Abstract
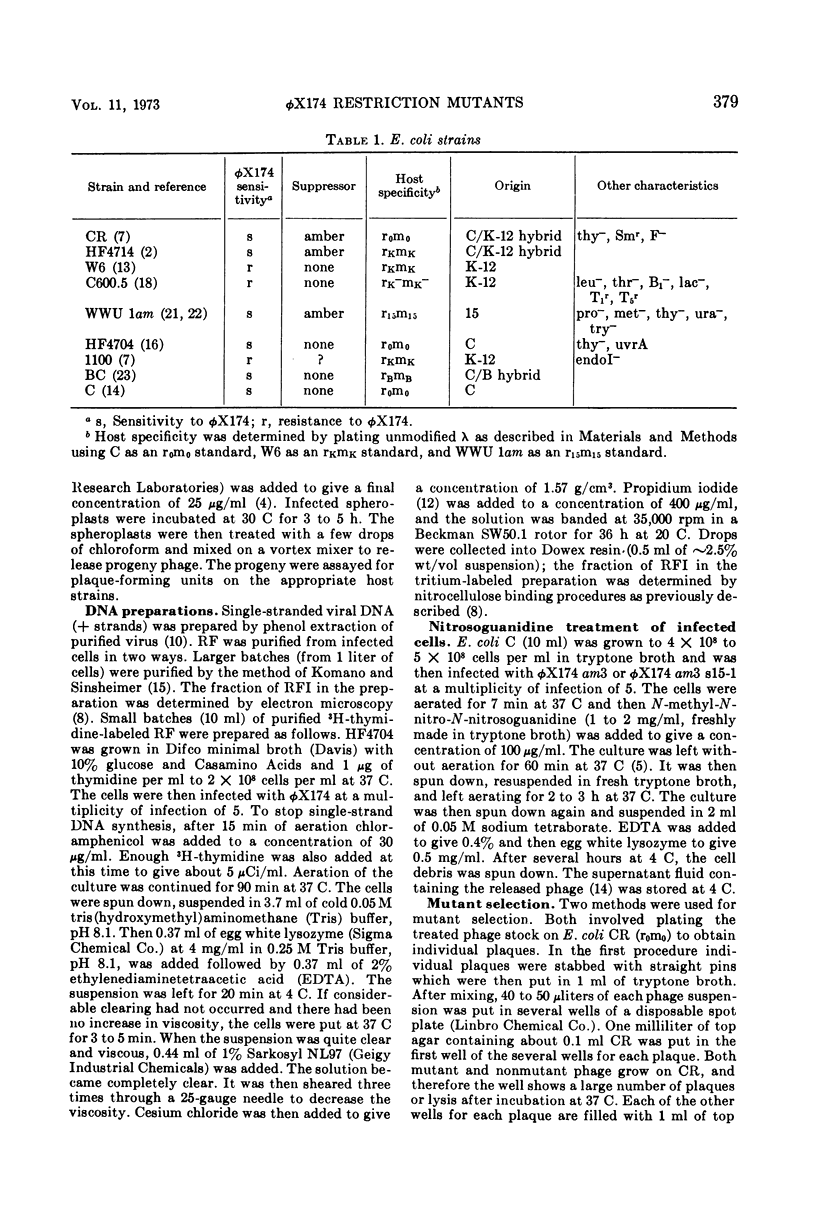
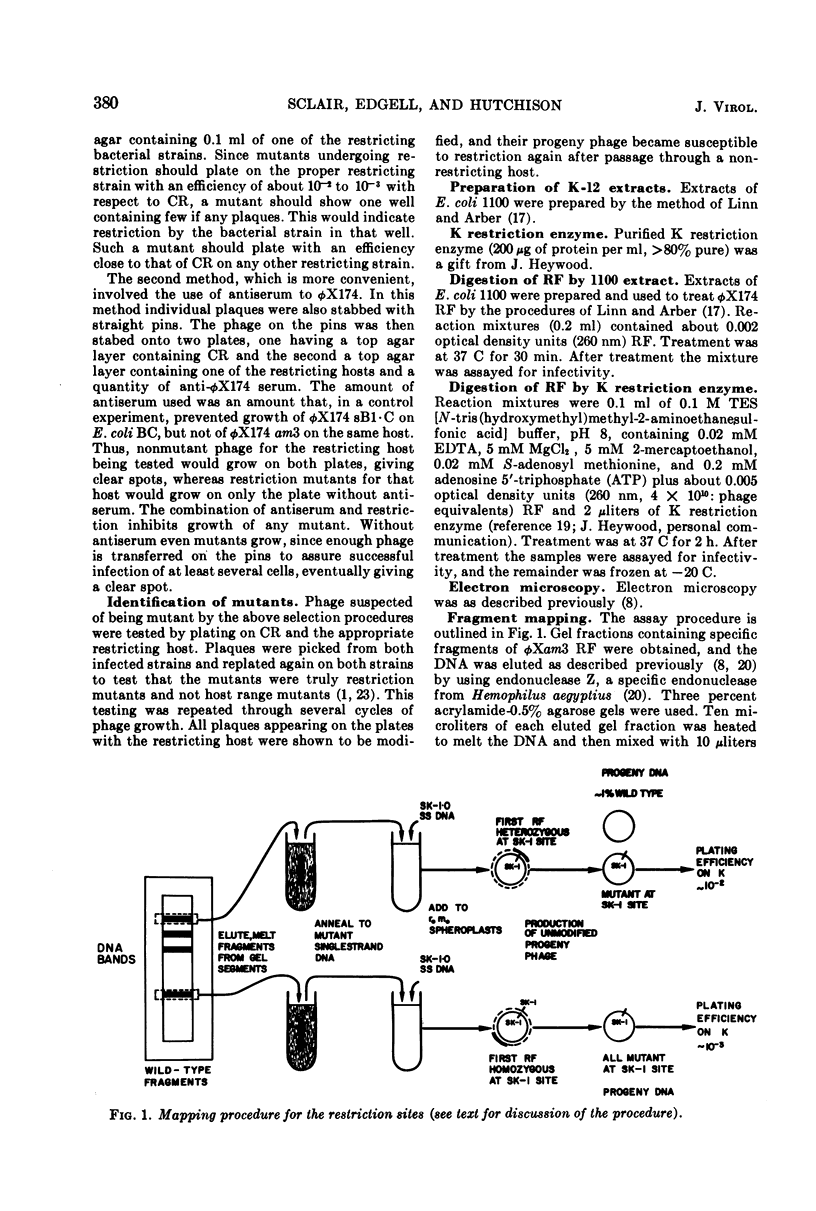
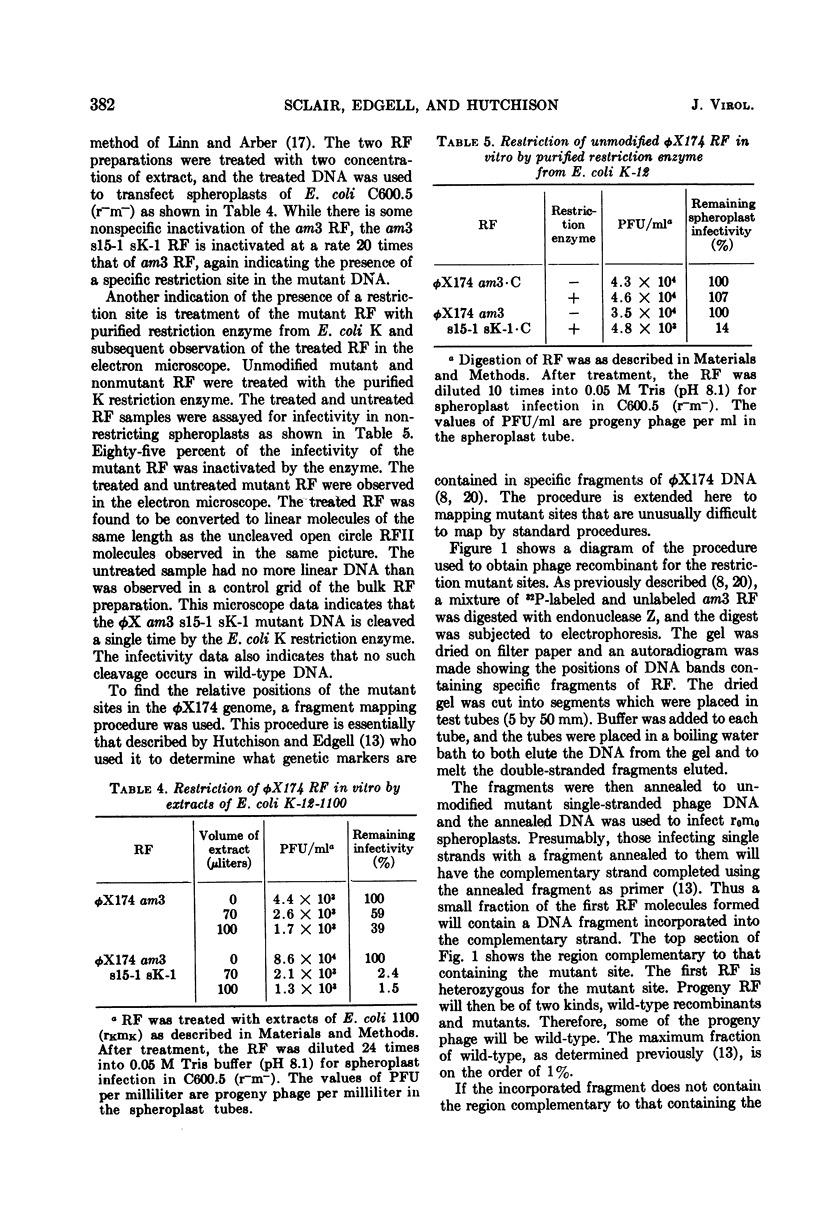
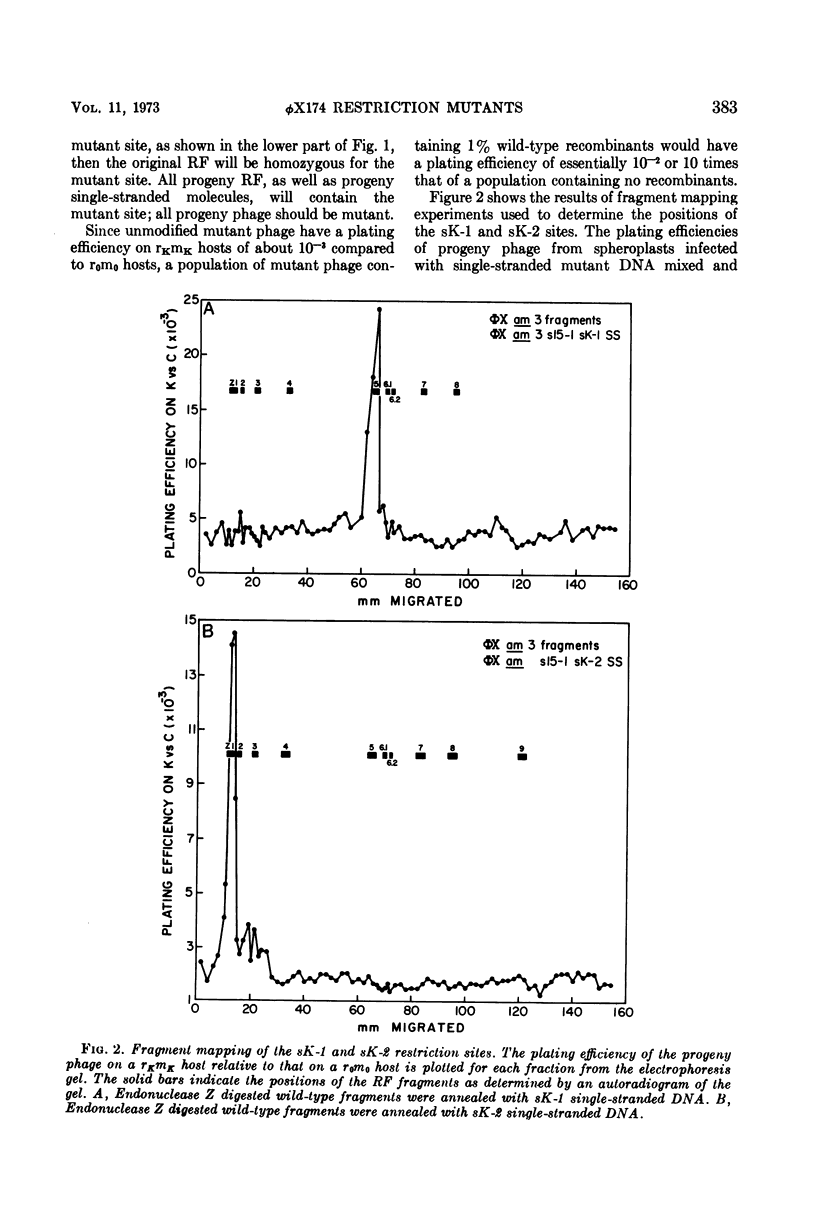
We have isolated several new φX174 mutants which contain sites sensitive to restriction by Escherichia coli. One contains an E. coli 15 restriction site and three are double mutants containing an E. coli K site as well as the E. coli 15 site. The replicative form (RF) DNA of one of the mutants containing a K site has been shown to be restricted in spheroplasts of a K-12 strain. The infectivity of this RF, but not wild-type RF, has also been shown to be inactivated by an E. coli K extract and by purified K restriction enzyme in vitro. The product of the RF treated with purified K restriction enzyme in vitro is a full length linear molecule. The mutant sites have also been localized to specific regions of the φX174 genome by a fragment mapping technique, making use of specific fragments of φX174 RF DNA obtained by digestion with a specific endonuclease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W., Linn S. DNA modification and restriction. Annu Rev Biochem. 1969;38:467–500. doi: 10.1146/annurev.bi.38.070169.002343. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Hutchison C. A., Fabricant J. D., Sinsheimer R. L. Genetic Map of Bacteriophage phiX174. J Virol. 1971 May;7(5):549–558. doi: 10.1128/jvi.7.5.549-558.