on RI, RT and rottage
| ‘Pusa Ruby’ | ‘Pusa Gaurav’ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RI, % | RT, % | Rottage, % | RI, % | RT, % | Rottage, % | |||||||||
| 5 | 8 | 10 | 5 | 8 | 10 | 14 | 5 | 8 | 10 | 5 | 8 | 10 | 14 | |
| C | 86.9* | 89.6* | 97.5* | 59.1* | 63.3* | 93.3* | 10.0 | 53.0** | 65.1** | 79.5** | 0.0 NS | 0.0 NS | 14.6** | 3.0 |
| T | 52.6 | 71.0 | 77.5 | 9.9 | 36.2 | 43.0 | 29.2* | 19.4 | 23.5 | 28.7 | 0.0 | 0.0 | 0.0 | 46.5** |
(n = 3 lots with 15 fruits in each lot)
*p ≤ 0.05, **p ≤ 0.01 for each column based on the comparison of 2 means by t-test. RI, RT, DAT, C, T: As in Table 1. 5, 8, 10, 14 represent DAT
Respiration rate was reduced significantly in treated fruits (Table 3). Except at breaker stage (10 DAT), the reduction was highly significant in treated fruits of ‘Pusa Ruby’. In ‘Pusa Gaurav’ the reduction was significant at 3 DAT for both the ripening stages but not at 10 DAT. ‘Pusa Gaurav’ exhibited lower rate of respiration irrespective of treatments and ripening stages (Table 3).
Table 3.
Effect of complete blockage of stem scar region of tomato fruits stored at 30 ± 10 C and 35 ± 6% RH on rate of respiration (μmole CO2 g-1 fr. wt. h-1) at different DAT
| Treatment (T) | ‘Pusa Ruby’ | ‘Pusa Gaurav’ | ||||
|---|---|---|---|---|---|---|
| Control | Treated | Mean (RS) | Control | Treated | Mean (RS) | |
| Green mature [3 DAT] | 20.4a | 9.0c | 14.7a | 11.6b | 3.1c | 7.4b |
| Green mature [10 DAT] | 15.2b | 4.8d | 10.0b | 3.8c | 2. 8 cd | 3.3c |
| Breaker [3 DAT] | 18.7ab | 9.4c | 14.0a | 20.6a | 1.7d | 11.1a |
| Breaker [10 DAT] | 5. 4 cd | 3.6d | 4.5c | 4.1c | 3.3c | 3.7c |
| Mean (T) | 14.9a | 6.7b | 10.0a | 2.7b | ||
| RS = ** | T = **, | RS × T = ** | RS = **, | T = **, | RS × T = ** | |
(n = 3 lots with 15 fruits in each lot)
Values followed by different superscript/s are significant over one another at
**p ≤ 0.01. DAT: As in Table 1, RS: Ripening stage
The extent of ripening suppressed by the treatment decreased gradually with the advancement in the ripening stage (Table 4). The differences in RI between control and treated fruits became lesser with the progress in the ripening stages of fruits. Respiration rate of treated fruits was significantly lower at green mature and breaker stages but not at the turning stage (Table 4). Thus, the suppressive effect of complete blockage treatment of stem scar region on RI and rate of respiration was dependent on the ripening stage at which the fruits were treated. Fruits of ‘Pusa Early Dwarf’ and ‘Pusa Sheetal’ (fast ripening varieties) when treated at turning stage showed the response (Table 5) comparable to the results presented for the same stage in Table 4. This confirmed that the treatment was less effective in delaying ripening at later stages of ripening.
Table 4.
