Abstract
In vitro starch digestibility and glycemic indices of three rice varieties- ‘Njavara’, ‘Jyothi’ (pigmented rice verities) and ‘IR 64’ (non-pigmented rice) with similar amylose content were studied. Starch digestibility studies showed differences in glycemic response in three types of rice. The rate of starch hydrolysis was maximum (67.3%) in ‘Njavara’ rice compared to other two rice varieties. ‘Njavara’ exhibited the lowest kinetic constant (k) indicating inherent resistance to enzymatic hydrolysis. The glycemic load (GL) and glycemic index (GI) of ‘Njavara’ were similar to ‘Jyothi’ and ‘IR 64’. Resistant starch content was high in pigmented rice varieties compared to ‘IR 64’. The resistant starch content of dehusked and cooked rice increased with the storage time at refrigeration temperature (4°C). ‘Njavara’ is an easily digestible rice and can be used for baby and geriatric foods.
Keywords: Rice, Njavara, Jyothi, Resistant starch, In vitro starch digestibility, Glycemic index, Glycemic load
Introduction
Carbohydrates are a major source of energy in human diet. In recent years, obesity and diabetes are the foremost problems of mankind. Carbohydrates are not just a source of calories but specific types of carbohydrates are included in the diet depending on the physiological disorder. People are aware of the alterations in blood glucose levels or glycemic index after consuming carbohydrate rich food. Currently, consumption of whole grain diet has received considerable attention due to its health benefits in attenuating chronic diseases including cardiovascular disease, type II diabetes, cancer and gastrointestinal disorders (Marquart et al. 2002). Whole grain cereals are a unique source of dietary fibre containing several bioactive compounds (Englyst et al. 1995) and nutrients (Marquart et al. 2002). Resistant starch is the residual fractions of starch, resistant to enzyme hydrolysis, entering the large intestine along with dietary fibre. Though resistant starch accounts only a small proportion of the total calorie intake, its effect is similar to those of other fibre components (Bjorck 1996). The glycemic index (GI) and resistant starch (RS) content have been established as important indicators of starch digestibility.
Rice is the most important cereal crop and one of the staple foods of the world’s population. It is an easily digestable fine cereal, producing high glycemic index (Wolever et al. 1990) and low colonic fermentation (Kerlin et al. 1984). The GI of brown rice has been reported to be 96, white rice is 83, while the freshly cooked rice is 64–93 (Miller et al. 1992). However, discrepancies in GI of rice have been reported due to differences in varieties, amylose content (Sagum and Arcot 2000; Frei et al. 2003), processing and cooking (Frei et al. 2003), particle size (Snow and O’Dea 1981), physicochemical characters like gelatinization (Chung et al. 2006; Panlasigui et al. 1991), amylose/ amylopectin ratio (Juliano and Goddard 1986), lipid-amylose complex (Guraya et al. 1997) and differential susceptibility to amylolytic enzymes (Jenkins et al. 1982). Rice is considered as a good source of insoluble fibre. There are limited studies on brown rice or dehusked rice compared to milled rice.
‘Njavara’ var. rice (Oryza sativa L.) is a medicinal red rice variety endemic to Kerala, in India. Morphologically, ‘Njavara’ is similar to ordinary rice with husk colour varying from golden yellow to brownish black, depending upon the edaphic and climatic conditions (Menon 2004). The medicinal quality of ‘Njavara’ is preserved by using only dehusked rice. It is the main component of ‘Njavara’ kizhi, an Ayurvedic treatment where a bolus is prepared by cooking rice with milk and certain herbs like Sida rectusa and Alpinia galangal and massaged over entire body for treatment of paralysis, arthritis and neurological problems. The local uses of ‘Njavara’ include consumption of rice cooked in copper vessel, to prevent rheumatic complaints. The rice is consumed to give high energy and gain weight. ‘Njavara’ is also recommended for lactating mothers and growing babies. ‘Njavara’ rice cooked along with medicinal herbs (Monsoon porridge) is consumed during monsoon season to increase immunity. ‘Jyothi’ (‘PTB 39’) is a hybrid, red rice variety consumed as a staple food in Kerala. ‘IR 64’ is a non-pigmented hybrid variety, known for its palatability and high yield. Our previous study reports the nutrient composition and physicochemical properties of ‘Njavara’ rice (Deepa et al. 2008). This study reports the starch digestibility, GI and RS of brown rice flour of a medicinal rice –‘Njavara’ and two non-medicinal rice varieties ‘Jyothi’ and ‘IR 64’.