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger R., Kleber I., Huskey R. Transfection of Escherichia coli spheroplasts. I. General facilitation of double-stranded deoxyribonucleic acid infectivity by protamine sulfate. J Virol. 1971 May;7(5):646–650. doi: 10.1128/jvi.7.5.646-650.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger R. Restriction of infectious bacteriophage fd DNA's and an assay for in vitro host-controlled restriction and modification. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1294–1299. doi: 10.1073/pnas.59.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá-Olmedo E., Hanawalt P. C. Diazomethane as the active agent in nitrosoguanidine mutagenesis and lethality. Mol Gen Genet. 1968 May 3;101(3):191–202. doi: 10.1007/BF00271621. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. IV. Replication of the viral DNA in a synchronized infection. J Mol Biol. 1965 Jul;12(3):647–662. doi: 10.1016/s0022-2836(65)80319-9. [DOI] [PubMed] [Google Scholar]
- Dürwald H., Hoffmann-Berling H. Endonuclease-I-deficient and ribonuclease I-deficient Escherichia coli mutants. J Mol Biol. 1968 Jul 14;34(2):331–346. doi: 10.1016/0022-2836(68)90257-x. [DOI] [PubMed] [Google Scholar]
- Edgell M. H., Hutchison C. A., 3rd, Sclair M. Specific endonuclease R fragments of bacteriophage phiX174 deoxyribonucleic acid. J Virol. 1972 Apr;9(4):574–582. doi: 10.1128/jvi.9.4.574-582.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell M. H., Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. 28. Removal of the spike proteins from the phage capsid. J Mol Biol. 1969 Jun 28;42(3):547–557. doi: 10.1016/0022-2836(69)90242-3. [DOI] [PubMed] [Google Scholar]
