Abstract
We have isolated 4 antibacterial substances that were active against the oral bacteria that cause dental caries and periodontitis, such as Streptococcus mutans, Prevotella intermedia, and Porphyromonas gingivalis, from lemon peel, a waste product in the citrus industry. The isolated substances were identified as 8-geranyloxypsolaren, 5-geranyloxypsolaren, 5-geranyloxy-7-methoxycoumarin, and phloroglucinol 1-β-D-glucopyranoside (phlorin) upon structural analyses. Among these, 8-Geranyloxypsolaren, 5-geranyloxypsolaren, and 5-geranyloxy-7-methoxycoumarin exhibited high antibacterial activity. These 3 compounds were effectively extracted using ethanol and n-hexane, whereas phlorin was extracted with water. Further, the above 3 compounds were present in lemon essential oil and abundantly present in the residue produced upon the cooling treatment of concentrated lemon essential oil.
Keywords: Antibacterial substance, Lemon peel, Oral bacteria, 8-geranyloxypsolaren, 5-geranyloxypsolaren, 5-geranyloxy-7-methoxycoumarin, Phlorin
Introduction
Pathogenic bacteria can cause dental caries (e.g., Streptococcus mutans) and periodontitis (e.g., Prevotella intermedia and Porphyromonas gingivalis). Inhibition of the growth of these bacteria is considered one of the most important factors for the prevention and treatment of these oral diseases (Takahashi and Schachtele 1990). Polyphenols in foods such as red grape skins and green tea reportedly inhibit the growth of the oral bacteria that cause dental caries and periodontitis (Kakuda et al. 1994; Smullen et al. 2007). Antibacterial substances in functional foods have been investigated from a practical standpoint.
Citrus fruits such as orange, lemon, and lime, have been cultured widely and processed into juice (Olaniyan 2010). During the manufacture of citrus juice, citrus peels are discarded as waste products. Lemon peel is a useful source of flavonoids, which have high antioxidative activity; pectin; and essential oil; further, it is expected to contain other functional compounds (Miyake et al. 1997). In this study, we attempted to isolate antibacterial substances from lemon peel that were active against oral bacteria that cause dental caries and periodontitis. We attempted also to examine for the condition of effective extraction for the active substances.
Materials and methods
Microorganisms
Streptococcus mutans ATCC7270, Prevotella intermedia, and Porphyromonas gingivalis 381, which were isolated from the human buccal capsule, were supplied by Dr. T. Noguchi, Department of Periodontology, School of Dentistry, Aichi Gakuin University, Nagoya, Japan.
Isolation of substances from methanol extracts
To extract of the antibacterial substances, 1 kg lemon peel was treated with 1 l methanol at room temperature for 3 days. The extract thus obtained was applied to a Cosmosil 75 C 18-OPN ODS column (Nacalai Tesque Inc., Kyoto, Japan; column size: ϕ37 × 500 mm). The column was washed with 2 l water and successively eluted with 2 l methanol. The compounds in the concentrated eluate were isolated using preparative HPLC and assayed for antibacterial activity against oral bacteria. Preparative HPLC was conducted using the following: column, Shim-pack PREP ODS (L) (Shimadzu Co. Ltd., Kyoto, Japan; ϕ15 × 250 mm) equipped with a UV detector (280 nm); elution solvent, methanol and water (linear gradient, from 0% to 100% methanol in 60 min); and flow rate, 10 ml/min.
Identification of the antimicrobial compounds
The isolated compounds were identified by instrumental analysis. UV absorption spectra were recorded on a spectrophotometer (HITACHI U-2000), and IR spectra were recorded on an FT/IR-8200RC (Shimadzu Co. Ltd., Japan). Fast atom bombardment mass spectra (FAB-MS) were recorded on a JEOL JMS-DX-705 L column. 1H NMR and 13C NMR spectra were obtained using a JEOL JNM-EX-400 NMR instrument (400 MHz for 1H and 100 MHz for 13C).
Antibacterial assay
Streptococcus mutans was cultured anaerobically in BHI medium (Brain-Heart Infusion medium, Nissui Pharmaceutical Co. Ltd., Tokyo, Japan) in a Gas Pak anaerobic jar (BBL, Cockeysville, MD) at 37 °C, and Prevotella intermedia and Porphyromonas gingivalis were cultured anaerobically in the GAM (Gifu Anaerobic Medium) broth medium (Nissui Pharmaceutical Co. Ltd.) under the same conditions. Each bacterium was inoculated into the medium at a density of 105 cells/ml, and the minimum inhibitory concentration (MIC) of the substances isolated from lemon peel was determined using the tube-dilution technique (Sakanaka et al. 1989).
