Abstract
Phytase in brown rice will be activated and accumulated during seed germination. Changes of phytase activity in brown rice during two stages of germination (steeping and sprouting) affected by process conditions were studied. It was shown that steeping led to significant decrease of phytase activity (p < 0.01), varying with steeping temperature and steeping medium composition. Steeping respectively in demineralized water, 0.5 mM CaCl2 and 0.1 M H2O2 at 20 °C for 24 h led to the lowest phytase activity in brown rice, which was only 25% of that in raw rice. At steeping stage, steeping media had no significant effect. During the sprouting stage, phytase activity increased with prolonged time and gradually reached stable levels, and with higher temperature in the range of 15–25 °C. Phytase activity in brown rice reached 320–340 U kg−1 after 5 d sprouting. The evolution pattern of phytase activity during sprouting differed with the solutes previously used during steeping. Steeping either with CaCl2 or H2O2 caused a delay followed by a rapid activation of phytase, while for control, a gradual accumulation of phytase activity was observed. Compared with acidic and alkaline steeping solutions, demineralized water at neutral (6.8) pH provided the best pre-treatment prior to sprouting at 25 °C, to activate maximum levels of phytase. Extreme conditions, either strong acidic or alkaline inhibited activation of phytase, and changed appearance of brown rice kernel as well.
Keywords: Phytase activity, Brown rice, Steeping, Sprouting
Introduction
Phytic acid (myo-inositol 1,2,3,4,5,6 hexakis dihydrogen phosphate) widely occurs in plant foods, such as cereals, legumes, fruits and vegetables. It represents 50–85% of the total phosphorus in plant seeds (Pallauf and Rimbach 1997). At neutral pH, phytic acid in foods is negatively charged and has capacity to bind proteins and cations including Ca2+, Fe2+, Zn2+, Mg2+, resulting in low bioavailability of bound components (Liang et al. 2008).
Phytic acid can be degraded by phytase, either occurring as endogenous enzyme in seeds and accumulated during germination, or as exogenous microbial enzyme (Duhan et al. 2001). Endogenous phytase in grains plays an important role in the utilization of nutrients by the embryo during germination of seeds and growth of plants (Reddy et al. 1978, 1982; Mulimani et al. 2003). Phytase will be activated and accumulates during seed germination, and acts on phytic acid (Murugkar and Jha 2009). It releases inorganic phosphate, which is then utilized for plant growth, and serves as a natural buffer in grains as well. Microbial phytase has similar effects as endogenous phytase. It has been widely applied as an additive to animal feed to improve bioavailability of phytate phosphorous and minerals in swine and poultry diets (Żyła et al. 1999). With the release of phosphate from phytic acid by phytase, lower inositol phosphate esters, such as pentakis-, tetrakis-, tris-, bis- and mono-phosphate, and even inositol are generated (Lönnerdal et al. 1989). Lower inositol phosphate esters are less effective to chelate minerals, thus have less inhibitory effects to other components (Sandberg et al. 1989; Brune et al. 1992; Greiner and Konietzny 1999). Compared with microbial phytases, plant endogenous phytases are preferred as food additives because of their better acceptance by consumers and the lower risk of allergic reactions (Kyriakidis et al. 1998).
Endogenous phytase activity varies among different cereals (Houde and Kermasha 1990; Laboure et al. 1993; Konietzny and Greiner 1994; Greiner et al. 2000; Bartnik and Szafranska 1987), due to genetic and agronomical factors (Liu et al. 2006). Based on their reported phytase activity, we distinguish cereals into three categories, i.e. high (>2,000 U kg−1), medium (200–2,000 U kg−1) and low-level (< 200 U kg−1) of endogenous phytase. Rye is rich in phytase activity, ranging between 4,132 and 6,127 U kg−1; wheat and barley have medium phytase activity, from 915 to 1,581 U kg−1 and 408–882 U kg−1, respectively; maize, soy beans, oats and rice have low levels of 0–46, 36–183, 0–108 and 0–190 U kg−1, respectively (Eeckhout and De Paepe 1994). Phytase activity increases significantly during seed germination. In spring and winter wheats, phytase activities increased by 275% and 250%, respectively after germination (Centeno et al. 2003). Phytase activity in lentils, broad beans and runner beans reached maximum levels after 8 days of germination, around 10-fold (Kyriakidis et al. 1998). Sung et al. reported that phytase activity in barley reached up to 7.9-fold after 4 days of germination at 20 °C and 25 °C, and 7 days at 15 °C (Sung et al. 2005).