Effect of complete blockage of stem scar region of tomato fruits at different ripening stages stored at 25 ± 1°C and 70 ± 10% RH on rate of respiration (μmole CO2 g-1 fr. wt. h-1) at 5 DAT and RI% at 10 DAT in ‘Pusa Ruby’
| Ripening stage (RS) | Green mature | Breaker | Turning | Mean (T) |
|---|---|---|---|---|
| Respiration rate at 5 DAT | ||||
| C | 19.1a | 10.8b | 7.3bc | 12.4a |
| T | 4.8c | 6.2c | 7.5bc | 6.1b |
| Mean (RS) | 11.9a | 8.5b | 7.4b | |
| RS = **, T = **, RS × T = ** | ||||
| RI (%) at 10 DAT | ||||
| C | 86.0ab | 92.7ab | 98.1a | 92.3a |
| T | 26.6d | 68.2c | 81.1b | 58.6b |
| Mean (RS) | 56.3c | 80.4b | 89.6a | |
| RS = **, T = **, RS × T = ** | ||||
(n = 4 lots with 12 fruits in each lot)
Values followed by different superscript/s are significant over one another at **p ≤ 0.01. DAT, C, T, RI: As in Table 1
Table 5.
Effect of complete blockage of stem scar region of tomato fruits at turning stage stored at 25 ± 1°C and 70 ± 10% RH on rate of respiration (μmole CO2 g-1 fr. wt. h-1) at 5 DAT and RI % at 10 DAT
| ‘Pusa Early Dwarf’ | ‘Pusa Sheetal’ | |||
|---|---|---|---|---|
| Respiration rate | RI, % | Respiration rate | RI, % | |
| C | 8.0NS | 92.1** | 7.1NS | 3.4** |
| T | 6.0 | 60.7 | 5.0 | 65.5 |
(n = 3 lots with 12 fruits in each lot)
**p ≤ 0.01 for each column based on the comparison of 2 means by t-test. NS: Not significant. DAT, RI, C, T: As in Table 1
The respiration rates were lowest in tomato fruits with complete blockage treatment in ‘Pusa Ruby’ at green mature stage (Fig. 1a). The rates of respiration in partially blocked (1/4th and 1/2 blocked treatments) fruits were comparable to the control except for 5 DAT at which, the rates of respiration in partially blocked fruits were also significantly lower in comparison to control (Fig. 1a). The RI was negatively related to the extent of blockage (Fig. 1b). Likewise, the extent of suppression in respiration rate, climacteric peak and RI were also negatively related with the degree of blockage in ‘Pusa Ruby’. ‘Pusa 120’ also showed similar trend for respiration and ripening (Table 6). This indicates the role of available surface area of stem scar region in determining not only the rate of respiration but also the progress of ripening.
Fig. 1.
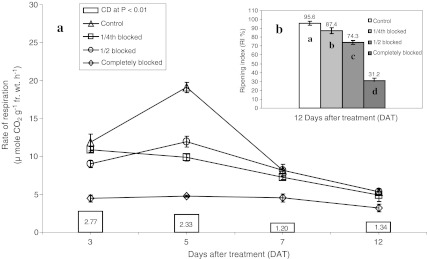
Effect of different degrees of blocking the stem scar region of tomato fruits at green mature stage on rate of respiration (a) and RI % (b) in 'Pusa Ruby'. Fruits were stored at 25 ± 1°C and 70 ± 10% RH. Histograms with different alphabetic letter are significant over one another at p ≤ 0.01. Bars indicate ± SE. (n = 4 lots with 12 fruits in each lot)
Table 6.