Materials and methods
‘Njavara’ paddy was brought from Padma Ayurveda, Mannar (Kerala) while ‘Jyothi’ and ‘IR 64’ paddy were procured from Agriculture Products Marketing Cooperative market in Bandipalya, Mysore. Paddy harvested in December 2003 was obtained and stored at room temperature (27 ± 2°C) for one year and five months and then shifted to cold (4–6°C) until use. Amylases (Bacillus amyloliquefaciens), glucosidase (Rhizopus sp.) and pepsin were purchased from Sigma Chemical (USA). Glucose oxidase peroxidase kit was purchased from Monozyme India Pvt. Ltd. (Secundrabad, India). All other chemicals used were of analytical grade. The paddy samples were dehusked using rubber roll dehusker (Satake Corporation, Tokyo, Japan) and ground into flour (−60 mesh) using a rice mill (Surabhi, India). The dehusked rice and brown rice flour were stored at 4°C until use.
Sample preparation
Brown rice flour (10%), dry basis, was made into slurry in glass distilled water and made up to 500 ml. The slurry was poured into Brabender viscograph bowl and heated from 30 to 95°C, maintained at 95°C for 20 min and then cooled to 30°C, at a rate of 1.5o C per min with constant stirring. The cooked rice flour paste was then cooled to room temperature and then processed as.
Freeze dried (0 h), (b) Kept at 4°C for 24 h and then freeze dried (24 h) and (c) Kept at 4°C for 48 h and then freeze dried (48 h).
The samples were stored at 4°C until used for the experiments.
Total starch
Brown rice flour (−60 mesh) and processed rice flour (100 mg db), were dispersed in 50 ml of water and treated with Termamyl (100 μl) and incubated in boiling water bath for 10 min, cooled and equilibrated at 60°C. Solubilized starch was then hydrolyzed by adding glucosidase (6 mg in 0.6 ml acetate buffer pH 4.6) and incubated in shaking water bath at 60°C for 2 h. The samples were centrifuged and filtered. The supernatant was made up to a known volume. The glucose concentration in the supernatant was determined using glucose oxidase peroxidase kit at 505 nm. Starch was calculated as glucose x 0.9.
Resistant Starch (RS)
Native and processed rice flour (100 mg, db) were suspended in water (50 ml) and treated with Termamyl (100 μl) at 95°C for 45 min, cooled, centrifuged and supernatant was discarded. The residue was hydrolyzed with protease (10 mg in phosphate buffer pH 7.5) and amyloglucosidase (10 mg 0.1 M acetate buffer pH 4.75) to remove proteins and hydrolyze starch, respectively. The residues were dissolved in 2 M KOH, incubated with amyloglucosidase for 35 min at 60°C to hydrolyze RS. Glucose content in the above samples was determined using glucose oxidase peroxidase kit. Digestible starch was calculated as the difference between total starch and RS.
Starch kinetics
Brown rice flour (50 mg, db) was cooked in 5.0 ml of water for 30 min and incubated with 10 ml of pepsin solution prepared in HCl-KCl buffer pH 1.5 at 40° C for 1 h in a shaking water bath. The volume of the samples was made to 25 ml using Tris-Maleate buffer (pH 6.9). Reaction was started by adding α-amylase (2.6 units in 5 ml of buffer pH 6.9) and the samples were incubated at 37o C in a shaking water bath. One ml of the sample aliquot was collected at intervals of 30 min for 3 h. The enzyme activity in these aliquots was inactivated by heating at 100°C for 5 min and refrigerated until the end of the incubation period. To these aliquots, 3 ml of 0.4 M sodium acetate buffer (pH 4.75) and 60 μl amyloglucosidase were added to hydrolyze the digested starch to glucose. The samples were incubated at 60 °C for 45 min. The glucose content in each aliquot was estimated using glucose oxidase peroxidase kit. Glucose was converted into starch by multiplying with 0.9. All the experiments were conducted thrice and with triplicates in each analysis. The kinetics of starch digestion was estimated by non-linear first order equation established by Goni et al. (1997).
