Abstract
Chemical composition of black cumin (Nigella sativa L.) seed extracts obtained by supercritical carbon dioxide at two different conditions that result in total extract (28 MPa/50°C, SFE 1) and major volatile part (12 MPa/40°C, SFE 2) and essential oil obtained by hydrodistillation of SFE-1 (HD SFE). SFE have been carried out to characterize the compounds and the variation of quinones and phenolics. The extracts were analysed by GC and GC-MS and the presence of phenolic compounds was further confirmed by 2D HSQCT 1H and 13C NMR spectroscopy. Forty-seven volatile compounds were detected where sixteen compounds were reported for the first time in the oil of this seed. Moreover, thymoquinone (TQ), dithymoquinone (DTQ), thymohydroquinone (THQ) and thymol (THY) were the major phenolic compounds. It can be concluded that the chemical composition of extracts obtained by SC CO2 extraction of the seeds showed better recovery of phenolic compounds than HD SFE and proved the occurrence of thermally labile or photosensitive bioactive volatiles of four major quinonic phenol compounds.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-010-0109-y) contains supplementary material, which is available to authorized users.
Keywords: Nigella sativa, Supercritical CO2, GC-MS, 2D HSQCT NMR, Thymoquinone
Introduction
A large number of medicinal plants and their purified constituents have been shown beneficial therapeutic potentials. Seeds of Nigella sativa (black cumin), a dicotyledon of the Ranunculaceae family, have been used for thousands of years as a spice and food preservative. Black cumin is an annual herbaceous plant widely grown in the Mediterranean countries, Middle East, Eastern Europe and Western Asia. The seeds have been added as a spice to a variety of Persian foods such as bread, yogurt, pickles, sauces and salads (Hajhashemi et al. 2004). In the Middle East, Northern Africa and India, it has been used traditionally for centuries for the treatment of asthma, cough, bronchitis, headache, rheumatism, fever, influenza and eczema and for its antihistaminic, antidiabetic and anti-inflammatory activities (Burits and Bucar 2000).
The oil and the seed constituents, in particular thymoquinone (TQ), have shown potential medicinal properties; they exhibit potent anti-inflammatory effects on several inflammation-based models including experimental encephalomyelitis, colitis, peritonitis, oedama, and arthritis through suppression of the inflammatory mediators prostaglandins and leukotriens (Chakrabarty et al. 2003). The oil and active ingredient of TQ showed beneficial immunomodulatory properties, augmenting the T cell and natural killer cell-mediated immune responses (Haq et al. 1999). Most importantly, both the oil and its active ingredients expressed anti-microbial and anti-tumor properties toward different microbes and cancers (Topozada et al. 1965; Badary et al. 1999). Coupling these beneficial effects with its use in folk medicine, Nigella sativa seed is a promising source for active ingredients that would be with potential therapeutic modalities in different clinical settings. More than 150 studies have been conducted and confirmed the pharmacological effectiveness of Nigella sativa seed constituents. Though, Nigella sativa seed is a complex substance of more than 100 compounds, some of which have not yet been identified or studied (Salem 2005).
TQ, derived from the medicinal spice Nigella sativa has been shown to exhibit anti-inflammatory (Mutabagani and El-Mehdy 1997) and anti-cancer activities (Worthen et al. 1998). TQ in nanoparticles were more potent than TQ in suppressing proliferation of colon cancer, breast cancer, prostate cancer, and multiple myeloma cells and also that encapsulation of TQ enhances its anti-proliferative, anti-inflammatory, and chemosensitizing effects (Ravindran et al. 2010). Several components of black cumin have been identified, including thymoquinone, thymol, thymohydroquinone, and dithymoquinone (Morikawa et al. 2004). The most abundant component of black seed oil, TQ has been reported to exhibit antioxidant (Mansour et al. 2002; Badary et al. 2003, 2007), anti-inflammatory, chemosensitization and chemopreventive potential effects (Badary et al. 1999; Badary and Gamal El-Din 2001; Gali-Muhtasib et al. 2004) .
Beneficial effects of Nigella sativa (NS) and TQ on histopathological changes of sciatic nerves in streptozotocin (STZ) induced diabetic rats were studied. The treatment with both NS and TQ caused sharp decrease in the elevated serum glucose, and an increase in the lowered serum insulin concentrations, in STZ induced diabetic rats. No histopathological changes of sciatic nerves in STZ induced diabetic rats by NS and TQ treatment have been reported (Kanter 2008).