Effect of different degrees of blocking stem scar region of tomato fruits at green mature stage stored at 25 ± 1°C and 70 ± 10% RH on RI % at 12 DAT and rate of respiration at 5 DAT in ‘Pusa 120’
| Respiration rate (μmole CO2 g-1 fr. wt. h-1) | RI, % | |
|---|---|---|
| C | 6.7a | 49.7a |
| 1/4th blocked | 3.3b | 17.1b |
| 1/2 blocked | 2.8b | 7.4c |
| Complete blocked | 2.9b | 2.3d |
(n = 4 lots with 12 fruits in each lot)
Values (for each column) followed by different superscript/s are significant over one another at p ≤ 0.01. RI, DAT, C: As in Table 1
Yang and Shewfelt (1999) observed that leaving the pedicel attached to the harvested tomato fruit lowered its rate of ripening. They also reported rapid reduction in O2 level (dropped below 3%) and increase in CO2 level (up to 6%) by a few days of sealing the stem scar region of fruits. Hypoxia or low O2 to CO2 ratio and anaerobic condition decreased the synthesis as well as action of ethylene (Kanellis et al 1989a, Gorny and Kader 1996, Mathooko 1996). Hypoxia condition in the microenvironment of the tomato fruit was reported to increase the activity of pyruvate decarboxylase and alcohol dehydrogenase (Chen and Chase 1993, Longhurst et al 1994). As a result, production of acetaldehyde and ethanol was also triggered in tomato (Longhurst et al 1994). Likewise, mandarins accumulate larger amount of acetaldehyde and ethanol after harvest than the grapefruit because of higher activity of alcohol dehydrogenase in the juice and lesser permeability of peel towards the exit of gases in mandarins (Shi et al 2007). Since, ethanol and acetaldehyde have been already reported to delay ripening in tomato and other fruits (Beaulieu et al 1997, Pesis 2005, 2006), the delayed ripening of treated fruits in the present investigation could also be due to the accumulation of ethanol and acetaldehyde. Lower availability of O2 may also lead to decrease in activities of cellulase and polygalacturonase enzymes (Kanellis et al 1991) as fruit softening is known to require oxygen (Knee 1982).
The cultivars with slow ripening (‘Pusa Gaurav’ and ‘Pusa 120’) responded better than the fast ripening variety (‘Pusa Ruby’) towards the treatment-mediated delay in ripening. Zagory and Kader (1988) have reported that resistance of plant organ to gaseous diffusion depends on type, varieties, organ and the stage of development or maturity of plants. Our earlier works have demonstrated that surface morphology and tendency of conversion of trichome base cells into lenticels and density of lenticels differed in fruits of 2 contrasting varieties of tomato (‘Pusa Ruby’ and ‘Pusa Gaurav’) (Paul and Srivastava 2006). Morphological and mechanical properties of cuticle as well as epidermis undergo considerable change during growth and ripening of tomato fruit (Bargel and Neinhuis 2005). Gas transport in fruit tissue is governed by diffusion as well as permeation. The permeation ability is determined by not only the overall pressure gradient of a given gas (Ho et al 2006a, b) but also by structural arrangement of cells and intercellular spaces of fruit (Cloetens et al 2006, Mendoza et al 2007, Verboven et al 2008). Existence of varietal differences in above said parameters will not only influence the internal gaseous environment of fruits but also the final effect of blockage treatments as noticed in this study.
The gradual reduction in the effectiveness of treatment in delaying the ripening beyond the green mature stage (Table 5) could be explained in view of the differences in the resistance to the gaseous diffusion on the maturity stage in different fruits (Zagory and Kader 1988, Kader and Saltveit 2003a, b), in tomato (Bargel and Neinhuis 2005), in mango (Paul et al 2007) and in pear (Ho et al 2008). Saltveit (1999) reported that with the onset of ripening in climacteric fruits (including tomato) the internal ethylene concentration increases quickly due to the stronger diffusion resistance at later stages of fruit ripening. So, possibly the lower levels of internal ethylene in fruits treated at early ripening stage (green mature) can be one of the reasons for more pronounced delay in ripening of treated fruits at this stage in comparison to the advanced ripening stages (breaker and turning).
Sanders and de Wild (2003) reported lower O2 partial pressure inside the fruit than outside due to O2 consumption and resistance to O2 diffusion into tomato. It has been already reported that blockage of stem scar region of tomato reduced the internal O2 to CO2 ratio (Yang and Shewfelt 1999). Stem scar region of tomato has been demonstrated as the major site for gaseous exchange (Cameron and Yang 1982). Lower rate of respiration in fruits where stem scar portion was completely or partially blocked (Tables 3, 4 and 6 and Fig. 1a) could be due to lower ratio of O2 to CO2 concentration inside the fruit due to the higher resistance to the net influx of O2 and net out flux of CO2 in the modified atmosphere packaging as reported by Banks (1984) and Smith et al (1987) for apple fruits. This change was shown to shift the respiration in favour of glycolytic fermentation in tomato (Klieber et al 1996). Besides this, higher CO2 concentration reduces the activity or synthesis of various enzymes of the respiratory metabolism (Lange and Kader 1997a) by altering the intercellular pH (Lange and Kader 1997b).