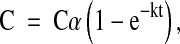 |
where, C corresponds to the concentration of starch hydrolyzed at time t. Cα represents the equilibrium concentration i.e. the percentage of starch hydrolyzed after 180 min. k is the kinetic constant. The parameters Cα and k were estimated for each cultivar based on the data obtained from the in vitro starch hydrolysis procedure. Parameters were estimated using SYSTAT (Sigma Plot 10) software, MS Office version.
The hydrolysis index (HI) was calculated as the percentage of total glucose released from the samples as compared to that released from standard glucose (0–180 min). The glycemic indices of the samples were estimated according to the equation of Goni et al. (1997), with the use of glucose as the reference food:
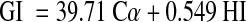 |
Glycemic load (GL) was estimated indirectly by multiplying the amount of carbohydrate contained in a nominal serving size (150 g) of rice with GI value of specific rice variety, divided by 100 (Salmeron et al. 1997).The available carbohydrate per serving (33 g carbohydrate/ serving) of boiled brown rice in India was obtained from literature (Kurup and Krishnamurthy 1992; Foster-Powell et al. 2002).
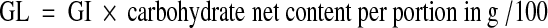 |
Statistical analysis
Analysis of variance (ANOVA) was performed by using SPSS system for windows version 7.5. Duncan’s multiple range tests were conducted for comparison of means at p < 0.05. Simple correlation coefficients were calculated for the relationships between nutrient composition (carbohydrate, protein, lipids and dietary fibre) and food indexes like total starch content, resistant starch and digestible starch and among the food indices.
Results and discussion
The total starch content was 79–89% in three rice varieties (Table 1) ‘IR 64’ had more starch content than the two red rice varieties, ‘Njavara’ and ‘Jyothi’. The resistant starch content was 0.6–1% in three rice varieties. The pigmented rice varieties showed more resistant starch content than the un-pigmented variety, ‘IR 64’. Resistant starch of all the rice varieties increased with storage at refrigeration (4°C). Various physical factors like stirring, water-starch ratio, cooking and cooling regimes affect resistant starch formation (Garcia-Alonso et al. 1999). In order to avoid these discrepancies all the rice varieties were cooked in Brabender Viscograph Type 801202 (Duisburg, FRG). Resistant starch formation is also influenced by amylose-amylopectin ratio in rice (Frei et al. 2003). Starch when cooked and cooled, rearrangement of amylose and amylopectin chains occur (retrogradation), which leads to increase in crystalline nature (B-type) of starch granules (Jane and Robyt 1984) and decreased starch digestibility. During retrogradation the amylose chains form double helix structure (Jane and Robyt 1984) while amylopectin crystallization occurs by re-association of the outermost short chains (Ring et al. 1987). Retrogradation of amylose is a more rapid process, occurring immediately, while cooling, but amylopectin requires longer time and hence storage conditions are important factors affecting retrogradation (Garcia-Alonso et al. 1999).
Table 1.
