Abstract
Using partial least square regression, calibration for non-destructive estimation of erucic acid and glucosinolate contents in seeds of rapeseed-mustard by Fourier transform near infrared reflectance spectroscopy (FT-NIRS) was developed. The calibration developed showed a very close relationship between the reference method for erucic acid (gas chromatography) and glucosinolate content (palladium complex formation) and NIR spectral data from 7502.1 to 5444.6 cm−1. The coefficients of determinations were 97.16% and 98.34% for erucic acid and glucosinolate contents, respectively.
Keywords: Fourier transform near infrared reflectance spectroscopy, Calibration, Erucic acid, Glucosinolate, Rapeseed-mustard
Rapeseed-mustard is an important source of edible oil in Indian diet especially in Eastern and North-Western India. The major fatty acids of rapeseed-mustard oil are oleic, linoleic, linolenic, eicosenoic and erucic acid. Erucic acid in oil of Indian rapeseed-mustard varieties is quite high (Chauhan et al. 2007). High amount of erucic acid in edible oils has been reported to impair myocardial conductance, causes lipidosis in children and increases blood cholestrol (Gopalan et al. 1974; Renard and McGregor 1976; Ackman et al. 1977). Rapeseed-mustard cultivars grown in India also have high level of glucosinolate content (Chauhan et al. 2007). Glucosinolates, a group of plant thioglucosides, found principally among members of family Brassicaceae are responsible for the characteristic pungency of rapeseed-mustard oil. The glucosinolates are broken down by the enzyme thioglucoside glucohydrolase commonly known as myrosinase to yield sulphate, glucose and other aglucon products. Cleavage products from hydrolysis are detrimental to animal health as they reduce the feed palatability and affect the iodine uptake by the thyroid glands thus reducing feed efficiency and weight gains (Bille et al. 1983; Fenwick et al. 1983; Bell 1984) especially in non-ruminants such as pigs and poultry.
Because of the adverse effects of high erucic acid in oil (35.7–51.4%) and glucosinolates in seed meal (49.9–120.3 μmole/g defatted seed meal) of Indian rapeseed-mustard varieties (Chauhan et al. 2007), rapeseed-mustard varietal improvement programme in India aims at reducing erucic acid level up to 2% and glucosinolate content up to 30 μ moles/g defatted seed meal. Internationally accepted norms necessitate screening of a large number of samples with limited seed availability especially in early segregating generations. Erucic acid in oil is currently being analysed in most of the breeding programmes in the country by gas-liquid chromatography, an expensive, time consuming and destructive method. Glucosinolate content can be accurately estimated by chromatographic as well as colorimetric methods. But these methods are cumbersome, time consuming, expensive and destructive in nature and hence are not suitable for screening a large number of breeding materials with limited seed quantity in early segregating generations. Palladium complex method (Kumar et al. 2004) based on the formation of a complex between hydrolytic products of glucosinolates and sodium tetrachloro palladate (II) has been extensively used at the DRMR, Bharatpur and several other research centres in the country. Although this method is rapid as compared to colorimetric methods but is destructive in nature. Furthermore, inadequacy of the selfed seeds (seeds produced by covering part of flowering raceme with cloth bag to avoid out crossing to maintain purity of seeds) especially from single plants in F2 generation limits the effectiveness of these methods. Therefore, rapid and non-destructive method for mass screening with small quantity of seeds is needed to facilitate and accelerate the pace of rapeseed-mustard quality breeding programme.
Near infrared reflectance spectroscopy (NIRS) provides a rapid, non-destructive, and simultaneous analysis of fatty acids and glucosinolates of Brassica intact seeds without the need of sample preparation (Daun and Williams 1995; Velasco and Becker 1998; Font et al. 2005; Koprna et al. 2006; Niewitetzki et al. 2007), for the analysis of sinapic acid esters in Brassica species (Velasco and Mollers 1998; Velasco et al. 1998), fatty acids of soybean flour (Sato et al. 2002); oil, protein and fatty acids in sunflower seed (Biskupek-Korell and Moschner 2006), oil content in cotton seed (Kohel 1998) and maize kernel (Orman and Schumann 1992), essential oil components of cinnamon and clove (Juliani et al. 2006), rutin and D-chiro-inositol in tartary buckwheat (Yang and Ren 2008) and free fatty acid content and peroxide value in virgin olive oils (Bendini et al. 2007). The NIRS, a non-destructive analytical method offers a practical solution for the analysis of erucic acid and glucosinolate contents, the two most commonly used quality factors besides oil and protein contents. And it will also greatly help in the identification of desirable segregants especially in the early segregating generations where availability of seed is a limiting factor. However, development of calibration equation for transforming NIRS spectral data into chemical information is essentially required. In India, information of NIRS for oil and meal quality analysis of Brassica is meagre (Kumar et al. 2003, 2004, 2009). The objective of the present investigation was to develop calibration parameters for erucic acid and glucosinolate content in the seeds of rapeseed-mustard.