Extent of reduction in rate of respiration and delay in ripening were related with the degree to which the stem scar region was blocked (Fig. 1 and Table 6). Gorny and Kader (1996), Solomos and Kanellis (1997) and Pech et al (2003) showed that low O2 and high CO2 in the microenvironment of fruits delayed the onset of rise in ethylene and reduced the respiration rate. Further, the potential shelf-life of plant tissue or plant part after harvest is closely related to its rate of respiration (Kader 1987, Kader and Saltveit 2003b, Varoquaux and Ozdemir 2005).
Lower reduction in the rate of respiration of tomato fruits treated either at breaker or turning stages in comparison with green mature stage (Table 4) could be due to the changes in resistance to gaseous diffusion with the maturity of fruit (Zagory and Kader 1988). In tomato, varietal dependent increase in the density of lenticels was reported with the progress of ripening (Paul and Srivastava 2006). Further, hypertrophy of lenticels on mango fruit was documented with the progress of ripening (Larson et al 1993) and it was also dependent on mango variety (Paul et al 2007). Hagenmaier (2005) reported that exchange of gases in fruits through the peel by diffusion from openings (stomates or lenticels) was proportional to the area of such openings.
Banks (1984) reported that deterioration of fruits by application of artificial diffusion barriers was because of altered endogenous concentrations of O2, CO2 and ethylene. Tomato fruits blocked at stem scar end showed delay and decrease in the evolution of ethylene (Yang and Shewfelt 1999). Low O2 to CO2 ratio was also reported to suppress not only the biosynthesis but also the action of ethylene (Kanellis et al 1989a, b). There are various factors that affect the production and release of ethylene by fruit. In this regard, a theory of ethylene emission was developed and used as the base to develop simulation model called ‘ETHY’ by Genard and Gouble (2005). This model was highly sensitive to factors like permeability of skin surface, internal concentration of O2, CO2 and 1-amino-cyclopropane-1-carboxylic acid (ACC), change in fruit growth and temperature, activities of ACC-oxidase and ACC-synthase, concentration of ethylene itself and production status of ATP. Since the level of ethylene has been positively involved in defense against pathogen (Saltveit 1999, Van Loon et al 2006), its lower endogenous levels (Gorny and Kader 1996, Solomos and Kanellis 1997, Yang and Shewfelt 1999) and suppressed action (Kanellis et al 1989a, b, Pech et al 2003) explain the higher rottage in tomato fruits under the influence of blockage of stem scar region.
Conclusion
Blocking the stem scar region of tomato fruits either completely or to different extent reduced the available or effective surface area of this region, which suppressed respiration and ripening. This suppression might have been due to the changes in the internal gaseous composition of fruit in view of reduced gaseous exchange under the influence of blockage. For delaying ripening it would be worthy to make an attempt for an overall reduction in the permeance ability of the tomato fruit through the breeding strategies.