Total starch, resistant starch and digestible starch of brown rice flour (% w/w dry weight) after cooking and stored at refrigerated temp
| Parameters | Njavara | Jyothi | IR 64 |
|---|---|---|---|
| Total starch | |||
| Native | 79.56 ± 0.28 a | 80.67 ± 2.52 a | 84.30 ± 1.29 a |
| 0 h | 85.58 ± 3.84 b | 84.69 ± 0.60 b | 88.89 ± 0.43 a |
| 24 h | 85.50 ± 3.29 a,b | 86.62 ± 0.40 b | 89.34 ± 0.65 a |
| 48 h | 85.24 ± 2.67 b | 86.33 ± 0.27 b | 89.30 ± 0.64 a |
| Resistant starch | |||
| Native | 0.80 ± 0.06 b | 0.83 ± 0.01 a | 0.68 ± 0.08 c |
| 0 h | 0.80 ± 0.02 a | 0.83 ± 0.01 a | 0.64 ± 0.04 b |
| 24 h | 0.94 ± 0.15 a,b | 0.98 ± 0.17 a | 0.68 ± 0.02 b |
| 48 h | 1.05 ± 0.03 a | 1.10 ± 0.27 a | 0.71 ± 0.05 b |
| Digestible starch | |||
| Native | 79.16 ± 0.42 a | 80. 67 ± 1.78 a | 83.96 ± 0.83 a |
| 0 h | 84.78 ± 2.70 b | 83.86 ± 0.42 b | 88.24 ± 0.35 a |
| 24 h | 84.05 ± 2.48 b | 85.64 ± 0.11 b | 88.66 ± 0.45 a |
| 48 h | 84.19 ± 1.86 b | 85.23 ± 0.08 b | 88.60 ± 0.50 a |
Values within the same row with different superscripts are significantly different (p < 0.05) (n = 3)
The digestible starch among the three varieties varied from 79 to 84% with ‘IR 64’ having more digestible fraction of starch while ‘Njavara’ showing the least (Table 1). In processed dehusked rice, the digestibility increased by 5–6%. Native starches are mostly indigestible. Cooking in excess of water leads to swelling of starch granules followed by disintegration, exposing the starch chains and making them more accessible to the action of digestive enzymes. In this study also the observed total starch and digestible starch after gelatinization (0, 24 and 48 h) is due to exposure of amylose and amylopectin chains to the action of enzyme (amylase and glucosidase) leading to its breakdown to glucose.
Correlation among total starch (TS), resistant starch (RS) and digestible starch (DS) is presented in Table 2. Starch indices (TS, RS and DS) were correlated with carbohydrate, protein, lipid and dietary fibre content of the three rice varieties reported elsewhere (Deepa et al. 2008). As shown in Table 3, TS and DS released were observed to be inversely related to protein, insoluble and total dietary fibre content. However, it was positively correlated to the carbohydrate and soluble dietary fibre content (Table 3). The release of TS and DS is dependent on starch, lipid and protein complexes which make them less susceptible to the action of amylolytic enzymes (Holm et al. 1986). Similar results have been reported in other rice varieties by Urooj and Puttraj (1999). RS was inversely proportional to the amount of starch digested. Further, RS was directly proportional to protein, insoluble and total dietary fibre content while negatively correlated to carbohydrate content and soluble dietary fibre content. Yao et al. (2002) reported that lipid-amylose complex decreases the amount of amylose available to interact with the external chains of amylopectin to form resistant starch.
Table 2.
Correlation coefficients among total starch, resistant starch and digestible starch in vitro
| Total starch | Resistant starch | Digestive starch | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Native | 0 h | 24 h | 48 h | Native | 0 h | 24 h | 48 h | Native | 0 h | 24 h | 48 h | |
| Total starch | ||||||||||||
| Native | 1 | |||||||||||
| 0 h | 0.483 | 1 | ||||||||||
| 24 h | 0.579 | 0.884# | 1 | |||||||||
| 48 h | 0.615 | 0.912# | 0.992# | 1 | ||||||||
| Resistant starch | ||||||||||||
| Native | −0.566 | −0.811# | –0.713* | –0.793* | 1 | |||||||
| 0 h | −0.735* | −0.731* | –0.681* | –0.762* | 0.966# | 1 | ||||||
| 24 h | −0.540 | −0.820# | –0.755* | –0.795* | 0.770* | 0.704* | 1 | |||||
| 48 h | −0.690* | −0.565 | −0.538 | −0.612 | 0.801# | 0.822# | 0.866# | 1 | ||||
| Digestive starch | ||||||||||||
| Native | 0.996# | 0.478 | 0.593 | 0.620 | −0.518 | –0.690* | −0.524 | −0.648 | 1 | |||
| 0 h | 0.497 | 1.00# | 0.885# | 0.915# | –0.825# | –0.748* | –0.823# | −0.580 | 0.491 | 1 | ||
| 24 h | 0.586 | 0.894# | 0.999# | 0.995# | –0.730* | –0.695* | –0.787* | −0.572 | 0.598 | 0.895# | 1 | |
| 48 h | 0.646 | 0.910# | 0.982# | 0.997# | –0.823# | –0.797* | –0.832# | –0.675* | 0.645 | 0.915# | 0.987# | 1 |
*Correlation is significant at 0.05 level
#Correlation is significant at 0.01 level
Table 3.