- Eskridge R. W., Weinfeld H., Paigen K. Susceptibility of different coliphage genomes to host-controlled variation. J Bacteriol. 1967 Mar;93(3):835–844. doi: 10.1128/jb.93.3.835-844.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTHRIE G. D., SINSHEIMER R. L. Observations on the infection of bacterial protoplasts with the deoxyribonucleic acid of bacteriophage phi X174. Biochim Biophys Acta. 1963 Jun 25;72:290–297. [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Edgell M. H. Genetic assay for small fragments of bacteriophage phi X174 deoxyribonucleic acid. J Virol. 1971 Aug;8(2):181–189. doi: 10.1128/jvi.8.2.181-189.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. X. Mutations in a phi-X Lysis gene. J Mol Biol. 1966 Jul;18(3):429–447. doi: 10.1016/s0022-2836(66)80035-9. [DOI] [PubMed] [Google Scholar]
- Komano T., Sinsheimer R. L. Preparation and purification of phi X-RF component I. Biochim Biophys Acta. 1968 Jan 29;155(1):295–298. doi: 10.1016/0005-2787(68)90360-2. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XIV. Studies on macromolecular synthesis during infection with a lysis-defective mutant. J Mol Biol. 1967 Aug 28;28(1):87–94. doi: 10.1016/s0022-2836(67)80079-2. [DOI] [PubMed] [Google Scholar]