Determination of antibacterial substances
Freeze-dried lemon peel powder (0.5 g) was mixed with 10 ml each of water, ethanol, and n-hexane and subjected to ultrasonic treatment for 20 min. The amount of the isolated antibacterial compounds in the resultant solutions was determined using HPLC. Lemon essential oil (Aruba Co. Ltd., Sapporo, Japan; production in USA) was concentrated to one-fifth its volume under reducing pressure in order to remove volatile substances and then cooled to −30 °C for 7 days. The concentrated solution was centrifuged at 20,627 g for 15 min, and the pellet was dissolved in n-hexane and analyzed using HPLC. HPLC (LC-10A, Shimadzu Co., Ltd., Kyoto, Japan) was conducted using a Shim-pack CLC-ODS (M) column (ϕ4.6 × 150 mm, 5 μm) equipped with a UV detector (280 nm) at 40 °C and a flow rate of 1.0 ml/min. The elution solvent for the determination of 8-geranyloxypsolaren, 5-geranyloxypsolaren, and 5-geranyloxy-7-methoxycoumarin was 90% methanol and 10% water, and that for phlorin was 2% methanol and 98% water.
Statistical analysis
The amount of antibacterial substances in the extracts obtained using each solvent was expressed as the mean ± SD (N = 4). Statistical analysis was conducted using two-way ANOVA followed by the Fisher PLSD test in order to identify significant differences. A 5% significance level (P < 0.05) was used.
Results and discussion
Isolation and identification of antibacterial substances
Three compounds were isolated from the hydrophobic fraction of lemon peel extract and identified as 8-geranyloxypsolaren, 5-geranyloxypsolaren, and 5-geranyloxy-7-methoxycoumarin by MS, 1H-NMR, and 13C-NMR analyses (Fig. 1). The data of structural analyses of 8-geranyloxypsolaren were shown as follows. UV (MeOH) nm: λmax 213, 250, 264, 300. IR (KBr) cm-1: 1720, 1624, 1448, 1152. 1H-NMR (d-DMSO, 400 MHz) δ: 1.56 (3 H, s, H9'), 1.64 (3 H, s, H10'), 1.69 (3 H, bs, H4'), 2.01 (2 H, m, H5'), 4.99 (1 H, m, H7'), 5.03 (2 H, d, J = 7.0, H1'), 5.60 (1 H, dt, J = 7.0 & 1.0, H2'), 6.37 (1 H, d, J = 9.5, H3), 6.81 (1 H, d, J = 2.5, H6), 7.36 (1 H, s, H5), 7.69 (1 H, d, J = 2.5, H7), 7.76 (1 H, d, J = 9.5, H4), 2.01 (2 H, m, H6'). 13C-NMR (d-DMSO, 100 MHz) δ: 16.51(C4'), 17.62(C9'), 25.62(C10'), 26.32(C6'), 39.54(C5'), 70.06(C1'), 106.70(C6), 113.18(C5), 114.68 (C3), 116.45(C10), 119.41(C2'), 123.74(C7'), 125.80(C11), 131.55(C3'), 131.69(C8'), 143.12(C8), 143.93(C9), 144.31(C4), 146.58(C7), 148.74(C12). FAB-MS (MH+) m/z:339 (C21H22O4). Yield: 5.6 mg. The data of structural analyses of 5-Geranyloxypsolaren were shown as follows. UV (MeOH) nm: λmax 213, 250, 258, 267, 304. IR (KBr) cm-1: 1720, 1624, 1448, 1152. 1H-NMR (d-DMSO, 400 MHz) δ: 1.60 (3 H, s, H9'), 1.68 (3 H, s, H10'), 1.69 (3 H, d, J = 1.0, H4'), 2.10 (2 H, m, H5'), 2.10 (2 H, m, H6'), 4.95 (2 H, d, J = 7.0, H1'), 5.07 (1 H, m, H7'), 5.54 (1 H, tq, J = 7.0 & 1.0, H2'), 6.28 (1 H, d, J = 9.5, H3), 6.96 (1 H, dd, J = 2.5 & 1.0, H6), 7.16 (1 H, bs, H8), 7.60 (1 H, d, J = 2.5, H7), 8.17 (1 H, dd, J = 9.5 & 0.5, H4). 13C-NMR (d-DMSO, 100 MHz) δ: 16.65(C4'), 17.68(C9'), 25.65(C10'), 26.19(C6'), 39.47(C5'), 69.73(C1'), 94.19(C8), 105.05(C6), 107.48(C10), 112.52(C3), 114.17(C11), 118.84(C2'), 123.47(C7'), 130.00(C8'), 139.59(C4), 143.02(C3'), 144.85(C7), 148.95(C5), 152.63(C9), 158.10(C12). FAB-MS (MH+) m/z:339 (C21H22O4). Yield: 3.8 mg. The data of structural analyses of 5-Geranyloxy-7-methoxycoumarin were shown as follows. UV (MeOH) nm: λmax 213, 247, 255, 318. IR (KBr) cm-1: 1740, 1608, 1440, 1160. 