A total of 92 previously analyzed genotypes of rapeseed-mustard having a range of variability in erucic acid (0.5–57.3%) and glucosinolate content (15–130 μmole/g meal) were taken. Erucic acid content was determined using gas liquid chromatograph (Nucon Model 5765, New Delhi, India) using SP 2300+2310 SS columns. The detailed method for fatty acid analysis has been described earlier (Chauhan et al. 2002). Erucic acid was identified on the basis of comparison of retention time with the standard samples and expressed as the percentage of total fatty acids present in the oil. Total glucosinolate content in the seed meal was estimated by complex formation between glucosinolates and sodium teterachloropalladate solution. The intensity of the colour produced was measured using ELISA reader at 405 nm (Kumar et al. 2004). ‘Hyola 401’, a double low hybrid of gobhi sarson (Brassica napus) and ‘Varuna’, non-canola variety of Indian mustard were used as checks for the analysis of fatty acid and glucosinolates. Spectra were also collected in the NIR regions in reflectance mode (800–2500 nm i.e 12500–4000 cm−1) at 1 nm interval using NIRS system (Matrix I, Bruker, Germany). Dry samples were scanned in a small circular quartz cup. Original reflectance spectra were corrected prior to calibration by applying mathematical transformations. Reflectance data were stored as the logarithm of reflectance; log 1/R. The spectrum of each sample was the average of 32 successive scans. Spectral data collection and manipulation were performed using OPUS software from Bruker, Germany.
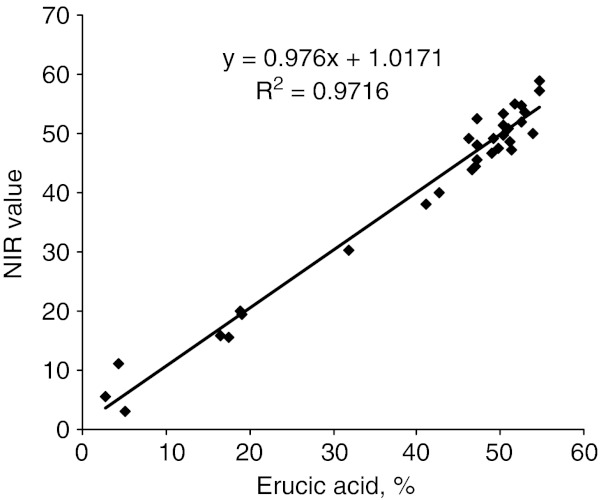
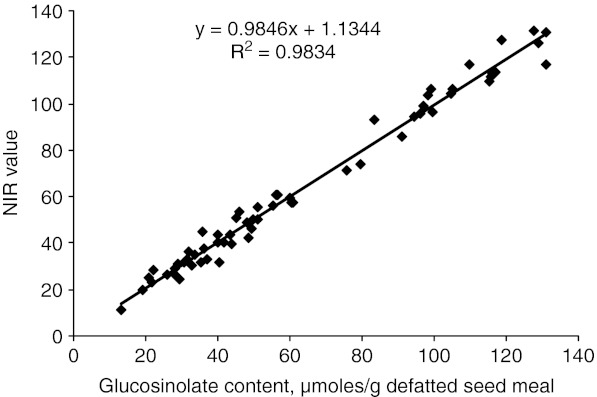
The best spectral region for screening erucic acid was between 7502.1 and 5444.6 cm−1 (1332.98–1836.68 nm), while for glucosinolates the optimum spectral region was 7502.1–5444.6 cm−1 and 4601.6–4246.7 cm−1 (2173.16–2355 nm). Accordingly calibration parameters were developed by using the information from the best spectral region for erucic acid and glucosinolates and partial least squares regression. Using stepwise elimination of out liers from the calibration graph, the efficiency of the method was gradually improved. Only 32 and 61 samples for erucic acid and glucosinolates corresponding to 534 and 627 selected data points, respectively were finally used to develop the calibration equations which are 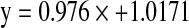 for erucic acid and
for erucic acid and 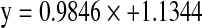 for glucosinolates. The calibration developed showed a very close relationship between the reference method and NIRS data for both erucic acid with coefficient of determination (r2) of 97.16% (Fig. 1) and glucosinolate content with coefficient of determination (r2) of 98.34%(Fig. 2). The standard error of cross validation was 2.7% for erucic acid and 4.5% for glucosinolate content. Velasco et al. (1998) also reported highly accurate (r2 = 0.98) calibration between gas chromatograph and NIRS values for erucic acid.