Whereas phytase activities in various raw and germinated cereals seeds were reported elsewhere, this study is focused on the change of endogenous phytase activity in brown rice during the steeping and sprouting stages, as a function of physico-chemical processing conditions. From the point of food safety, it would be of interest to carry out steeping and sprouting under acidic conditions, as these discourage the survival or multiplication of several pathogenic bacteria (Beumer 2001).
The objectives were to investigate simple interventions aimed to increase and accelerate phytase activity accumulation in brown rice, thus to provide effective ways to improve the nutritional value of brown rice. Variables investigated are steeping media composition, pH, and temperature.
Materials and methods
Brown rice
Brown rice was obtained after dehulling of paddy (II-838, harvested in 2005, collected from Wulong Seeds Institute, Chongqing, China) using a dehuller (THU-35C, Japan). Only intact and mature brown rice kernels were selected for germination.
Steeping
About 20 g (accuracy 0.01 g) brown rice was soaked in 70 mM sodium hypochlorite for 15 min, followed by three-times washing with demineralized water. Steeping of washed brown rice was done either by:
Steeping in demineralized water (1:5, w/v) under 15 °C, 20 °C and 25 °C, respectively;
Steeping (1:5, w/v) at 20 °C in different steeping media as follows: demineralized water, 0.5 mM CaCl2, or 0.1 M H2O2;
Steeping at 25 °C in buffer (1:5, w/v) with different pH values (2.2, 5.4, 6.8, 9.4, 10.5). Buffers were prepared as follows: buffer pH 2.2: 21 g citric acid and 8.4 g NaOH were mixed into 16 mL HCL (w/v 37%) and add demineralized water to 1,000 mL; buffer pH 5.4: 24.5 g citric acid and 14.4 g NaOH were mixed into 6.8 mL HCL (w/v 37%) and add demineralized water to 1,000 mL; buffer pH 6.8: 26.6 g citric acid and 15.6 g NaOH were mixed into 12.6 mL HCL (w/v 37%) and add demineralized water to 1,000 mL; buffer pH 9.4: 5.724 g Na2CO3 ·10H2O and 6.72 g NaHCO3 were mixed into demineralized water to 1,000 mL; buffer pH 10.5: 22.896 g Na2CO3 ·10H2O and 1.68 g NaHCO3 were mixed into demineralized to 1,000 mL.
All steeping treatments had a duration of 24 h and were carried out in triplicate. After steeping, brown rice was separated by decanting liquids, and either followed by sprouting, or dried at 40 °C for 2 h and then ground to pass through a 1 mm sieve for further analysis.
Sprouting
Steeped brown rice was sprouted in plastic boxes under a top cover of cotton cloth and boxes were closed with perforated lids. Brown rice kernels were moistened by spraying demineralized water on the cotton cloth every 8 h and were cleaned by washing with demineralized water every 2 d. Samples of sprouted rice were collected at intervals of 1, 2, 3 and 4 d. After sprouting, brown rice was dried at 40 °C and ground for further analysis. Sprouting treatments were done in triplicate.
Determination of phytase activity
Phytase activity of steeped and sprouted brown rice was determined by spectrophotometric analysis based on AOAC (Engelen et al. 2001) with minor modification.