Correlation coefficients between nutrient composition and total starch release, resistant starch and digestive starch during in vitro starch digestion
| Dietary fibre | ||||||
|---|---|---|---|---|---|---|
| Parameters | Carbohydrate | Protein | Lipid | Soluble | Insoluble | Total |
| Total starch | ||||||
| Native | 0.592 | −0.518 | −0.526 | 0.063 | −0.526 | −0.522 |
| 0 h | 0.623 | −0.316 | −0.657 | 0.377 | −0.463 | −0.448 |
| 24 h | 0.541 | −0.710* | −0.629 | 0.037 | −0.780* | −0.776* |
| 48 h | 0.569 | −0.660* | −0.642 | 0.148 | −0.773* | −0.764* |
| Resistant starch | ||||||
| Native | -0.505 | 0.316 | 0.504 | −0.673 | 0.590 | 0.568 |
| 0 h | −0.516 | 0.369 | 0.475 | −0.579 | 0.624 | 0.600 |
| 24 h | −0.732 | 0.452 | 0.851# | −0.491 | 0.491 | 0.471 |
| 48 h | −0.638 | 0.433 | 0.697* | −0.603 | 0.523 | 0.499 |
| Digestive starch | ||||||
| Native | 0.519 | −0.543 | −0.530 | −0.011 | −0.525 | −0.524 |
| 0 h | 0.625 | −0.321 | −0.656 | 0.389 | −0.473 | −0.457 |
| 24 h | 0.565 | −0.702 | −0.657 | 0.072 | −0.772 | −0.767 |
| 48 h | 0.598 | −0.666 | −0.672 | 0.202 | −0.775 | −0.765 |
*Correlation is significant at 0.05 level
#Correlation is significant at 0.01 level
In vitro method for measuring the rate of hydrolysis of starch has been suggested as an inexpensive and less time consuming method compared to measuring in vivo starch digestion (Jenkins et al. 1987). O’Dea et al. (1980) reported that in rice postprandial glucose and insulin responses correlate closely to the in vitro rates of hydrolysis. The present study on in vitro starch digestion showed that ‘Njavara’ is easily digestible (starch released 67% in 180 min) than ‘Jyothi’ and ‘IR 64’ (Table 4). The starch hydrolyzed in first 30 min was similar (58–59%) in all the three varieties of rice. However, after 30 min a gradual increase in starch digestion was observed in ‘Njavara’. ‘Njavara’, ‘Jyothi’ and ‘IR 64’ exhibited a plateau at approximately 60 min of hydrolysis. ‘Njavara’ and ‘Jyothi’ have been reported to have similar amylose content of 23% but they differed in their rate of digestion (Deepa et al. 2008). This is in agreement with the earlier reports that rice varieties with similar amylose content differ in their digestibility due to differences in their physiochemical properties like gelatinization temperature (Panlasigui et al. 1991). The rate of digestion also depends on the granule size, the amylose/amylopectin ratio, starch protein interaction, amylose/lipid complexes and the level of resistant starch (Sagum and Arcot 2000). In the present study we observed difference in the gelatinization temperatures (communicated elsewhere) and resistant starch (Table 1). According to the studies of Snow and O’Dea (1981) digestibility of starch is affected by the size of the granule and surface area to starch ratio for action of hydrolytic enzymes. The easier digestibility of ‘Njavara’ may be due to its smaller granular size (Deepa et al. 2008) rendering more surface area for the action of hydrolytic enzymes.