1H-NMR (d-DMSO, 400 MHz) δ: 8.01 (1 H, dd, J = 9.5 & 0.5, H4), 6.41 (1 H, dd, J = 2.0 & 0.5, H8), 6.29 (1 H, d, J = 2.0, H6), 6.15 (1 H, d, J = 9.5, H3), 5.48 (1 H, tq, J = 6.5 & 1.0, H2'), 5.09 (1 H, m, H7'), 4.60 (2 H, d, J = 6.5, H1'), 3.85 (3 H, s, OCH3), 2.12 (2 H, m, H5'), 2.12 (2 H, m, H6'), 1.75 (3 H, bs, H4'), 1.68 (3 H, s, H10'), 1.61 (3 H, s, H9'). 13C-NMR (d-DMSO, 100 MHz) δ: 16.65(C4'), 17.68(C9'), 25.65(C10'), 26.19(C6'), 39.47(C5'), 55.74(OCH3), 69.73(C1'), 94.19(C8), 107.48(C10), 112.52(C3), 114.17(C6), 118.84(C2'), 123.47(C7'), 130.00(C8'), 139.59(C4), 143.02(C3'), 148.95(C5), 152.63(C9), 158.10(C7). FAB-MS (MH+) m/z:329 (C20H24O4). Yield: 8.8 mg. The spectral properties of the isolated compounds were consistent with those described in the literature (Dreyer and Huey 1973; Herpol-Borremans et al. 1985; Chang et al. 1997; Murakami et al. 1999).
Fig. 1.
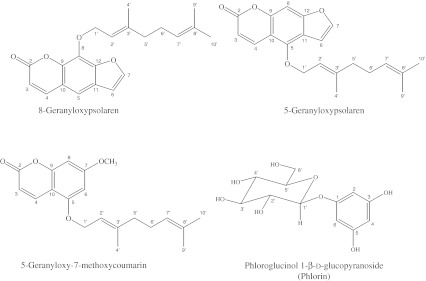
Chemical structures of antibacterial substances isolated from lemon peel
One antibacterial compound was isolated from the hydrophilic fraction of lemon peel extract and identified as phloroglucinol 1-β-D-glucopyranoside (phlorin) on the basis of MS, 1H-NMR, and 13C-NMR spectral data (Fig. 1). The data of structural analyses of phloroglucinol 1-β-D-glucopyranoside (phlorin) were shown as follows. UV (MeOH) nm: λmax 268, 233, 191. IR (KBr) cm-1: 1613, 3399. 1H-NMR (CD3OD, 400 MHz) δ: 3.71 (dd, J = 12.0, 4.8, C-6'α), 3.83 (dd, J = 12.0, 1.6, C-6'β), 5.95 (t, J = 2.1, C-4), 6.08 (2 H, d, J = 2.2 Hz, C-2), 6.08 (2 H, d, J = 2.2 Hz, C-6). 13C-NMR (CD3OD, 100 MHz) δ: 62.5 (C-6'), 71.3 (C-4'), 74.8 (C-2'), 78.0 (C-3'), 78.1 (C-5'), 96.7 (C2), 96.7 (C6), 98.0 (C4), 102.1 (C-1'), 160.1 (C3), 160.1 (C5), 160.9 (C1). FAB-MS (MH+) m/z: 289 (C12H16O8). Yield: 10.2 mg. The 1H-NMR and 13C-NMR spectral data of the isolated compound were consistent with those previously reported (Foo and Karchesy 1989). In the present study, 4 compounds with activity against oral bacteria were isolated from lemon peel.
Activity against oral bacteria
The 4 isolated compounds were examined for activity against Streptococcus mutans, Prevotella intermedia, and Porphyromonas gingivalis (Table 1). The antibacterial activity of 8-geranyloxypsolaren, 5-geranyloxypsolaren, and 5-geranyloxy-7-methoxycoumarin was higher than that of phlorin. Coumarin and psoralen derivatives obtained from plants have been reported to possess antibacterial activity (Céspedes et al. 2006). 7-Geranyloxycoumarin, isolated from the oil of hassaku (Citrus hassaku), has been reported to show activity against bacteria such as Bacillus subtilis (Nakatani et al. 1987). However, 8-geranyloxypsolaren, 5-geranyloxypsolaren, and 5-geranyloxy-7-methoxycoumarin have never before been reported to show any antibacterial activity. Phlorin exhibited antibacterial activity against Prevotella intermedia and weak antibacterial activity against Streptococcus mutans and Porphyromonas gingivalis. The present study is the first to report that the above 4 compounds isolated from lemon peel exhibit activity against oral bacteria.