One activity unit (1 U) is defined as the quantity of enzyme that will liberate 1 μmol phosphate from phytic acid per min at 37 °C at pH 5.50. A standard curve was made with solutions of potassium dihydrogen phosphate (KH2PO4) prepared as follows. Dissolved 0.6804 g (accuracy 0.0001 g) pre-dried KH2PO4 into 100 mL acetate buffer and diluted 16-fold. Then 1, 1.5, 2, 2.5, 3, 4 mL of diluted solution were pipetted into centrifugal tubes and made to 4 mL with 0.22 M sodium acetate (pH5.5 ± 0.01) followed by addition of 4 mL mixed colour solution (consisting of 100 g L−1 heptamolybdate, 2.35 g L−1 vanadate, and 5 M nitric acid 1:1:2) to terminate the reaction. The mixture was then centrifuged at 4,400 × g for 10 min and the absorbance of the supernatant was measured at 415 nm (UNICO 7200, Shanghai, China). Sodium acetate buffer was used as blank.
About 3 g (accuracy 0.0001 g) ground rice powder was mixed with 40 mL of 0.22 M sodium acetate buffer (pH5.4 ± 0.1) in a flask and shaken at 4 °C for 1 h. The mixture was centrifuged at 4,400 g for 10 min and the supernatants were used for the analysis of phytase activity. Sodium phytate (1 mL, 10 mM) was used as substrate and mixed with 3 mL supernatants followed by incubation at 37 °C for 1 h. Then 4 mL mixed heptamolybdate solution was added to terminate the reaction. The mixture was then centrifuged at 4,400 × g for 10 min and absorbance of supernatant was measured at 415 nm. Phytase activity was then calculated as follows:
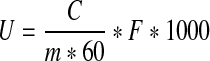 |
1 |
- U
phytase activity of germinated brown rice, U kg−1
- M
weight of test sample, g
- C
phytase activity calculated by the linear regression equations and the absorbance of samples, U
- F
total dilution factor of test sample
Phytase activity determinations were carried out in triplicate.
Statistical analysis
The data were analyzed by SPSS 10.0 one way analysis of variance (ANOVA). A multiple comparison procedure of the treatment means was performed by Duncan’s new multiple range tests. Significance of the differences was defined as p < 0.01.
Results and discussion
Phytase activities in brown rice after steeping in different liquids
Phytase activities in brown rice after steeping in different liquids were presented in Table 1.
Table 1.
Phytase activity of brown rice after 24 h steeping
| Steeping variables | Phytase activity (U kg−1)* | |
|---|---|---|
| Control | raw brown rice | 214.3 ± 1.8 |
| Demineralized water (temperature, °C) | 15 | 163.3 ± 1.3 b |
| 20 | 57.6 ± 1.2 a | |
| 25 | 171.5 ± 0.9 c | |
| Steeping media (20 °C) | 0.5 mM CaCl2 | 53.5 ± 0.4 a |
| 0.1 M H2O2 | 55.6 ± 3.2 a | |
| Steeping pH (buffer, 25 °C) | pH 2.2 | 184.5 ± 1.1 d |
| pH 5.4 | 171.1 ± 6.4 c | |
| pH 6.8 | 57.6 ± 1.2 a | |
| pH 9.4 | 144.7 ± 6.1 b | |
| pH 10.5 | 172.1 ± 1.4 c |
* The results are expressed as mean ± SD. Each observation is a mean of triplicate experiments. Mean values bearing different letters (a, b, c) in the column are significantly different (p < 0.01) on application of Duncan’s multiple-range test.
Phytase activity in raw brown rice was 214 U kg−1, which falls within the category of low phytase activity of cereals mentioned before. This activity was 13% higher than in brown rice reported by Egli et al. (190 U kg−1) (2002), but is within the same order of magnitude. Temperature and pH value during activity measurement affect the activity of enzymes significantly. We measured phytase activity at pH 5.5 and 37 °C, while Egli et al. used pH 5.0 and 45 °C.