Table 4.
Rate of starch hydrolysis in brown rice flour
| Time, min | ‘Njavara’ | ‘Jyothi’ | ‘IR 64’ |
|---|---|---|---|
| 30 | 58.5 ± 0.50 a | 58.2 ± 4.86 a | 59.5 ± 1.79 a |
| 60 | 62.4 ± 0.04 a | 59.5 ± 2.99 a | 59.9 ± 1.96 a |
| 90 | 63.6 ± 0.83 a | 60.5 ± 3.50 a | 60.3 ± 3.98 a |
| 120 | 65.6 ± 1.64 a | 61.8 ± 4.83 a | 61.7 ± 1.96 a |
| 150 | 66.0 ± 2.06 a | 62.3 ± 4.13 a | 62.2 ± 2.69 a |
| 180 | 67.3 ± 0.41 a | 63.1 ± 3.63 b | 62.7 ± 2.93 b |
Values within the same row with different superscripts are significantly different
(p < 0.05) (n = 3)
The hydrolysis kinetics (Table 5) showed that the equilibrium constant (Cα) of ‘Njavara’ was relatively higher (65.2) than ‘Jyothi’ and ‘IR 64’ (~ 61). However, ‘Njavara’ exhibited significantly low kinetic constant (0.073) compared to Jyothi (0.095) and IR 64 (0.114), indicating that ‘Njavara’ has an inherent resistance to enzymatic hydrolysis.
Table 5.
Percentage of starch hyrolysis in 90 min (H 90), equilibrium constant (Cα) and kinetic constant (k) of brown rice flour
| Sample | Ha90 (Form) | Hb90 (Expt) | Cαa | ka |
|---|---|---|---|---|
| ‘Njavara’ | 65.2 | 63.6 ± 0.83 | 65.2 | 0.073 |
| ‘Jyothi’ | 61.5 | 60.5 ± 3.50 | 61.5 | 0.095 |
| ‘IR 64’ | 61.4 | 60.3 ± 3.98 | 61.4 | 0.114 |
aExperimental results
bAs per equation C = Cα (1 − e − kt )
Currently, nutritionists recommend that a whole-food approach rather than a GI approach to measure the glycemic potency of foods (Monro 2003). HI, GI and GL obtained for the three varieties are presented in Table 6. All the three varieties were observed to have similar HI, GI and GL. Further studies are warranted to understand in vivo digestibility of ‘Njavara’ rice, as it has high dietary fibre content compared to ‘Jyothi’ and ‘IR 64’ (Deepa et al. 2008). Dietary fibres are believed to enfold the food, hinder the action of hydrolytic enzymes in the gut, increase the viscosity of intestinal contents and thereby reduce the absorption of carbohydrates, in vivo (Jenkins et al. 1977). Moreover, the beneficial effects of dietary fibre are nullified when whole grains are ground. The whole grain flours are hydrolyzed at the same rate as polished grain flour.
Table 6.
Estimated hydrolysis index (HI), glycemic index (GI) and glycemic load (GL) of brown rice
| Sample | HI | GI | GL |
|---|---|---|---|
| ‘Njavara’ | 63.9 ± 0.75 | 74.8 ± 0.41 | 24.7 ± 0.34 |
| ‘Jyothi’ | 60.9 ± 3.99 | 73.1 ± 2.19 | 24.1 ± 5.24 |
| ‘IR 64’ | 61.1 ± 2.55 | 73.2 ± 1.40 | 24.2 ± 6.54 |
(n = 3)
Conclusion
The study suggests that pigmented whole grain rice (‘Njavara’ and ‘Jyothi’) is a better source of dietary fibre and resistant starch. The RS content in cooked rice can be increased by storing at low temperatures. The starch retrogradation property of these pigmented rice varieties can be exploited in preparation of healthy food products. ‘Njavara’ rice is observed to be easily digestible than ‘Jyothi’ and ‘IR 64’ based on in vitro starch hydrolysis study. Thus, Njavara rice could be considered for baby and geriatric foods.