- Linn S., Arber W. Host specificity of DNA produced by Escherichia coli, X. In vitro restriction of phage fd replicative form. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1300–1306. doi: 10.1073/pnas.59.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MESELSON M. ON THE MECHANISM OF GENETIC RECOMBINATION BETWEEN DNA MOLECULES. J Mol Biol. 1964 Sep;9:734–745. doi: 10.1016/s0022-2836(64)80178-9. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Middleton J. H., Edgell M. H., Hutchison C. A., 3rd Specific fragments of phi X174 deoxyribonucleic acid produced by a restriction enzyme from Haemophilus aegyptius, endonuclease Z. J Virol. 1972 Jul;10(1):42–50. doi: 10.1128/jvi.10.1.42-50.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Person S., Phillips S., Funk F. A determination of mutagen specificity in bacteria using nonsense mutants of bacteriophage T4. J Mol Biol. 1967 Jun 28;26(3):437–447. doi: 10.1016/0022-2836(67)90314-2. [DOI] [PubMed] [Google Scholar]
- PERSON S., BOCKRATH R. C., Jr DIFFERENTIAL MUTATION PRODUCTION BY THE DECAY OF INCORPORATED TRITIUM COMPOUNDS IN E. COLI. Biophys J. 1964 Sep;4:355–365. doi: 10.1016/s0006-3495(64)86788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnegg B., Hofschneider P. H. Mutant of phi X174 accessible to host-controlled modification. J Virol. 1969 May;3(5):541–542. doi: 10.1128/jvi.3.5.541-542.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- Weisbeek P. J., van de Pol J. H. Bological activity of phi X174 replicative form DNA fragments. Biochim Biophys Acta. 1970 Dec 14;224(2):328–338. [PubMed] [Google Scholar]