Despite the availability and use of numerous antiepileptic drugs, nearly 15% of childhood epilepsy cases are resistant to treatment. However, in traditional medicine, Nigella sativa has been known for its anticonvulsant effects. In this double-blinded crossover clinical trial conducted on children with refractory epilepsy, the aqueous extract of black seed was administered as an adjunct therapy and the effects were compared with those of a placebo. It can be concluded that the water extract of Nigella sativa has antiepileptic effects in children with refractory seizures (Akhondian et al. 2007).
TQ converted to DTQ via photodimerization, as a consequence of exposure to heat and sunlight during separation and extraction procedures. Various storage conditions are also expected to make a difference in the amounts of the quinone constituents especially TQ of the oil (El-Dakhakhny 1963).
SFE is an attractive alternative to conventional methods due to its use of environmentally compatible fluids, reduced solvent consumption, oxygen-free extraction environment, the ease of separation of solute from supercritical fluid (SCF) solvent by simple expansion and shorter extraction time. Supercritical fluid extraction (SFE) of bioactive compounds from plant material is a promising field for the industrial application of SFE, since it has many advantages over steam-distillation and solvent extraction as it prevents the transformation of bioactive compounds during extraction. Supercritical CO2 extraction has been considered as a possible applied field of SFE, because CO2 is nontoxic and nonflammable, the lack of a chemical residue problem and low critical temperature (31.2°C) is important (Machmudah et al. 2005).
Currently, black cumin seeds have been extensively studied particularly, which justifies its broad traditional therapeutic and nutritional value. In consideration of potential utilization, detailed knowledge on the chemical composition of the seed is of major importance for food and pharmaceutical industries. The reason might be found in the complex chemical composition of the seeds. SC CO2 technology has provided an impetus for their improvement in terms of quality as well as quantity of the extracted bioactive compounds than other conventional extractions. Thus the SC CO2 technology described here may find utility as a superior technology for determine the composition of pharmacologically active quinones in this N. sativa seed oil. The aim of the present study was to explore the chemical composition of extracts isolated from Nigella sativa seeds by Supercritical CO2 besides identifying all the compounds present in the volatile oils by GC MS and NMR Spectroscopy.
Materials and methods
Plant material
Seeds of Nigella sativa were obtained from Supreem Pharmaceuticals Mysore Pvt. Ltd, Mysore, India. A voucher specimen authenticated and has been deposited at the Central Food Technological Research Institute, Mysore. The seeds were stored in polythene bags and maintained at 4°C until extraction. Seeds material was dried and ground into a fine powder using an IKA-10 mini laboratory mill.
Chemicals
Sodium sulphate (anhydrous) and silica gel (60–120 mesh) for column chromatography was purchased from SD Fine Chemicals, India. Silica gel G for thin layer chromatography was purchased from Loba Chemicals, India. All the solvents used were of analytical grade and dimethyl sulfoxide (DMSO-d6) from Merck Co, Mumbai, India. The solvents were distilled once before use. For the determination of retention indices, a hydrocarbon mixture (Sigma, India) ranging from n-octane to n-docosane was used. Food grade CO2 cylinder (99.9% purity) was obtained from Kiran Corporation, Mysore, India.
Supercritical fluid extraction
A Nova Swiss high pressure extractor (Nova Werke, AG, model Ex 1000–1.4–1.2) was used for the extractions. Food grade CO2 was pumped into the system by diaphragm pump until the required pressure was obtained. Back pressure regulators were used to set the system pressure (in extractor and separator). The extractor vessel was loaded with 1 kg of the powdered material of black cumin seeds. Heat exchangers were provided in the system and on the extractor and separator vessel for temperature elevation. SC CO2 flows through the extractor and enters the separator vessel through an expansion valve and was re-circulated. Samples of the extracted substance were collected by opening the valve located at the bottom of the separator vessel. A flow meter was provided to monitor the flow rate of CO2 circulating in the system. Extractions were carried out at two different conditions of pressures and temperatures, namely 28 MPa at 50°C (SFE 1) and 12 MPa at 40°C (SFE 2) at a CO2 mass flow rate of 3.3–8.05 × 10−4 kg/s (Udaya Sankar 1989).
Hydrodistillation
The Supercritical CO2 extract (SFE 1) was subjected to hydrodistillation (HD) for 6 h using a Clevenger-type apparatus. The essential oil (HD SFE) obtained was yellow color with aromatic odour in a yield of 1.5% (v/v) which was dried using anhydrous sodium sulphate and then stored at 4°C in dark until analysis.