References
- Banks NH. Internal atmosphere modification in Pro-Long coated apples. Acta Hort. 1984;157:105–112. [Google Scholar]
- Bargel H, Neinhuis C. Tomato (Lycopersicon esculentum Mill.) fruit growth and ripening as related to the biomechanical properties of fruit skin and isolated cuticle. J Exp Bot. 2005;56:1049–1060. doi: 10.1093/jxb/eri098. [DOI] [PubMed] [Google Scholar]
- Beaulieu JC, Peiser G, Saltveit ME. Acetaldehyde is a causal agent responsible for ethanol induced ripening inhibition in tomato fruits. Plant Physiol. 1997;113:431–439. doi: 10.1104/pp.113.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yehoshua S, Rodov V. Transpiration and water stress. In: Bartz JA, Brecht JK, editors. Postharvest physiology and pathology of vegetable. New York: Marcel Dekker; 2003. pp. 111–159. [Google Scholar]
- Blanke MM. Comparative SEM study of stomata on developing quince, apple, grape and tomato fruit. Angewandte Bot. 1986;60:209–214. [Google Scholar]
- Burg SP, Burg EA. Gas exchange in fruits. Physiol Plant. 1965;18:870–886. doi: 10.1111/j.1399-3054.1965.tb06946.x. [DOI] [Google Scholar]
- Cameron AC, Yang SF. A simple method for the determination of resistance to gas diffusion in plant organs. Plant Physiol. 1982;70:21–23. doi: 10.1104/pp.70.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ARS, Chase J., Jr Alcohol dehydrogenase and pyruvate decarboxylase induction in ripening and hypoxia tomato fruit. Plant Physiol Biochem. 1993;31:875–885. [Google Scholar]
- Clendenning KA. Studies of the tomato in relation to its storage. II. The effects of altered internal atmosphere upon the ripening and respiratory behaviour of tomato fruit stored at 12.5 0 C. Can J Res. 1941;19:500–518. [Google Scholar]
- Cloetens P, Mache R, Schlenker M, Lerbs-Mache S. Quantitative phase tomography of Arabidopsis seeds reveals intercellular void network. Proc Nat Acad Sci USA. 2006;103:14626–14630. doi: 10.1073/pnas.0603490103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadzie BK, Banks NH, Cleland DJ, Hewett EW. Role of skin resistance to gas diffusion in the response of fruits to modified atmosphere. Acta Hort. 1993;343:129–134. [Google Scholar]
- Das DJ, Barringer SA. Use of organic solvents for improving peelability of tomatoes. J Food Proc Preserv. 1999;23:93–202. doi: 10.1111/j.1745-4549.1999.tb00379.x. [DOI] [Google Scholar]
- de Varies HSM, Harren FJM, Voesenek LACJ, Blom CWPM, Woltering EJ, van der Valk HCPM, Reuss J. Investigation of local ethylene emission from intact cherry tomatoes by means of photothermal deflection and photoacoustic detection. Plant Physiol. 1995;107:1371–1377. doi: 10.1104/pp.107.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genard M, Gouble B. ETHY. A theory of fruit climacteric ethylene emission. Plant Physiol. 2005;139:531–545. doi: 10.1104/pp.105.063339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny JR, Kader AK. Controlled atmosphere suppression of ACS and ACC-oxidase in ‘Golden Delicious’ apple during long-term cold storage. J Am Soc Hort Sci. 1996;121:751–755. [Google Scholar]
- Hagenmaier RD. A comparison of ethane, ethylene and CO2 peel permeance for fruits with different coatings. Postharvest Biol Technol. 2005;37:56–64. doi: 10.1016/j.postharvbio.2005.02.012. [DOI] [Google Scholar]
- Ho QT, Verboven P, Verlinden BE, Lammertyn J, Vandewalle S, Nicolai BM. A continuum model for metabolic gas exchange in pear fruit. PLoS Computational Biol. 2008;4:e1000023. doi: 10.1371/journal.pcbi.1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho QT, Verlinden BE, Verboven P, Nicolai BM. Gas diffusion properties at different positions in the pear. Postharvest Biol Technol. 2006;41:113–120. doi: 10.1016/j.postharvbio.2006.04.002. [DOI] [Google Scholar]