In general, compared to raw brown rice, steeping caused significant decrease of phytase activity (p < 0.01), which varied with process conditions. Steeping of brown rice respectively in demineralized water, 0.5 mM CaCl2 and 0.1 M H2O2 at 20 °C for 24 h resulted in the lowest phytase activity in brown rice, which was about 25% of that in raw brown rice. Phytase losses in these different steeping media did not differ significantly (p > 0.01). These 75% losses correspond well with phytase losses recorded in other cereals such as millet, rye, maize and oat, treated similarly (Egli et al. 2002). The decrease of phytase activity in steeped brown rice could be explained by diffusion since phytase is mainly located in the cereal aleurone layer, and can thus diffuse easily into steeping media. Carlson and Poulsen reported that soaking of feed based on barley and wheat at 20 °C induced significantly lower endogenous phytase activity than soaking at 10 °C (Carlson and Poulsen 2003). Solutions of 0.5 mM CaCl2 and 0.1 M H2O2 were selected since both were reported previously to improve sprouting rates of cereal seeds (Gu et al. 2003; Wu et al. 1996). However, during steeping these solutions had the same effect on phytase activity in brown rice as demineralized water. The effect of pH of steeping media was significant (p < 0.01) with the highest phytase losses occurring at neutral pH. The reduced losses at low or high pH values could be attributed to different properties of cell membranes, or to effects on metabolic pathways of seeds (Taylorson and Hendricks 1979).
Effect of sprouting temperature on the phytase activity of brown rice
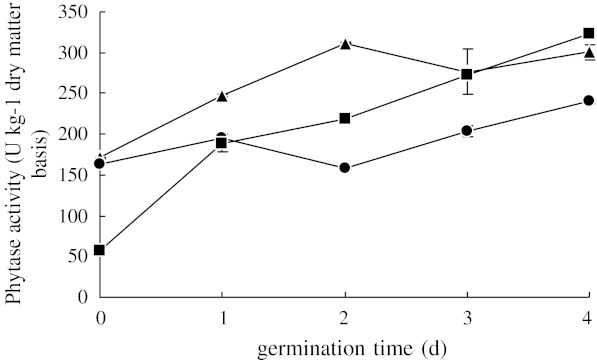
After steeping in demineralized water, brown rice was sprouted for 4 d at identical temperatures as during steeping. Changes of phytase activity during sprouting are presented in Fig. 1.
Fig. 1.
Phytase activity in brown rice during sprouting at different temperatures after 24 h steeping in demineralized water. Steeping and sprouting temperatures were kept the same, at: (●) 15 °C, (■) 20 °C, (▲) 25 °C. Each observation is a mean of triplicate experiments
Phytase activity tended to increase during the sprouting period. Except after 4 d, higher sprouting temperatures led to higher phytase activity. Sprouting temperature had significant effect on the phytase activity (p < 0.01). When sprouted at 25 °C, phytase activity would reach a relatively stable level (312.1 ± 1.0 U Kg−1) after 2 d of germination, which was 82% higher than that of non-sprouted steeped rice.
For each sprouting time, the phytase activity in brown rice was ranked by temperatures as 25 °C >20 °C >15 °C. The phytase activity in dry brown rice is low. When sprouting starts, phytase will be activated and accumulated, and will hydrolyze phytates, releasing inorganic phosphorus for seedling growth. Therefore, sprouting increases phytase activity. Meanwhile, several other enzymes will be activated when sprouting starts. It was reported that protease activity will affect phytase (Houde and Kermasha 1990). Proteases will cause the degradation of stored protein reserves for growth, and this may contribute to the lowering of phytase activity (Houde and Kermasha 1990). In our study, 15 °C was not suitable for sprouting and had less negative impact on the activation of phytase. At 25 °C, phytase activity declined after 2 d probably due to degradation of the enzyme by activated proteases.