Column chromatography
5 ml of SFE 1 sample was subjected to purification, using a glass column (40 mm i.d × 450 mm length) packed with silica gel (60–120 mesh) and eluted with hexane and ethyl acetate at 99:1 ratio. Fractions of volume 250 ml were collected and concentrated. They were monitored by TLC in 9:1 ratio of hexane and ethyl acetate. The spots were located by exposing the plates to iodine vapours. Fractions having the similar pattern of the spots with similar Rf values on the TLC plates were collected and pooled. From chromatographic separation, four fractions were detected. The fraction weights were: fraction 1; 1.3 L, fraction 2; 0.8 L, fraction 3; 0.4 L and fraction 4; 0.7 L.
GC and GC-MS analysis
GC analyses were performed using a Fisons GC 8000 gas chromatograph equipped with FID detector. All the analyses were carried out by a fused silica DB-5MS column (30 m × 0.32 mm i.d., film thickness 0.25 μm). The oven temperature was increased from 70°C to 220°C at 4°C min and held isothermal for 15 min. The injector and detector temperatures were maintained at 220°C and 240°C respectively. 10% of samples were prepared in chloroform. The injection volume was 0.5 μL with a split ratio of 1:30 and nitrogen used as carrier gas at a flow rate of 1 ml/min. GC-MS recordings were made on a Shimadzu gas chromatograph (Shimadzu, Japan) coupled with QP-5000 mass spectrometer. A 0.5 μL sample was injected in the split mode ratio of 1:15. Helium was used as carrier gas at a flow rate of 1 ml/min. All the other parameters remained unchanged relative to GC analyses. Mass spectra were obtained by EI at 70 eV. Mass scanning was performed from 40 to 400 amu.
1H and 13C NMR analysis
Two-dimensional Heteronuclear Single Quantum Coherence Transfer Spectra (2D HSQCT) were recorded using a Bruker Avance AQS 500 MHz (Bruker Biospin, Fallanden, Switzerland) NMR spectrometer operating at 500.18 MHz for 1H and 125.78 MHz for 13C at 20°C. Proton and carbon 90° pulse widths were 12.25 and 10.5 μs, respectively. Chemical shifts were expressed in ppm relative to tetramethylsilane (TMS) as an internal standard. 5 mg of samples dissolved in DMSO-d6 was used for recording the spectra in magnitude mode with sinusoidal-shaped z-gradients of strength 25.7, 15.42 and 20.56 G/cm with a gradient recovery delay of 100 μs to defocus unwanted coherences. Increment of t1 was in 256 steps. About 50–200 scans and 500–6000 scans were accumulated with a recycle period of 2–3 seconds to obtain good spectra for 1H and 13C NMR, respectively. A region from 0–10 ppm and 0–200 ppm were scanned for all the samples for 1H and 13C NMR, respectively. The size of the computer memory used to accumulate the data was 4 kB. The spectra were processed using unshifted and π/4 shifted sine bell window function in F1 and F2 dimensions, respectively.
Compounds identification
Compounds were identified based on comparison of their Kovats retention indices (RI) relative to C8-C22n-alkanes and matching of the mass spectra with those detailed in the NIST, Wiley commercial libraries, data from Chemistry web book and literature data (Joulain and Konig 1998; Adams 2007).
Results and discussion
The SFE 1 extract carried out at pressure of 28 MPa and temperature of 50°C resulted in total extract yield of 26.02% contained both volatile and nonvolatile fraction. The SFE 2 extract found to contain a major fraction of steam volatile components only at lower pressure and temperature of extraction (Udaya Sankar 1989). In this study, extract yield is expressed in % and defined as weight of the extract divided by weight of the sample. SFE1 obtained higher extract yield than the SFE 2. The extraction yield was increased with increasing pressure and temperature at certain levels along with better recovery of quinones and phenolics. The hydrodistillation of Nigella sativa seed powder with Clevenger distillation yielded 1.0–1.2% of essential oil with 30–32% of thymoquinone (TQ). In SFE 1, the extract yield was 26.02% with 7.11% of TQ whereas in SFE 2 the yield of extract was 7.8% with TQ content of 12.27%. The recovery of TQ on the seed basis was 0.4–0.5% in hydrodistillation, 1.85% in SFE 1 and 0.95% in SFE 2. Hence, by SC CO2 extraction the recovery of TQ was much better.