for glucosinolates. The calibration developed showed a very close relationship between the reference method and NIRS data for both erucic acid with coefficient of determination (r2) of 97.16% (Fig. 1) and glucosinolate content with coefficient of determination (r2) of 98.34%(Fig. 2). The standard error of cross validation was 2.7% for erucic acid and 4.5% for glucosinolate content. Velasco et al. (1998) also reported highly accurate (r2 = 0.98) calibration between gas chromatograph and NIRS values for erucic acid.
Fig. 1.
Relationship between erucic acid content estimated by gas chromatograph and FT-NIRS
Fig. 2.
Relationship between glucosinolate content by palladium complex method and FT-NIRS
The present investigation revealed that NIRS is a highly accurate and powerful technique that could be utilized successfully for rapid mass screening in early segregating generations for selecting low erucic acid in oil and glucosinolates in seed meal of rapeseed-mustard and thus enhance the effectiveness of quality breeding programme aiming at developing canola rapeseed-mustard in India.
References
- Ackman RG, Eaton CA, Sipos JC, Loew FM, Hancock D. Comparison of fatty acids from high levels of erucic acid of RSO and partially hydrogenated fish oil in non-human primate species in a short-term exploratory study. Nutr Diet. 1977;25:170–185. doi: 10.1159/000400508. [DOI] [PubMed] [Google Scholar]
- Bell JM. Nutrients and toxicants in rapeseed meal: a review. J Anim Sci. 1984;58:996–1010. doi: 10.2527/jas1984.584996x. [DOI] [PubMed] [Google Scholar]
- Bendini A, Cerretani L, Virgillio FD, Belloni P, Belloni-Carbognin M, Lercker G. Preliminary evaluation of the application of the FTIR spectroscopy to control geographic origin and quality of vergin olive oils. J Food Qual. 2007;30:424–437. doi: 10.1111/j.1745-4557.2007.00132.x. [DOI] [Google Scholar]
- Bille N, Eggum BO, Jacobsen I, Olsen O, Sorengen N. Antinutritional and toxic effect in rats of individual glucosinolates (+) myrosinases added to a standard diet. I. Effects on protein utilization and organ weights. Tierphysiol Tierernahar Futtermittelkd. 1983;49:195–210. doi: 10.1111/j.1439-0396.1983.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Biskupek-Korell B, Moschner CR. Near infrared spectroscopy (NIRS) for quality assurance in breeding, cultivation and marketing of high-oleic sunflowers. Helia. 2006;29:73–80. doi: 10.2298/HEL0645073B. [DOI] [Google Scholar]
- Chauhan JS, Tyagi P, Tyagi MK. Inheritance of erucic acid in two crosses of Indian mustard (Brassica juncea L.) SABRAO J Breed Genet. 2002;34:19–26. [Google Scholar]
- Chauhan JS, Bhadauria VPS, Singh M, Singh KH, Kumar A. Quality characteristics and their interrelationship in Indian rapeseed-mustard (Brassica sp) varieties. Indian J Agric Sci. 2007;77:616–620. [Google Scholar]
- Daun JK, Williams PC (1995) Use of NIR spectroscopy to determine quality factors in harvest survey of canola. In: Proc. 9th Int Rapeseed Congr, Cambridge, UK, Henry Ling Ltd, Dorchester, pp 864-866
- Fenwick GR, Heaney RK, Mullin WJ. Glucosinolates and their breakdown products in food and food plants. CRC Crit Rev Food Nutr. 1983;18:123–201. doi: 10.1080/10408398209527361. [DOI] [PubMed] [Google Scholar]
- Font R, Rio-Celestino MD, Cartea E, Harow-Bailon AD. Quantification of glucosinolates in leaves of leaf rape (Brassica napus ssp. pabularia ) by near-infrared spectroscopy. Phytochem. 2005;66:175–185. doi: 10.1016/j.phytochem.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Gopalan CD, Krishanamurthy D, Shenolikar IS, Krshnamurthy KAVR. Myocardial changes in monkey fed on mustard oil. Nutr Metabol. 1974;16:352–365. doi: 10.1159/000175508. [DOI] [PubMed] [Google Scholar]