Table 1.
Minimum inhibitory concentrations (MICs; mM) of the substances that were isolated from lemon peel and showed activity against oral bacteria
| Substances isolated from lemon peel | Minimum inhibitory concentration (mM) | ||
|---|---|---|---|
| S. mutans | P. Intermedia | P. gingivalis | |
| 8-Geranyloxypsolaren | 0.15 | 0.30 | 0.30 |
| 5-Geranyloxypsolaren | 0.15 | 0.30 | 0.15 |
| 5-Geranyloxy-7-methoxycoumarin | 0.30 | 0.30 | 0.45 |
| Phlorin | 3.50 | 0.90 | 3.50 |
Extraction of antibacterial substances from lemon peel
Water, ethanol, and n-hexane were used as extraction solvents for food materials. The amount of the 4 compounds extracted from lemon peel by using each of these solvents was determined (Table 2). Because of their hydrophobic properties, 8-geranyloxypsolaren, 5-geranyloxypsolaren, and 5-geranyloxy-7-methoxycoumarin were effectively extracted with ethanol and n-hexane but not water. Ethanol was superior to n-hexane as an extraction solvent for the above compounds. Phlorin was effectively extracted from lemon peel by using water because of its hydrophilic property. Phlorin is a major phenolic constituent of molasses, which are by-products generated during citrus fruit processing (Fisher and Trama 1979; Manthey and Grohmann 2001). The results of the present study suggest that phlorin may be effectively extracted from molasses.
Table 2.
Concentrations of antibacterial substances isolated from lemon peel by using different extraction solvents
| Substances isolated from lemon peel | Concentration of the antimicrobial substance (μg/ml) | ||
|---|---|---|---|
| Water | Ethanol | n-Hexane | |
| 8-Geranyloxypsolaren | 0a | 207 ± 15.1b | 161 ± 4.9c |
| 5-Geranyloxypsolaren | 0a | 108 ± 9.5b | 97.2 ± 3.0c |
| 5-Geranyloxy-7-methoxycoumarin | 0a | 121 ± 14.9b | 101 ± 3.0b |
| Phlorin | 709 ± 39.9a | 16.0 ± 4.7b | 0c |
The results are the mean±SD of 3 replicates. Means with common letters (a–c) within a column are not significantly different (P < 0.05, LSD test)
The substances of 8-geranyloxypsolaren, 5-geranyloxypsolaren, and 5-geranyloxy-7-methoxycoumarin are the main coumarin derivatives isolated from lemon peel (Stanley and Jurd 1971; Fisher and Trama 1979). These 3 compounds were detected in lemon essential oil and were present in abundant amounts in the residue obtained upon cooling treatment of the concentrated oil (Table 3). The removal of residue produced upon the cooling treatment of essential oil is known as “wintering” and is a part of the purification process during the manufacture of high-quality oil. The residue (by-product) produced during the wintering of lemon essential oil possibly consists of 8-geranyloxypsolaren, 5-geranyloxypsolaren, and 5-geranyloxy-7-methoxycoumarin.
Table 3.
Concentrations of antibacterial substances in lemon essential oil and the residue produced upon cooling treatment of lemon essential oil
| Substances isolated from lemon peel | Concentration of the antimicrobial substance | |
|---|---|---|
| Lemon essential oil (μg/ml) | Lemon essential oil residue (μg/g) | |
| 8-Geranyloxypsolaren | 333 ± 36 | 1,911 ± 39 |
| 5-Geranyloxypsolaren | 550 ± 18 | 5,761 ± 163 |
| 5-Geranyloxy-7-methoxycoumarin | 502 ± 43 | 5,376 ± 149 |
| Phlorin | 0 | 0 |
Data are presented as means±SD (N = 3)
Conclusion
In this study, 8-geranyloxypsolaren, 5-geranyloxypsolaren, 5-geranyloxy-7-methoxycoumarin, and phlorin were isolated from lemon peel and found to be active against oral bacteria. Therefore, these compounds may be used in oral care materials; further, the development of such materials will enable the effective utilization of by-products produced in the citrus industry, such as molasses or the residue of lemon essential oil.