Hydrodistillation of SFE 1 extract yielded 1.5% of essential oil (HD SFE) with characteristic transparent yellow color with typical aromatic odour. A total of 47 different compounds were identified in Nigella sativa seed oils extracted by supercritical CO2 (SFE 1 and SFE 2) and hydrodistillation of SFE 1 (HD SFE). About 31 compounds were identified in SFE 1 oil, 22 compounds in SFE 2 oil and 23 compounds in HD SFE oil. Among the 47 compounds, the occurrences of 16 volatile compounds were reported for the first time in Nigella sativa seeds (Table 1). They are n-nonane, allo-ocimenol, terpinen-1-ol, 1,5,8-ρ-menthatriene, dihydrocarvone, ocimenone (E), n-octyl isobutyrate, citronellyl acetate, thymohydroquinone methyl ether, (Z)-caryophyllene, thymohydroquinone dimethyl ether, aromadendrene, davanone, 8-heptadecene, dihydro farnesyl acetate and pimaradiene. The lower number of compounds occurred in HD SFE compared to SFE is mainly related to the possible degradation of volatile compounds by higher temperature and longer distillation time.
Table 1.
Chemical composition (%) of black cumin seed extracts isolated by supercritical CO2 extraction
| Compound | RIexp | RIlit | SFE 1 | SFE 2 | HD SFE | Identification |
|---|---|---|---|---|---|---|
| n-Nonanea | 905 | 900 | 0.12 | ── | ── | RI, MS |
| Tricyclene | 926 | 926 | tr | ── | ── | RI, MS |
| Camphene | 953 | 953 | ── | ── | 1.64 | RI, MS |
| β-Pinene | 958 | 959 | ── | ── | 0.40 | RI, MS |
| 2,4,(10)-Thujadiene | 967 | 960 | 4.74 | 0.19 | ── | RI, MS |
| Sabinene | 978 | 977 | 1.05 | ── | ── | RI, MS |
| β-Myrcene | 990 | 991 | 0.31 | ── | ── | RI, MS |
| 1,8-Cineole | 1013 | 1010 | ── | ── | 0.98 | RI, MS |
| α-Terpinene | 1025 | 1026 | 2.34 | ── | ── | RI, MS |
| Limonene | 1034 | 1034 | 0.18 | 0.38 | 1.03 | RI, MS |
| γ-Terpinene | 1054 | 1056 | 27.46 | 13.20 | 12.87 | RI, MS |
| cis-Sabinene hydrate | 1063 | 1068 | ── | 0.38 | tr | RI, MS |
| allo-Ocimenola | 1079 | 1071 | ── | 0.11 | ── | RI, MS |
| Linalool | 1087 | 1080 | 0.25 | 0.19 | ── | RI, MS |
| Terpinolene | 1091 | 1088 | ── | ── | tr | RI, MS |
| trans-Sabinene hydrate | 1099 | 1097 | 0.37 | ── | ── | RI, MS |
| Terpinen-1-ola | 1124 | 1120 | ── | ── | 0.11 | RI, MS |
| 1,5,8-p-Menthatrienea | 1130 | 1135 | 0.43 | 0.38 | ── | RI, MS |
| Borneol | 1152 | 1152 | ── | ── | 1.02 | RI, MS |
| Pinocarvone | 1167 | 1165 | 2.96 | 3.00 | ── | RI, MS |
| trans-Dihydrocarvone | 1208 | 1202 | ── | 0.19 | ── | RI, MS |
| Dihydrocarvonea | 1215 | 1214 | 0.37 | 2.06 | ── | RI, MS |
| Ocimenone (E)a | 1249 | 1239 | 1.54 | 1.50 | ── | RI, MS |
| Thymoquinone | 1250 | 1250 | 35.05 | 33.12 | 38.41 | RI, MS,NMR |
| Thymol | 1283 | 1288 | 7.43 | 5.30 | 16.95 | RI, MS,NMR |
| Carvacrol | 1299 | 1299 | 1.98 | 1.73 | 0.81 | RI, MS |
| 2-Undecanone | 1312 | 1315 | ── | ── | 13.72 | RI, MS |
| n-Octyl isobutyratea | 1323 | 1326 | ── | ── | 0.12 | RI, MS |
| α-Longipinene | 1330 | 1334 | 0.26 | ── | ── | RI, MS |
| Citronellyl acetatea | 1339 | 1336 | ── | ── | 0.50 | RI, MS |
| Thymohydroquinone methyl ethera | 1353 | 1351 | ── | ── | tr | RI, MS |
| Cyclosativene | 1367 | 1366 | ── | ── | 1.43 | RI, MS |
| α-Longicyclene | 1381 | 1380 | 0.43 | 5.25 | ── | RI, MS |
| α-Copaene | 1385 | 1383 | 1.54 | 2.00 | 0.41 | RI, MS |
| α-Longifolene | 1391 | 1387 | ── | ── | 0.51 | RI, MS |