- Ho QT, Verlinden BE, Verboven P, Vandewalle S, Nicolai BM. A permeation-diffusion-reaction model of gas transport in cellular tissue of plant material. J Exp Bot. 2006;57:4215–4224. doi: 10.1093/jxb/erl198. [DOI] [PubMed] [Google Scholar]
- Kader AA. Respiration and gas exchange of vegetable. In: Weichmann J, editor. Postharvest physiology of vegetables. New York: Marcel Dekker; 1987. pp. 25–43. [Google Scholar]
- Kader AA, Saltveit ME. Atmosphere modification. In: Bartz JA, Brecht JK, editors. Postharvest physiology and pathology of vegetables. New York: Marcel Dekker; 2003a. pp. 229–246. [Google Scholar]
- Kader AA, Saltveit ME. Respiration and gas exchange. In: Bartz JA, Brecht JK, editors. Postharvest physiology and pathology of vegetables. New York: Marcel Dekker; 2003b. pp. 7–29. [Google Scholar]
- Kanellis AK, Solomos T, Mattoo AK. Hydrolytic enzyme activities and protein pattern of avocado fruit ripened in air and in low oxygen with and without ethylene. Plant Physiol. 1989;90:259–266. doi: 10.1104/pp.90.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellis AK, Solomos T, Mattoo AK. Changes in sugar, enzymatic activities and acid phophatse isozyme profile of bananas ripened in air or stored in 2.5% O2 with and without ethylene. Plant Physiol. 1989;90:251–258. doi: 10.1104/pp.90.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellis AK, Solomos T, Roubelakis-Angelakis KA. Suppression of cellulase and polygalacturonase and induction of alcohol dehydrogenase isoenzymes of avocado fruit mesocarp subjected to low oxygen stress. Plant Physiol. 1991;96:269–274. doi: 10.1104/pp.96.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klieber A, Ratanachinakorn B, Simons DH. Effect of low oxygen and high carbon dioxide on tomato cultivar ‘Bermuda’ fruit physiology and composition. Sci Hort. 1996;65:251–261. doi: 10.1016/0304-4238(96)00881-3. [DOI] [Google Scholar]
- Knee M. Fruit softening III. Requirement for oxygen and pH effects. J Exp Bot. 1982;33:1263–1269. doi: 10.1093/jxb/33.6.1263. [DOI] [Google Scholar]
- Lange DL, Kader AA. Effects of elevated carbon dioxide on key mitochondrial respiratory enzymes in ‘Hass’ avocado fruit and fruit disks. J Am Soc Hort Sci. 1997;122:228–244. [Google Scholar]
- Lange DL, Kader AA. Elevated carbon dioxide exposure alters intracellular pH and energy charge in avocado fruit tissue. J Am Soc Hort Sci. 1997;122:253–257. [Google Scholar]
- Larson KD, Schaffer B, Davies FS. Flood water oxygen content, ethylene production and lenticel hypertrophy in flooded mango (Mangifera indica L.) trees. J Exp Bot. 1993;44:665–671. doi: 10.1093/jxb/44.3.665. [DOI] [Google Scholar]
- Longhurst TJ, Lee E, Brady CJ, Speirs J. Structure of the tomato adh2 gene and adh2 pseudo-gene and the study of adh2 gene expression in fruit. Plant Mol Biol. 1994;26:1073–1084. doi: 10.1007/BF00040690. [DOI] [PubMed] [Google Scholar]
- Mathooko FM. Regulation of ethylene biosynthesis in higher plants by carbon dioxide. Postharvest Biol Technol. 1996;7:1–26. doi: 10.1016/0925-5214(95)00026-7. [DOI] [Google Scholar]
- Mendoza F, Verboven P, Mebatsion HK, Kerckhofs G, Wevers M, Nicolai B. Three-dimensional pore space quantification of apple tissue using X-ray computed microtomography. Planta. 2007;226:559–570. doi: 10.1007/s00425-007-0504-4. [DOI] [PubMed] [Google Scholar]
- Nuevo PA, Lizada MCC, Pantastico Er B. Gas diffusion factors in fruits III. Oxygen and carbon dioxide. Postharvest Res Notes. 1984;1:86–89. [Google Scholar]
- Paul V, Malik SK, Srivastava GC. Intervarietal differences in the surface morphology and anatomy of mango (Mangifera indica L.) fruits. Phytomorphol. 2007;57:1–10. [Google Scholar]
