Abstract
Fruiting bodies of 6 mushrooms including Agaricus bisporus, Hyspizygus ulmarius, Pleurotus florida PF-01, Pleurotus florida PF-01 R5, Pleurotus platypus and Pleurotus sajor-caju PSC-04 were analyzed for antioxidative enzymatic profile during low temperature storage. Colour, rehydration ratio and moisture were taken as indices of accessing their shelf-life/marketability, of which, colour contributed significantly while rehydration ratio and moisture did not change considerably during storage. Mushrooms were stored at 5 and 10 °C and activities and isozyme profile of antioxidative enzymes, superoxide dismutase (SOD) and peroxidase (POX) were analyzed after every 48 h interval till the fruiting bodies remained marketable. SOD activity increased generally at 5 and 10 °C while POX activity first increased and then decreased under similar conditions. Isozyme profile of SOD and POX did not show any new isozyme during storage, the only difference was in the intensity of bands.
Keywords: Mushrooms, Superoxide dismutase, Peroxidase, Low temperature storage, Isozymes, Shelf-life
Introduction
Mushrooms are now a days popularly known as functional foods (Liu and Wang 2009). World production of cultivated edible mushroom increased from 170 MT in 1960 to 10,995.5 × 103 MT in 2007 (Chang 2007). China has become a giant producer and consumer of mushrooms and a large share (70%) of the total world production comes from China (Chang 2007). India’s share is estimated to be around 70,000 MT per annum in which major share is contributed by button mushroom (Agaricus spp.), while the rest being speciality one (Singh et al. 2003). Diversified agro-climatic conditions in India offer vast potential for growing different types of mushrooms. There are about 20 varieties of mushrooms being cultivated throughout the world for food. In India, only white button mushroom (Agaricus bisporus), oyster mushroom (Pleurotus spp.) and paddy straw mushroom (Volvariella volvacea) are grown commercially. Out of these, white button mushroom contributes ~90% of the total production. Mushrooms have been recognized as the alternate source of good quality protein and produce the highest quantity of protein per unit area from agro-wastes. Besides, they provide potentiality for generating employment, improving economic status of growers, help in checking pollution and earn foreign exchange (Rai and Arumuganathan 2005). Mushrooms contain ~90% moisture and are highly perishable in nature.
Edible mushroom suffers major post harvest loss due to short shelf-life mainly due to enzymatic browning, high metabolic rate and environmental stress factors (Beelman 1987). The tropical temperature being the major limiting factor in India for production as well as productivity of mushroom. The intrinsic antioxidative defense mechanism protects the produce from temperature induced stress to some extent. These include various antioxidative enzymes like superoxide dismutase (SOD), peroxidase (POX) and antioxidants like glutathione and ascorbate. The changes in shelf-life and the defense mechanism countering them occur at molecular level (Misra and Fridovich 1972; Ravindranath and Fridovich 1975). Molecular markers are used for analyzing genetic variations in a number of organisms. Techniques include either DNA or enzyme based systems or both. But isoenzyme based system is the cheapest method yielding comparable results with DNA based system for genetic investigations (Packia et al. 2000). Many edible mushrooms are reported and thousands of unidentified strains are present especially in Aravali region of Rajasthan. The present paper describes the activity and isozyme profile of two antioxidative enzymes, SOD and POX along with other indices of marketability during low temperature storage till mushrooms remain marketable.
Materials and methods
Fruiting bodies and storage conditions
Fruiting bodies of 6 strains of mushrooms, wild (Pleurotus florida PF-01, Pleurotus florida PF-01 R5, Pleurotus platypus and Pleurotus sajor-caju PSC-04) and cultivated (Agaricus bisporus, Hyspizygus ulmarius), were obtained fresh just after harvesting from All India Co-ordinated Mushroom Improvement Project, Department of Plant Pathology of the College and stored at 5 and 10 °C in polypropylene bags of thickness 150 gauze. Activity and isozyme profile of antioxidative enzymes SOD and POX were investigated at 5 and 10 °C at every 48 h interval till the fruiting bodies remained edible/ marketable in order to know the shelf-life of mushrooms during storage.