Effect of steeping solutes on phytase activity of brown rice during sprouting
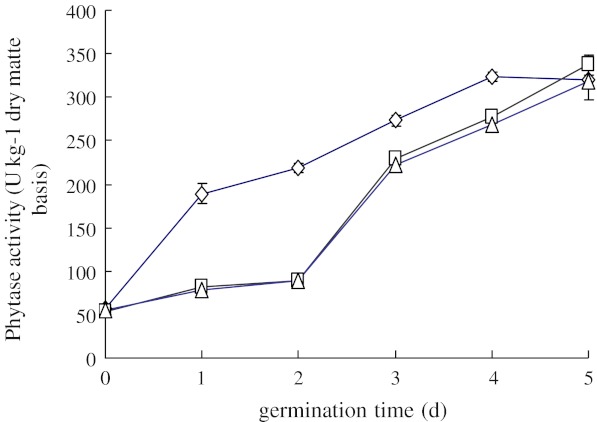
The effect of steeping on the evolution of phytase activity during sprouting at 20 °C is shown in Fig. 2.
Fig. 2.
Phytase activity in brown rice during sprouting at 20 °C after 24 h steeping in different media. Steeping media: (◊) demineralized water (control), (□) 0.5 mM CaCl2, (∆) 0.1 M H2O2. Each observation is a mean of triplicate experiments
After 5 d sprouting, the phytase activity in brown rice reached 320–340 U kg−1, which was about 1.5 times higher than in the raw material. Phytase activity during sprouting was affected by steeping solutions. Steeping both in 0.5 mM CaCl2 and in 0.1 M H2O2 resulted in similar patterns of phytase activation, which differed markedly from the control (demineralized water). Whereas in the control a gradual accumulation of phytase activity was observed, steeping with CaCl2 or H2O2 caused a delay followed by a rapid activation of phytase. Enhancing effects of CaCl2 or H2O2 were expected due to GABA (γ-Aminobutyric acid) induced activation (Gu et al. 2003) and oxidative degradation of seed capsule barriers (Wu et al. 1996; Fidler 1968), respectively. However, the resulting phytase activities were not higher than in the control. Further experiments would be required to find appropriate conditions to enhance phytase activation in brown rice.
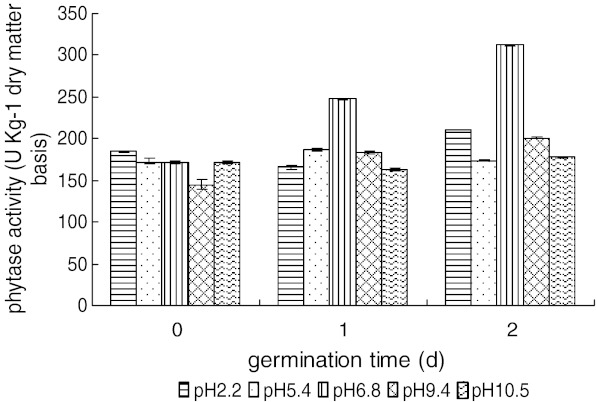
Effect of steeping pH on phytase activity of brown rice during sprouting
The phytase activity in sprouting brown rice at 25 °C after steeping at different pH values in control medium is shown in Fig. 3.
Fig. 3.
Phytase activity in brown rice during sprouting at 25 °C after 24 h steeping at different pH values. Each observation is a mean of triplicate experiments
It appears that neutral (6.8) pH is optimum for activation and accumulation of phytase activity in germinated brown rice, whereas acidic (pH 2.2) or alkaline conditions (pH 10.5) inhibited phytase activation. Extreme conditions, either strong acidic or alkaline, may harm sprouting seeds. In addition, the natural colour of brown rice was lost after steeping at pH 2.2 and 10.5, and undesirable flavour was formed during sprouting of brown rice after steeping at pH 5.4 and 9.4.
Conclusion
The data in this study indicates that steeping and sprouting in demineralized water at neutral pH at a temperature of 25 °C, provides the best conditions for phytase activation in brown rice. Although varying with steeping temperature and medium composition, significant decrease of phytase activity was observed during steeping process. Sprouting treatment promoted phytase activity effectively. And the evolution pattern of phytase activity during sprouting differed with the solutes previously used during steeping. Extreme conditions, either strong acidic or alkaline, inhibited activation of phytase.
Acknowledgement
Financial support was provided by INREF project from Wageningen University.