Acknowledgement
Authors thank Prakash V, Director and Salimath P V, Head, Biochemistry and Nutrition Department, Central Food Technological Research Institute, Mysore, India for their support and encouragement in the present study. Deepa Gopinath, CSIR-SRF, gratefully acknowledges the financial support from CSIR, New Delhi, in carrying out these investigations. KAN gratefully acknowledges the financial support in the form of a Project by the Department of Science and Technology, New Delhi, India.
References
- Bjorck I. Starch: Nutritional aspects. In: Eliasson AC, editor. Carbohydrates in foods. Sweden: University of Lund; 1996. pp. 505–553. [Google Scholar]
- Chung HJ, Lim SH, Lim ST. Effect of partial gelatinization and retrogradation on the enzymatic digestion of waxy rice starch. J Cereal Sci. 2006;43:353–359. doi: 10.1016/j.jcs.2005.12.001. [DOI] [Google Scholar]
- Deepa G, Singh V, Naidu KA. Nutrient composition and physicochemical properties of Indian medicinal rice—Njavara. Food Chem. 2008;106:165–171. doi: 10.1016/j.foodchem.2007.05.062. [DOI] [Google Scholar]
- Englyst HN, Quigley ME, Hudson GJ. Definition and measurement of dietary fibre. Eur J Clin Nutr. 1995;49:S48–S62. [PubMed] [Google Scholar]
- Foster-Powell K, Holt SHA, Brand-Miller JC. International table of glycemic index and glycemic load values. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- Frei M, Siddhuraju P, Becker K. Studies on the in vitro starch digestibility and the glycemic index of six different indigenous rice cultivars from the Philippines. Food Chem. 2003;83:395–402. doi: 10.1016/S0308-8146(03)00101-8. [DOI] [Google Scholar]
- Garcia-Alonso A, Jimenez-Escrig A, Martin-Carron N, Bravo L, Saura-Calixto F. Assessment of some parameters involved in the gelatinization and retrogradation of starch. Food Chem. 1999;66:181–187. doi: 10.1016/S0308-8146(98)00261-1. [DOI] [Google Scholar]
- Goni I, Garcia-Alonso A, Saura-Calixto F. A starch hydrolysis procedure to estimate glycemic index. Nutr Res. 1997;17:427–437. doi: 10.1016/S0271-5317(97)00010-9. [DOI] [Google Scholar]
- Guraya HS, Kadan RS, Champagne ET. Effect of rice starch—lipid complexes on in vitro digestibility, complexing index, viscosity. Cereal Chem. 1997;74:561–565. doi: 10.1094/CCHEM.1997.74.5.561. [DOI] [Google Scholar]
- Holm J, Bjorck I, Drews A, Asp NG. A rapid method for the analysis of starch. Starch. 1986;38:224–226. doi: 10.1002/star.19860380704. [DOI] [Google Scholar]
- Jane JL, Robyt JF. Structure studies of amylose complexes and retrograded amylose by action of alpha amylase and a new method for preparing amylodextrins. Carbohydr Res. 1994;132:105–118. doi: 10.1016/0008-6215(84)85068-5. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Leeds AR, Gassell MA, Cocket B, Alberti KGM. Decrease in post-prandial insulin and glucose concentrations by gaur and pectin. Ann Intern Med. 1977;86:20–23. doi: 10.7326/0003-4819-86-1-20. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Taylor RH, Wolever TMS. The diabetic diet, dietary carbohydrate and differences in digestibility. Diabetologia. 1982;23:477–480. doi: 10.1007/BF00254294. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Thorne MJ, Wolever TMS, Jenkins AL, Venketschwer R, Thompson LU. The effect of starch protein interaction in wheat on the glycemic response and rate of in vitro digestion. Am J Clin Nutr. 1987;45:946–951. doi: 10.1093/ajcn/45.5.946. [DOI] [PubMed] [Google Scholar]