Indices of marketability
Moisture content was determined by oven drying method (Rangana 2000) while for re-hydration ratio, method of Kumar et al. (2007) was followed where pre-weighed samples were soaked in ample amount of water for 30 min at room temperature (32 °C). The ratio of mass of re-hydrated to normal samples was used to find the re-hydration ratio whereas the colour observations were done visually.
Preparation of crude extract
After preliminary studies of standardizations with respect to buffer, pH and extraction conditions, the extractions were done by following procedure: 5 g of fresh tissue (mushroom fruiting body) was homogenized with 10 ml of 0.1 M Tris-HCl buffer (pH 7.5) containing 1 mM EDTA, 3% polyvinyl pyrrolidone and 1 mM CaCl2 in a pre-chilled pestle and mortar using acid washed sand as an abrasive. The homogenate was filtered through 4 layers of cheesecloth and the filtrate centrifuged at 12,000 rpm for 15 min in a refrigerated centrifuge (Remi, India) at 4 °C. The supernatant obtained was used as crude preparation for enzyme assays and other biochemical estimations. Soluble protein was estimated as described by Bradford (1976) using bovine serum albumin as standard.
Assay of SOD activity
SOD was assayed by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) adopting the method of Beauchamp and Fridovich (1971). The reaction mixture 3 ml contained 0.05 M Tris-HCl (pH 7.8), 14 mM L-methionine, 60 μM NBT, 3 μM riboflavin, 0.1 mM EDTA and 0.2 ml of enzyme extract. Riboflavin was added at the end. The tubes were properly shaken and placed 30 cm below light source consisting of two 100 W fluorescent lamps (Phillips, India). The reaction was started by switching on the light and terminated after 20 min of incubation by switching off the light. After terminating the reaction, the tubes were covered with black cloth to protect them from light. A non-illuminated reaction mixture that did not develop colour served as the blank. The reaction mixture developed maximum colour without enzyme extract and its absorbance decreased with the addition of enzyme. The absorbance was recorded by Spectrophotometer (Elico, India) at 560 nm. The enzyme activity was calculated by the formula of Gianopolitis and Ries (1977) and the activity expressed in units/mg protein.
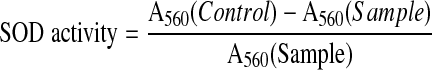 |
where Control = Rate of assay reaction without SOD, Sample = Rate of assay reaction with SOD.
Assay of POX activity
POX activity was assayed by the modified method of Seevers et al. (1971). The reaction mixture contained 0.1 ml of 2.0% o-dianisidine in methanol, 0.1 ml of 10 mM H2O2, 2.7 ml of 0.1 M sodium acetate buffer (pH 5) and properly diluted aliquots of enzyme extract (0.1 ml). The reaction was initiated by the addition of H2O2 and change in absorbance was followed at 470 nm spectrophotometrically (Systronics, India) up to 3 min. The linear change in absorbance was taken to calculate enzyme activity. One unit of enzyme activity is defined as 0.001 change in OD per min (Neves 2002) and the activity is expressed in units/mg protein.
Specific enzyme staining of SOD
Specific enzyme staining of SOD was done by the method of Beauchamp and Fridovich (1971). SOD was localized by soaking the native PAGE gel in 2.45 × 10−3 M NBT for 20 min followed by immersion for 15 min in a solution containing 0.028 M TEMED, 2.8 × 10−5 M riboflavin and 0.036 M Tris-HCl buffer (pH 7.8). The gel was then illuminated under fluorescent light for 1 h. During illumination, the gel became uniformly faint blue except at positions containing SOD. Illumination was discontinued when maximum contrast between the achromatic zones and the general blue colour had been achieved.
Specific enzyme staining of POX
After electrophoresis, the gels were stained for specific enzyme activity of POX with 100 ml benzidine reagent containing equal amounts of 0.3% benzidine in 25% acetic acid and 0.5% H2O2 for 20–30 min (Guikema and Shermen 1980). Blue coloured bands appeared which turned brown after 10–15 min. These brown coloured bands represented the POX activity.
Statistical analysis
The statistical analysis of the data was done by using complete randomized design (CRD). The 6 accessions during storage were studied replicating each observation thrice. The critical differences of stages, varieties and interactions were calculated at p ≤ 0.05 using the table ‘ANOVA’.