- Paul V, Pandey R, Srivastava GC. Ripening of tomato (Solanum lycopersicum L.). Part I: 1-methylcyclopropene mediated delay at higher storage temperature. J Food Sci Technol. 2010 doi: 10.1007/s13197-010-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul V, Srivastava GC. Role of surface morphology in determining the ripening behaviour of tomato (Lycopersicon esculentum Mill.) fruits. Sci Hort. 2006;110:84–92. doi: 10.1016/j.scienta.2006.06.023. [DOI] [Google Scholar]
- Pech JC, Bouzayen M, Latche A, Sanmartin M, Aggelis A, Kanellis AK. Physiological, biochemical and molecular aspects of ethylene biosynthesis and action. In: Bartz JA, Brecht JK, editors. Postharvest physiology and pathology of vegetables. New York: Marcel Dekker; 2003. pp. 247–285. [Google Scholar]
- Pesis E. The role of the anaerobic metabolites, acetaldehyde and ethanol in fruit ripening, enhancement of fruit quality and fruit deterioration. Postharvest Biol Technol. 2005;37:1–19. doi: 10.1016/j.postharvbio.2005.03.001. [DOI] [Google Scholar]
- Pesis E (2006) Postharvest treatments prior storage with anaerobiosis or anaerobic metabolites to improve fruit quality. In: Advances in postharvest technology for horticultural crops, Research Signpost, Trivandrum, India, p 251-274
- Saltveit ME. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol Technol. 1999;15:279–292. doi: 10.1016/S0925-5214(98)00091-X. [DOI] [Google Scholar]
- Sanders MG, de Wild HPJ. The relation between in vivo ethylene production and oxygen partial pressure. Postharvest Biol Technol. 2003;30:143–151. doi: 10.1016/S0925-5214(03)00102-9. [DOI] [Google Scholar]
- Shi JX, Goldschmidt EE, Goren R, Porat R. Molecular, biochemical and anatomical factors governing ethanol fermentation metabolism and accumulation of off-flavours in mandarins and grapefruit. Postharvest Biol Technol. 2007;46:242–251. doi: 10.1016/j.postharvbio.2007.05.009. [DOI] [Google Scholar]
- Smith S, Geeson J, Stow J. Production of modified atmospheres in deciduous fruits by the use of films and coatings. HortSci. 1987;22:772–777. [Google Scholar]
- Solomos T. Principles of gas exchange in bulky plant tissue. HortSci. 1987;22:766–771. [Google Scholar]
- Solomos T, Kanellis AK. Hypoxia and fruit ripening. In: Kannellis AK, Chang C, Kende H, Grierson D, editors. Biology and biotechnology of plant hormone ethylene. Dordrecht: Kluwer Academic; 1997. pp. 239–252. [Google Scholar]
- Thompson AK. Postharvest technology of fruits and vegetables. In: Thompson AK, editor. Fruits and vegetables: Harvesting handling and storage. Oxford: Blackwell; 2003. p. 354. [Google Scholar]
- UFFVA (1975) United Fresh Fruit and Vegetable Association. Colour classification requirements in tomatoes. USDA Visual Aid TM-L-1, The John Henry Co., Lansing, Minch
- Van Loon LC, Geraats BPJ, Linthorst HJM. Ethylene as a modulator of disease resistance in plants. Tr Plant Sci. 2006;11:185–191. doi: 10.1016/j.tplants.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Varoquaux P, Ozdemir IS. Packaging and produce degradation. In: Lamikanra O, Imam S, Ukuku D, editors. Produce degradation pathways and prevention. Boca Raton: CRC Press; 2005. pp. 117–153. [Google Scholar]
- Verboven P, Kerckhofs G, Mebatsion HK, Ho QT, Temst K, Wevers M, Cloetens P, Nicolai BM. Three-dimensional gas exchange pathways in pome fruit characterized by synchrotron X-ray computed tomography. Plant Physiol. 2008;147:518–527. doi: 10.1104/pp.108.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LA, Sterling C. Studies on the cuticle of tomato fruit. I. Fine structure of the cuticle. Z Pflanzenphysiol Bd. 1976;77S:359–371. [Google Scholar]
- Yang CX, Shewfelt RL. Effects of sealing of stem scar on ripening rate and internal ethylene oxygen and carbon dioxide concentrations of tomato fruits. Acta Hort. 1999;485:399–404. [Google Scholar]
- Zagory D, Kader AA. Modified atmosphere packaging of fresh produce. Food Technol. 1988;42:70–77. [Google Scholar]