- Juliano B, Goddard M. Cause of varietal differences in insulin and glucose responses to ingested rice. Plant Food Hum Nutr. 1986;36:35–41. doi: 10.1007/BF01091751. [DOI] [Google Scholar]
- Kerlin P, Wong L, Harris B, Capra S. Rice flour, breath hydrogen and malabsorption. Gastroenterology. 1984;87:578–585. [PubMed] [Google Scholar]
- Kurup PG, Krishnamurthy S. Glycemic index of selected foodstuffs commonly used in South India. Int J Vitam Nutr Res. 1992;62:266–268. [PubMed] [Google Scholar]
- Marquart L, Slavin JL, Fulcher RG. Whole grain foods in health and diseases. St. Paul: American Association of Cereal Chemists, Inc; 2002. pp. 187–200. [Google Scholar]
- Menon MV (2004) Njavara: The healing touch. Sci Report, Feb. 28–30
- Miller JB, Pang E, Bramall L. Rice: a high or low glycemic index food? Am J Clin Nutr. 1992;34:1034–1036. doi: 10.1093/ajcn/56.6.1034. [DOI] [PubMed] [Google Scholar]
- Monro J. Redefining the glycemic index for dietary management of postprandial glycemia. J Nutr. 2003;133:4256–4258. doi: 10.1093/jn/133.12.4256. [DOI] [PubMed] [Google Scholar]
- O’Dea K, Nestel PJ, Antonoff L. Physical factors influencing postprandial glucose and insulin responses in starch. Am J Clin Nutr. 1980;33:760–765. doi: 10.1093/ajcn/33.4.760. [DOI] [PubMed] [Google Scholar]
- Panlasigui LN, Thompson LU, Juliano BO, Perez CM, Yiu SH, Greenberg GR. Rice varieties with similar amylose content differ in starch digestibility and glycemic responses in humans. Am J Clin Nutr. 1991;54:871–877. doi: 10.1093/ajcn/54.5.871. [DOI] [PubMed] [Google Scholar]
- Ring SG, Collona P, Panson KJ, Kalicheversky MT, Miles MJ, Morris VJ, Oxford PD. The gelation and crystallization of amylopectin. Carbohydr Res. 1987;162:277–293. doi: 10.1016/0008-6215(87)80223-9. [DOI] [Google Scholar]
- Sagum R, Arcot J. Effect of domestic processing methods on the starch, non-starch polysaccharides and in vitro starch and protein digestibility of three varieties of rice with varying levels of amylase. Food Chem. 2000;70:107–111. doi: 10.1016/S0308-8146(00)00041-8. [DOI] [Google Scholar]
- Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL. Dietary fiber, glycemic load, and risk of NIDDM in men. Diab Care. 1997;20:545–550. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- Snow P, O’Dea K. Factors affecting the rate of hydrolysis of starch in foods. Am J Clin Nutr. 1981;34:2721–2727. doi: 10.1093/ajcn/34.12.2721. [DOI] [PubMed] [Google Scholar]
- Urooj A, Puttraj SH. Digestibility index and factors affecting rate of starch digestion in vitro in conventional food preparation. Starch. 1999;51:S430–S435. doi: 10.1002/(SICI)1521-3803(19990101)43:1<42::AID-FOOD42>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Wolever TMS, Jenkins DJA, Vuksan V, Josse RG, Wong GS, Jenkins AL. Glycemic index of foods in individual subjects. Diab Care. 1990;13:126–132. doi: 10.2337/diacare.13.2.126. [DOI] [PubMed] [Google Scholar]
- Yao Y, Zhang J, Ding X. Structure-retrogradation relationship of rice starch in purified starches and cooked rice grains: a statistical investigation. J Agric Food Chem. 2002;50:7420–7425. doi: 10.1021/jf020643t. [DOI] [PubMed] [Google Scholar]