Results and discussion
Indices of marketability
Greater shelf-life was observed at 5 °C than at 10 °C (Table 1). Mushroom respiratory rate and decay increased with the increase in storage temperature. Mushrooms at 10 °C have 3.5 times higher respiratory rate than at 0 °C (Hyun et al. 1996). Wijewardane and Guleria (2009) reported shelf life of 150 days in case of pre-treated apple fruits stored at 2 ± 1 °C and 85–90% RH by lowering the incidence of fruit softening and spoilage. Fresh colour of A. bisporus, P. florida PF-01, P. florida PF-01R5 and P. platypus were whitish or creamish white while colour of P. sajor-caju PSC-04 was bluish grey and that of H. ulmarius was blue and had excellent appearance (Table 2). As the time elapsed, colour started deteriorating. The deterioration was more at 10 °C than at 5 °C. Results indicated that colour changes reduced the marketability of mushrooms stored at 10 °C.
Table 1.
Shelf-life (h) of various accessions of mushrooms at 5 and 10 °C
| Strains | 5 °C | 10 °C |
|---|---|---|
| A. bisporus | 192 | 144 |
| H. ulmarius | 240 | 192 |
| P. florida PF-01 | 240 | 192 |
| P. florida PF-01 R5 | 192 | 144 |
| P. platypus | 240 | 192 |
| P. sajor-caju PSC-04 | 240 | 192 |
Table 2.
Colour pattern for all strains during sampling at 5 and 10 °C
| At 5 °C | At 10 °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 48 h | 96 h | 144 h | 192 h | 240 h | 48 h | 96 h | 144 h | 192 h | |
| A. bisporus | +++ | +++ | +++ | + | – | +++ | +++ | ++ | – |
| H. ulmarius | Blue | Blue + Brown | Blue + Brown | Brown | Brown | Blue | Blue + Brown | Blue + Cream | Light yellow + Brown |
| P. florida PF-01 | +++ | +++ | ++ | + | Dark cream | +++ | +++ | + | Dark cream |
| P. florida PF 01 R5 | +++ | +++ | Light white + Cream | Light white + Brown | – | +++ | +++ | + | Brown |
| P. platypus | +++ | +++ | +++ | ++ | + | +++ | +++ | ++ | + |
| P. sajor-caju PSC-04 | Bluish grey | Bluish grey | Light brown | Light brown | Light brown + Cream | Bluish grey | Bluish grey | Light brown | Light brown |
+++ excellent white, ++ good white, + creamy white (–) indicates samples spoiled
All samples spoiled at 240 h at 10 °C
Activity profile of SOD and POX
As the temperature stress increased, SOD activity increased (Table 3). There had been 8–10 times increase in specific activities with respect to fresh SOD activity in all the fungal accessions tested. Also, SOD activities were more at 10 °C storage as compared to 5 °C. This suggested that increased stress conditions might have created more SOD activity either at the level of expression or activity to counter temperature stress, as it is the very first enzyme involved in countering oxidative stress conditions. Among the accessions, maximum variation in SOD activity was observed in H. ulmarius while minimum in P. platypus. POX activity (Table 4) showed a mixed type of response. Generally, specific activity of POX increased during first sampling and then decreased both at 5 and 10 °C sampling, but, under certain conditions, activity again aggravated during second or third sampling. In all the accessions, POX activity decreased with respect to sampling time and high temperature stress at 10 °C. However, highest POX activities were observed with P. platypus. Rabinowitch et al. (1982) found role of SOD in supplementing the protective action of carotenoids against photo-oxidative injury in ripening tomato fruit. SOD activity increased with increased photo-oxidative damage while POX activity was not changed much. Neves (2002) while working on peach fruit, reported that at mature green stage, POX activity was low and increased at ripened stage. After harvest, POX activity first increased up to certain period and then decreased at senescence. Kumar (2009) reported higher ascorbate peroxidase and glutathione reductase activities at 4 °C than at 12 °C in case of potato tubers during storage at low temperature. However, Flurkey et al. (1994) observed low or undetectable POX activity in Agaricus, oyster and Shiitake mushrooms. Control of enzymatic browning in different mushroom types may depend upon the distribution of different oxidases in each of them. Gullen and Eris (2004) reported that heat stress enhances POX activity in strawberry plants indicative of countering stress condition. However, total protein content decreased at elevated temperature. POX activity also increased with increase in temperature. Our results are in conformity with Rabinowitch et al. (1982) and Neves (2002) while contradictory to Flurkey et al. (1994) and Gullen and Eris (2004). Gupta and Gupta (2005) incubated leaf discs of 15 day old seedlings of wheat genotypes ‘C-306’ (temperature tolerant) and ‘HD-2329’ (widely adapted) at 25, 35 and 45 °C. The genotype, ‘C-306’ exhibited lower accumulation of malondialdehyde and H2O2 due to increased activities of SOD, POX and catalase under high temperature conditions. SOD activity enhanced continuously with increasing temperature and POX also followed similar trend.