References
- Bartnik M, Szafranska I. Changes in phytate content and phytase activity during the germination of some cereals. J Cereal Sci. 1987;5:23–28. doi: 10.1016/S0733-5210(87)80005-X. [DOI] [Google Scholar]
- Beumer RR. Microbiological hazards and their control: Bacteria. In: Adams MR, Nout MJR, editors. Fermentation and food safety. Gaithersburg: Aspen; 2001. pp. 141–158. [Google Scholar]
- Brune M, Rossander-Hulten L, Hallberg L. Iron absorption from bread in humans: inhibiting effects of cereal fiber, phytate and inositol phosphates with different numbers of phosphate groups. J Nutr. 1992;122:442–449. doi: 10.1093/jn/122.3.442. [DOI] [PubMed] [Google Scholar]
- Carlson D, Poulsen HD. Phytate degradation in soaked and fermented liquid feed–effect of diet, time of soaking, heat treatment, phytase activity, pH and temperature. Anim Feed Sci Technol. 2003;103:141–154. doi: 10.1016/S0377-8401(02)00288-2. [DOI] [Google Scholar]
- Centeno C, Viveros A, Brenes A, Lozano A, De La Cuadra C. Effect of several germination conditions on total P, phytate P, phytase, acid phosphatase activities and inositol phosphate esters in spring and winter wheat. J Agric Sci. 2003;141:313–321. doi: 10.1017/S0021859603003666. [DOI] [PubMed] [Google Scholar]
- Duhan A, Khetarpaul N, Bishnoi S. Effect of soaking, germination and cooking on phytic acid and hydrochloric acid extractability of a pigeonpea cultivar. J Food Sci Technol. 2001;38:374–378. [Google Scholar]
- Eeckhout W, De Paepe M. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim Feed Sci Technol. 1994;47:19–29. doi: 10.1016/0377-8401(94)90156-2. [DOI] [Google Scholar]
- Egli I, Davidsson L, Juillerat MA, Barclay D, Hurrell RF. The influence of soaking and germination on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feeding. J Food Sci. 2002;67:3484–3488. doi: 10.1111/j.1365-2621.2002.tb09609.x. [DOI] [Google Scholar]
- Engelen AJ, Heeft FC, Randsdorp PHG, Somers WAC, Schaefer J, Vat BJC. Determination of phytase activity in feed by a colorimetric enzymatic method: collaborative interlaboratory study. J AOAC Int. 2001;84:629–633. [PubMed] [Google Scholar]
- Fidler JC. The metabolism of acetaldehyde by plant tissues. J Exp Bot. 1968;19:41–51. doi: 10.1093/jxb/19.1.41. [DOI] [Google Scholar]
- Greiner R, Konietzny U. Improving enzymatic reduction of myo-inositol phosphates with inhibitory effects on mineral absorption in black beans (Phaseolus vulgaris var. preto) J Food Process Preserv. 1999;23:249–261. doi: 10.1111/j.1745-4549.1999.tb00383.x. [DOI] [Google Scholar]
- Greiner R, Jany KD, Alminger ML. Identification and properties of myo-inositol hexakisphosphate phosphohydrolases (phytases) from barley (Hordeum vulgare) J Cereal Sci. 2000;31:127–139. doi: 10.1006/jcrs.1999.0254. [DOI] [Google Scholar]
- Gu Z, Chen Z, Duan Y, Liao M. Effect of Ca2+ on germination power and mobilization of stored reserves of brown rice. J Nanjing Agric Univ (in Chinese) 2003;26:97–100. [Google Scholar]
- Houde RL, Kermasha IAS. Purification and characterization of canola seed (Brassica sp.) phytase. J Food Biochem. 1990;14:331–351. doi: 10.1111/j.1745-4514.1990.tb00846.x. [DOI] [Google Scholar]
- Konietzny U, Greiner R. Purification and characterization of a phytase from spelt. J Food Biochem. 1994;18:165–183. doi: 10.1111/j.1745-4514.1994.tb00495.x. [DOI] [Google Scholar]