Acknowledgment
We are grateful to Dr. Y. Sugiyama and Dr. M. Isobe of Bioagricultural Sciences, Graduate School of Nagoya University, for the FAB-MS and NMR measurements and to T. Kato and Y. Tamaki of the Pokka Corporation Ltd. for their advice on this experiment.
References
- Céspedes CL, Avila JG, Martínez A, Serrato B, Calderón-Mugica JC, Salgado-Garciglia R. Antifungal and antibacterial activities of Mexican tarragon (Tagetes lucida) J Agric Food Chem. 2006;54:3521–3527. doi: 10.1021/jf053071w. [DOI] [PubMed] [Google Scholar]
- Chang C, Floss HG, Steck W. Carbon-13 magnetic resonance spectroscopy of coumarins. Carbon-13-proton long-range couplings. J Org Chem. 1997;42:1337–1340. doi: 10.1021/jo00428a014. [DOI] [PubMed] [Google Scholar]
- Dreyer DL, Huey PF. Coumarins of Citrus marcroptera. Phytochemistry. 1973;12:3011–3013. doi: 10.1016/0031-9422(73)80536-9. [DOI] [Google Scholar]
- Fisher JF, Trama LA. High-performance liquid chromatographic determination of some coumarins and psoralens found in citrus peel oils. J Agric Food Chem. 1979;27:1334–1337. doi: 10.1021/jf60226a001. [DOI] [PubMed] [Google Scholar]
- Foo LY, Karchesy JJ. Polyphenolic glycosides from Douglas fir inner bark. Phytochemistry. 1989;28:1237–1240. doi: 10.1016/0031-9422(89)80217-1. [DOI] [Google Scholar]
- Herpol-Borremans M, Masse MO, Grimee R. Furocoumarines dans les fuiles essentielles identification et dosage du 5 methoxy psoralene dans les produits solaires. J Pharm Belg. 1985;40:147–158. [PubMed] [Google Scholar]
- Kakuda T, Takihara T, Sakane I, Mortelmans K. Antimicrobial activity of tea extracts against periodontopathic bacteria. Nippon Nôgeikagaku Kaishi. 1994;68:241–243. [Google Scholar]
- Manthey JA, Grohmann K. Phenols in citrus peel byproducts. Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. J Agric Food Chem. 2001;49:3268–3273. doi: 10.1021/jf010011r. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Yamamoto K, Osawa T. Isolation of eriocitrin (eriodictyol 7-rutinoside) from lemon fruit (Citrus limon BURM. f.) and its antioxidative activity. Food Sci Technol Int Tokyo. 1997;3:84–89. doi: 10.3136/fsti9596t9798.3.84. [DOI] [Google Scholar]
- Murakami A, Gao G, Kim OK, Omura M, Yano M, Ito C, Furukawa H, Jiwajinda S, Koshimizu K, Ohigashi H. Identification of coumarins from the fruit of citrus hystrix DC as inhibitors of nitric oxide generation in mouse macrophage RAW 264.7 cells. J Agri Food Chem. 1999;47:333–339. doi: 10.1021/jf980523e. [DOI] [PubMed] [Google Scholar]
- Nakatani N, Yamada Y, Fuwa H. Epoxyaurapten and marmin from juice oil in Hassaku (Citrus hassaku) and the spasmolytic activity of 7-geranyloxycoumarin-related compounds. Agric Biol Chem. 1987;51:1105–1110. doi: 10.1271/bbb1961.51.1105. [DOI] [Google Scholar]
- Olaniyan AM. Development of a small scale orange juice extractor. J Food Sci Technol. 2010;47:105–108. doi: 10.1007/s13197-010-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka S, Kim M, Taniguchi M, Yamamoto T. Antibacterial substances in Japanese green tea extract against Streptococcus mutans, a cariogenic bacterium. Agric Biol Chem. 1989;53:2307–2311. doi: 10.1271/bbb1961.53.2307. [DOI] [Google Scholar]
- Smullen J, Koutsou GA, Foster HA, Zumbé A, Storey DM. The antibacterial activity of plant extracts containing polyphenols against Streptococcus mutans. Caries Res. 2007;41:342–349. doi: 10.1159/000104791. [DOI] [PubMed] [Google Scholar]
- Stanley WL, Jurd L. Citrus coumarins. J Agric Food Chem. 1971;19:1106–1110. doi: 10.1021/jf60178a007. [DOI] [Google Scholar]
- Takahashi N, Schachtele CF. Effect of pH on the growth and proteolytic activity of Porphyromonas gingivalis and Bacteroides intermedius. J Dent Res. 1990;69:1266–1269. doi: 10.1177/00220345900690060801. [DOI] [PubMed] [Google Scholar]