Table 3.
Superoxide dismutase activity (units/mg protein) profile of different accessions of mushroom during sampling at 5 and 10 °C
| At 5 °C | At 10 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 48 h | 96 h | 144 h | 192 h | 240 h | 48 h | 96 h | 144 h | 192 h | |
| A. bisporus | 1.69 | 1.98 | 12.11 | 16.48 | 29.20 | – | 2.52 | 15.10 | 20.51 | – |
| H. ulmarius | 12.27 | 28.87 | 35.02 | 80.92 | 109.50 | 140.96 | 31.63 | 66.67 | 96.62 | 138.56 |
| P. florida PF-01 | 8.33 | 10.71 | 12.44 | 15.80 | 16.14 | 17.02 | 12.81 | 15.46 | 15.88 | 16.32 |
| P. florida PF-01 R5 | 2.55 | 3.25 | 11.20 | 14.89 | 31.86 | – | 4.72 | 13.75 | 17.13 | – |
| P. platypus | 2.68 | 4.39 | 6.53 | 12.12 | 16.79 | 32.21 | 5.01 | 11.56 | 14.46 | 16.65 |
| P. sajor-caju PSC-04 | 19.06 | 19.93 | 24.73 | 33.21 | 37.81 | 40.63 | 23.78 | 32.32 | 34.01 | 38.45 |
| SEm ± | 0.0194 | 0.1234 | 0.4363 | 0.3684 | 1.1014 | 0.5832 | 0.1234 | 0.3684 | 0.4673 | 0.9945 |
| CD (p = 0.05) (n = 3) | 0.0598 | 0.3803 | 1.3443 | 1.1352 | 3.3937 | 0.7970 | 0.3803 | 0.1352 | 1.4400 | 3.0644 |
– samples spoiled
Table 4.
Peroxidase activity (units/mg protein) profile of different accessions of mushroom during sampling at 5 and 10 °C
| At 5 °C | At 10 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 48 h | 96 h | 144 h | 192 h | 240 h | 48 h | 96 h | 144 h | 192 h | |
| A. bisporus | 317 | 702 | 1,358 | 521 | 657 | – | 2,038 | 589 | 974 | – |
| H. ulmarius | 622 | 533 | 667 | 444 | 311 | 356 | 800 | 400 | 622 | 667 |
| P. florida PF-01 | 360 | 1,216 | 675 | 626 | 653 | – | 360 | 1,463 | 1,689 | 405 |
| P. florida PF-01 R5 | 777 | 1,554 | 691 | 669 | 583 | – | 820 | 2,331 | 820 | – |
| P. platypus | 1,990 | 2,674 | 1,244 | 1,306 | 435 | 470 | 653 | 870 | 777 | 404 |
| P. sajor-caju PSC-04 | 257 | 486 | 400 | 229 | 771 | 800 | 286 | 1,143 | 343 | 514 |
| SEm ± | 8.01 | 19.604 | 18.073 | 9.243 | 12.136 | 2.5586 | 19.4222 | 26.0800 | 12.8156 | 4.8237 |
| CD (p = 0.05) (n = 3) | 24.68 | 60.405 | 55.687 | 28.482 | 37.394 | 7.8838 | 59.8456 | 80.3605 | 39.4889 | 14.8634 |
– samples spoiled
Isozyme profile of SOD and POX
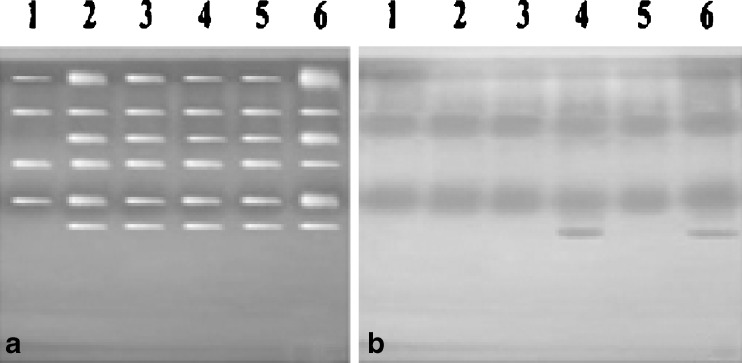
Isozyme profiles of SOD and POX (Fig. 1), as on second day of storage, have been reported. However, during storage, no new isozyme was reported. Isozyme profile of SOD in 6 accessions shown in Fig. 1a indicated 4 isozymes of SOD in A. bisporus and 6 each in H. ulmarius, P. florida PF-01, P. florida PF-01 R5, P. platypus and P. sajor-caju PSC-04. Most of the species had monomorphic isozymes and only 2 loci of isozymes (in case of A. bisporus) showed polymorphism on the basis of specific enzyme staining of SOD. The difference observed in SOD isozymes was in terms of intensity and size of bands. Less number of isozymes in A. bisporus as compared to other strains might be responsible for lesser shelf-life of A. bisporus and susceptibility to high temperature stress during fruiting body development as well as during storage. Two bands of each accession were more towards cathodic side, possibly having net positive