- Kyriakidis NB, Galiotou-Panayotou M, Stavropoulou A, Athanasopoulos P. Increase in phytase activity and decrease in phytate during germination of four common legumes. Biotechnol Lett. 1998;20:475–478. doi: 10.1023/A:1005488112582. [DOI] [Google Scholar]
- Laboure AM, Gagnon J, Lescure AM. Purification and characterization of a phytase (myo-inositol-hexakisphosphate phosphohydrolase) accumulated in maize (Zea mays) seedlings during germination. Biochem J. 1993;295:413–419. doi: 10.1042/bj2950413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Han B, Nout MJR, Hamer RJ. Effects of soaking, germination and fermentation on phytic acid, total and in vitro soluble zinc in brown rice. Food Chem. 2008;110:821–828. doi: 10.1016/j.foodchem.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wang H, Wang X, Zhang G, Chen P, Liu D. Genotypic and spike positional difference in grain phytase activity, phytate, inorganic phosphorus, iron, and zinc contents in wheat (Triticum aestivum L.) J Cereal Sci. 2006;44:212–219. doi: 10.1016/j.jcs.2006.06.001. [DOI] [Google Scholar]
- Lönnerdal B, Sandberg AS, Sandström B. Inhibitory effects of phytic acid and other inositol phosphates on zinc and calcium absorption in suckling rats. J Nutr. 1989;119:211–214. doi: 10.1093/jn/119.2.211. [DOI] [PubMed] [Google Scholar]
- Mulimani VH, Kadi NS, Thippeswamy S. Effect of processing on phytic acid content in different red gram (Cajanus cajan L.) varieties. J Food Sci Technol. 2003;40:371–373. [Google Scholar]
- Murugkar DA, Jha K. Effect of sprouting on nutritional and functional characteristics of soybean. J Food Sci Technol. 2009;46:240–243. [Google Scholar]
- Pallauf JR, Rimbach G. Nutritional significance of phytic acid and phytase. Arch Anim Nutr (Germany) 1997;50:301–319. doi: 10.1080/17450399709386141. [DOI] [PubMed] [Google Scholar]
- Reddy NR, Balkrishnan CV, Salunkhe DK. Phytate phosphorus and mineral changes during germination and cooking of black gram (Phaseolus mungo) seeds. J Food Sci. 1978;43:540–544. doi: 10.1111/j.1365-2621.1978.tb02349.x. [DOI] [Google Scholar]
- Reddy NR, Sathe SK, Salunkhe DK. Phytates in legumes and cereals. Adv Food Res. 1982;28:1–92. doi: 10.1016/s0065-2628(08)60110-x. [DOI] [PubMed] [Google Scholar]
- Sandberg AS, Carlsson NG, Svanberg U. Effects of inositol tri-, tetra-, penta-, and hexaphosphates on in vitro estimation of iron availability. J Food Sci. 1989;54:159–161. doi: 10.1111/j.1365-2621.1989.tb08591.x. [DOI] [Google Scholar]
- Sung H, Shin H, Ha J, Lai H, Cheng K, Lee J. Effect of germination temperature on characteristics of phytase production from barley. Bioresour Technol. 2005;96:1297–1303. doi: 10.1016/j.biortech.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Taylorson RB, Hendricks SB. Overcoming dormancy in seeds with ethanol and other anesthetics. Planta. 1979;145:507–510. doi: 10.1007/BF00380106. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou W, Chen H, Zheng G. Effect of H2O2 on water convolulus seed viability. J Fujian Acad Agric Sci (in Chinese) 1996;11:25–30. [Google Scholar]
- Żyła K, Gogol D, Koreleski J. Simultaneous application of phytase and xylanase to broiler feeds based on wheat: in vitro measurements of phosphorus and pentose release from wheats and wheat-based feeds. J Sci Food Agric. 1999;79:1832–1840. doi: 10.1002/(SICI)1097-0010(199910)79:13<1832::AID-JSFA441>3.0.CO;2-Q. [DOI] [Google Scholar]