- Juliani HR, Kapteyn J, Jones D, Korosh AR, Wang M, Charles D, Simon JE. Application of near-infrared spectroscopy in quality control and determination of adulteration of African essential oils. Phytochem Anal. 2006;17:121–128. doi: 10.1002/pca.895. [DOI] [PubMed] [Google Scholar]
- Kohel RJ. Evaluation of near-infrared reflectance spectroscopy for oil content. J Cotton Sci. 1998;2:23–26. [Google Scholar]
- Koprna R, Nerusil P, Kolovrat O, Kucera V, Kohoutek A. Estimation of fatty acid content in intact seeds of oilseed rape (Brassica napus L.) lines using near-infrared spectroscopy. Czech J Genet Plant Breed. 2006;42:132–136. [Google Scholar]
- Kumar S, Singh AK, Kumar M, Yadav SK, Chauhan JS, Kumar PR. Standardization of near infrared reflectance spectroscopy (NIRS) for determination of seed oil and protein contents in rapeseed-mustard. J Food Sci Technol. 2003;40:306–309. [Google Scholar]
- Kumar S, Yadav SK, Chauhan JS, Singh AK, Khan NA, Kumar PR. Total glucosinolate estimation by complex formation between glucosinolates and tetrachloropalladate (II) using ELISA reader. J Food Sci Technol. 2004;41:63–65. [Google Scholar]
- Kumar S, Mishra AP, Shukla AK, Singh YP, Bhaudharia VPS, Kumar S, Meena RC, Kumar A. Suitability of Soxhlet, NMR and NIR methods for oil estimation in rapeseed-mustard seeds. J Food Sci Technol. 2009;46:502–503. [Google Scholar]
- Niewitetzki O, Becker HC, Tillmann P, Mollers C (2007) A new NIRS method for high throughput analysis of oleic,linoleic and linolenic content of single seeds in oilseed rape. In: Quality, nutrition and processing. Proceedings. 12th Int Rapeseed Congr: Sustainable Development in Cruciferous Oilseed Crops Production. Wuhan, PR China, March 26-30, pp 55-57
- Orman BA, Schumann RA., Jr Nondestructive single-kernel oil determination of maize by near infrared transmission spectroscopy. J Food Qual. 1992;30:511–526. [Google Scholar]
- Renard S, McGregor L. Antithormbo genetic effects of erucic acid poor rapeseed oils in the rats. Rev Fr Cross. 1976;25:339–396. [Google Scholar]
- Sato T, Takahashi M, Matsunaga R. Use of NIR spectroscopy for estimation of fatty acid composition of soy flour. J Am Oil Chem Soc. 2002;79:535–537. doi: 10.1007/s11746-002-0517-3. [DOI] [Google Scholar]
- Velasco L, Becker HC. Estimating the fatty acid composition of the oil in intact seed rapeseeds (Brassica napus) by near-infrared reflectance spectroscopy. Euphytica. 1998;101:221–230. doi: 10.1023/A:1018358707847. [DOI] [Google Scholar]
- Velasco L, Mollers C. Nondestructive assessment of sinapic acid esters in Brassica species: II. Evaluation of germplasm and identification of phenotypes with reduced levels. Crop Sci. 1998;38:1650–1654. doi: 10.2135/cropsci1998.0011183X003800060039x. [DOI] [Google Scholar]
- Velasco L, Matthaus B, Mollers C. Nondestructive assessment of sinapic acid esters in Brassica species: I. Analysis by near-infrared reflectance spectroscopy. Crop Sci. 1998;38:1645–1650. doi: 10.2135/cropsci1998.0011183X003800060038x. [DOI] [Google Scholar]
- Yang N, Ren G. Application of near-infrared reflectance spectroscopy to the evaluation of rutin and D-chiro-Inositol contents in tartary buckwheat. J Agric Food Chem. 2008;56:761–764. doi: 10.1021/jf072453u. [DOI] [PubMed] [Google Scholar]