charge and high molecular weight, while rest bands were towards anode indicating net negative charge and corresponding low molecular weights. The number of isozymes of SOD had been reported to vary, viz., 2 in roots and needles of Picea abies (Kroniger et al. 1992), 3 in Spirulina (Lumsden and Hall 1974), kidney bean leaves (Kono et al. 1979), Brussels sprouts (Walker et al. 1991) and Fagopyrum tataricum cv. Wutai leaves (Wang et al. 1993), 4 in maize (Gianopolitis and Ries 1977) and tomato (Kumar et al. 2006) and 5 in maize (Baum et al. 1983). However, when studied the polymorphism of 15 species of the genus Pleurotus (oyster mushroom) at genetic level using electrophoretic separation of the isozymes esterase, POX, malate dehydrogenase and SOD, the differences observed in enzyme profiles between species were found to be adequate analyzing the extent of genetic variability at interspecific level. The isozyme pattern of SOD was unique in such a way that it was monomorphic and found in all the species studied including P. sajor caju PSC-04, P. platypus and P. florida (Pradeep et al. 2002).
Fig. 1.
Isozyme profile of (a) Superoxide dismutase and (b) Peroxidase during sampling 1. A. bisporus, 2. P. florida PF-01, 3. P. florida PF-01 R5, 4. P. platypus, 5. P. sajor-caju PSC-04, 6. H. ulmarius
Isozyme profile as observed for POX presented in Fig. 1b indicate 3 isozymes of POX each in A. bisporus and P. platypus, 2 of each in P. florida PF-01, P. florida PF-01 R5 and P. sajor-caju PSC-04 and 5 isozymes in H. ulmarius. Two bands were monomorphic while 3 bands were polymorphic; band 2 of H. ulmarius was exclusively present in this mushroom species. Two bands were towards cathodic side while most of the POX were of low molecular weight and towards anodic side. Three POX isozymes had been characterized in bean leaves (Racusen and Foote 1966), while 7 in horseradish roots (Shannon et al. 1966). Seven isozymes may be segregated into 2 groups on the basis of their chromatographic behaviour, electrophoretic migration, spectrophotometric properties and amino acid and carbohydrate composition. However, while studying genetic variation of 7 mutant lines of Pleurotus citrinopileatus, out of the 7 enzymes clearly resolved, 19 loci were ascertained for genetic analysis. Esterase, SOD, polyphenol oxidase and malate dehydrogenase were polymorphic, while acid phosphatase, lactate dehydrogenase and POX resolved to only one locus (Packia et al. 2000). A total of 6 isozymes of POX in Agrocybe aegerita had been observed and out of that, 2 fractions of the enzyme, Aap I and Aap II, had identical molecular masses (46 kDa) and isoelectric points of 4.6–5.4 and 4.9–5.6, respectively. Hence, POX are found in plants, fungi, bacteria and animals and have been grouped on the basis of sequence similarity into 2 superfamilies: animal POX form one superfamily and plant, fungal, and bacterial POX form another superfamily. POX of fungal superfamily are involved in white rot formation and degradation of ligno-cellulosic wastes (Ullrich et al. 2004).
Conclusion
As the temperature stress increases, shelf-life decreases i.e. more shelf-life at 5 °C as compared to 10 °C. SOD activity increased correspondingly while POX activity first increased and then decreased with respect to temperature stress and time. This may possibly be the major cause of fungal decay. Out of the total loci tested for both isozymes, 2 of SOD and 3 of POX showed polymorphism.
Acknowledgement
Authors are thankful to Dean, Rajasthan College of Agriculture for providing laboratory facilities.
References
- Baum JA, Chandlee JM, Scandalios JG. Purification and partial characterization of a genetically defined superoxide dismutase (SOD-I) associated with maize chloroplasts. Plant Physiol. 1983;73:31–35. doi: 10.1104/pp.73.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp CH, Fridovich I. Superoxide dismutase : improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beelman R. Factors influencing post harvest quality and shelf-life of fresh mushrooms. Mushroom News. 1987;35(7):12–18. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang ST. Development of the World Mushroom Industry and its roles in human health. In: Rai RD, Singh SK, Yadav MC, Tewari RP, editors. Mushroom biology and biotechnology. Solan: Mushroom Society of India, National Research Centre for Mushroom; 2007. [Google Scholar]
- Flurkey WH, Kuglin J, Dawley R. Tyrosinase, laccase and peroxidase in mushrooms (Agaricus crimini, Oyster and Shiitake) J Food Sci. 1994;59(4):824–825. doi: 10.1111/j.1365-2621.1994.tb08137.x. [DOI] [Google Scholar]
- Gianopolitis CN, Ries SK. Superoxide dismutase: purification and quantitative relationship with water-soluble protein in seedling. Plant Physiol. 1977;59:315–318. doi: 10.1104/pp.59.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema JA, Shermen LA. Electrophoretic profiles of cyanobacterial membrane polypeptides showing heme dependent peroxidase activity. Biochim Biophys Acta. 1980;637:189–201. [Google Scholar]
- Gullen H, Eris A. Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Sci. 2004;166:739–744. doi: 10.1016/j.plantsci.2003.11.014. [DOI] [Google Scholar]
- Gupta S, Gupta NK. High temperature induced antioxidative defense mechanism in seedling of contrasting wheat genotypes. Indian J Plant Physiol. 2005;10(1):73–75. [Google Scholar]
- Hyun CJ, Taemoon H, Ho KY, Ju YC. Effect of storage temperature and packing method for keeping freshness of fresh mushrooms. RDA Journal of Agricultural Science, Farm Management, Agricultural Engineering, Sericulture and Farm Products Utilization. 1996;38(1):915–921. [Google Scholar]
- Kono Y, Takahashi M, Asada K. Superoxide dismutase from kidney bean leaves. Plant Cell Physiol. 1979;20:1229–1235. [Google Scholar]
- Kroniger W, Rennenberg H, Polle A. Purification of two superoxide dismutase isozymes and their subcellular localization in needles and roots of Norway Spruce (Picea abies Z.) trees. Plant Physiol. 1992;33:334–340. doi: 10.1104/pp.100.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D. Changes in antioxidant system of potato tubers during low temperature storage in relation to sweetening development. J Food Sci Technol. 2009;46:392–394. [Google Scholar]
- Kumar S, Dhillon S, Singh D, Singh R, Kumar M. Isozyme profile and kinetic characterization of superoxide dismutase from tomato fruit. Prog Agric- An Int J. 2006;6(2):130–134. [Google Scholar]
- Kumar S, Mishra BK, Jain NK, Doharey DS. Effect of fluidized bed drying on quality characteristics of button and oyster mushroom. J Food Sci Technol. 2007;44:630–632. [Google Scholar]
- Liu GQ, Wang XL. Selection of a culture medium for reducing costs and intracellular polysaccharide production by Agaricus blazei AB2003. Food Technol Biotechnol. 2009;47:210–214. [Google Scholar]
- Lumsden J, Hall DO. Soluble and membrane bound superoxide dismutase in a blue green alga (Spirulina) and spinach. Biochem Biophys Res Commun. 1974;58:35–41. doi: 10.1016/0006-291X(74)90887-0. [DOI] [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The purification and properties of superoxide dismutase from Neurospora crassa. J Biol Chem. 1972;247:3410–3414. [PubMed] [Google Scholar]
- Neves VA. Ionically bound peroxidase from peach fruit. Braz Arch Biol Technol. 2002;45(1):7–15. doi: 10.1590/S1516-89132002000100002. [DOI] [Google Scholar]
- Packia J, Jacob S, Sabu KK, Abraham TK. Genetic variability of mutant strains of Pleurotus citrinopileatus based on isozyme electrophoresis. Mushroom Res. 2000;9(2):79–84. [Google Scholar]
- Pradeep NS, Sabu KK, Kumuthakalavally R, Abraham TK. Genetic variation in Pleurotus species (Oyster mushrooms) using actual and computer simulated data. Mushroom Res. 2002;11(2):65–71. [Google Scholar]
- Rabinowitch HD, Sklan D, Budowski P. Photooxidative damage in the ripening tomato fruit : Protective role of superoxide dismutase. Physiol Plant. 1982;54:369–374. doi: 10.1111/j.1399-3054.1982.tb00273.x. [DOI] [Google Scholar]
- Racusen D, Foote M. Peroxidase isozymes in bean leaves by preparative disc electrophoresis. Can J Bot. 1966;44:1633–1638. doi: 10.1139/b66-174. [DOI] [Google Scholar]
- Rai RD, Arumuganathan T (2005) Nutritive value of mushrooms. In: Frontiers in mushroom biotechnology. National Research Centre for Mushroom, Solan, pp 27–36
- Rangana S. Handbook of analysis and quality control for fruits and vegetable products. New Delhi: Tata Mcgraw Hill Publ Co Ltd; 2000. [Google Scholar]
- Ravindranath SD, Fridovich I. Isolation and characterization of a Mn-containing superoxide dismutase from yeast. J Biol Chem. 1975;250:6107–6112. [PubMed] [Google Scholar]
- Seevers PM, Daly JM, Catedral FF. The role of peroxidase isozymes in resistance to wheat stem rust disease. Plant Physiol. 1971;48:353–360. doi: 10.1104/pp.48.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon LM, Kay E, Lew JY. Peroxidase isozymes from horseradish roots: isolation and physical properties. J Biol Chem. 1966;241(9):2166–2172. [PubMed] [Google Scholar]
- Singh SK, Yadav MC, Upadhyay RC, Kamal S, Rai RD, Tewari RP. Molecular characterization of specialty mushroom germplasm of the national mushroom repository. Mushroom Res. 2003;12(2):67–78. [Google Scholar]
- Ullrich R, Nuske J, Scheibner K, Spantzel J, Hofrichter M. Novel haloperoxidase from the agric basidiomycete Agrocybe aegertia oxidizes aryl alcohols and aldehydes. Appl Environ Microbiol. 2004;70(8):4581. doi: 10.1128/AEM.70.8.4575-4581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JL, Mclellan KM, Robinson DS. Isolation and purification of superoxide dismutase purified from brussels sprouts (Brassica oleracea L. var. bullata sub var. gemnifera) Food Chem. 1991;41:1–9. doi: 10.1016/0308-8146(91)90126-9. [DOI] [Google Scholar]
- Wang ZH, Lin R, Zhang Z, Zhou M. Purification and characterization of superoxide dismutase from tartary buckwheat leaves. Fagopyrum. 1993;13:31–34. [Google Scholar]
- Wijewardane RMNA, Guleria SPS. Effect of post harvest coating treatments on apple storage quality. J Food Sci Technol. 2009;46:549–553. [Google Scholar]