| (Z)-Caryophyllenea | 1395 | 1395 | 0.23 | ── | ── | RI, MS |
| β-Caryophyllene | 1420 | 1417 | 2.89 | 5.07 | 4.80 | RI, MS |
| Thymohydroquinone dimethylethera | 1429 | 1425 | 0.43 | ── | ── | RI, MS |
| Aromadendrenea | 1437 | 1438 | ── | ── | 1.04 | RI, MS |
| Thymohydroquinone | 1515 | 1509 | 1.17 | 1.12 | 2.31 | RI,MS,NMR |
| Davanonea | 1587 | 1586 | 0.31 | ── | ── | RI, MS |
| 8-Heptadecenea | 1683 | 1680 | 1.23 | 1.13 | 0.86 | RI, MS |
| Dihydrofarnesyl acetatea | 1841 | 1840 | 2.28 | 4.69 | ── | RI, MS |
| Pimaradienea | 1934 | 1935 | 1.23 | 2.25 | ── | RI, MS |
| Palmitic acid | 1947 | 1946 | 0.18 | ── | ── | RI, MS |
| Pimara-8(14),15-diene | 1968 | 1966 | 0.92 | ── | ── | RI, MS |
| Octadecanoic acid | 2145 | 2157 | 0.26 | 12.31 | ── | RI, MS |
| Total identified | 99.94 | 95.55 | 99.92 | |||
| Grouped compounds: | ||||||
| Quinones | 44.08 | 39.54 | 57.67 | |||
| Monoterpene hydrocarbons | 36.51 | 14.15 | 15.94 | |||
| Oxygenated monoterpenes | 7.47 | 9.16 | 17.14 | |||
| Sesquiterpene hydrocarbons | 5.35 | 12.32 | 8.19 | |||
| Oxygenated sesquiterpenes | 2.59 | 4.69 | ── | |||
| Diterpenes | 2.15 | 2.25 | ── | |||
| Alkane | 0.12 | ── | ── | |||
| Alkenes | 1.23 | 1.13 | 0.86 | |||
| Fatty acids | 0.44 | 12.31 | ── | |||
| Fatty acid esters | ── | ── | 0.12 | |||
Identification has been through by comparing mass spectra (MS), retention indices (RI), NMR spectra, data from NIST, Wiley commercial libraries, Chemistry Web Book (www.nist.org/chemistrywebbook) and other reports (Joulain and Konig 1998; Adams 2007)
SFE 1 (28 MPa/50°C), SFE 2 (12 MPa/40°C) and hydrodistillation of SFE 1 (HD SFE)
The retention indices were calculated for all compounds using a homologous series of C8-C22n- alkanes. RIexp, experimental retention indices given for DB-5MS column; RIlit, literature retention indices given for DB-5MS column. tr, trace (<0.1%)
a Compounds identified for the first time in the extracts of Nigella sativa
The SFE oils could be distinguished from the HD SFE oil by their greater richness in monoterpene hydrocarbons (36.51% in SFE 1, 14.15% in SFE 2 and 15.94% in HD SFE), sesquiterpene hydrocarbons (5.35% in SFE 1, 12.32% in SFE 2 and 8.19% in HD SFE), oxygenated sesquiterpenes (2.59% in SFE 1 and 4.69% in SFE 2), diterpenes (2.15% in SFE 1 and 2.25% in SFE 2), fatty acids (0.44% in SFE 1 and 12.31% in SFE 2) and fatty acid esters (0.12% in HD SFE). The oxygenated monoterpenes were more represented in HD SFE (17.14%) than in SFE 1 (7.47%) and SFE 2 (9.16%). Quinones were present to greater extent in HD SFE (57.67%) than SFE 1 (44.08%) and SFE 2 (39.54%) oils. The main compounds in SFE 1, SFE 2 and HD SFE oils were thymoquinone (35.05% in SFE 1, 33.12% in SFE 2 and 38.41% in HD SFE), γ-terpinene (27.46% in SFE 1, 13.20% in SFE 2 and 12.87% in HD SFE), thymol (7.43% in SFE 1, 5.30% in SFE 2 and 16.95% in HD SFE), β-caryophyllene (2.89% in SFE 1, 5.07% in SFE 2 and 4.80% in HD SFE) and thymohydroquinone (1.17% in SFE 1, 1.12% in SFE 2 and 2.31% in HD SFE). In this study, dithymoquinone (DTQ) could not be traced by GC and GC-MS analysis of these oils, as confirmed by Burits and Bucar (2000) and Benkaci-Ali et al. (2007). The present analysis showed higher thymoquinone content than what has been reported in the literatures so far (Burits and Bucar 2000; El-Ghorab 2003).
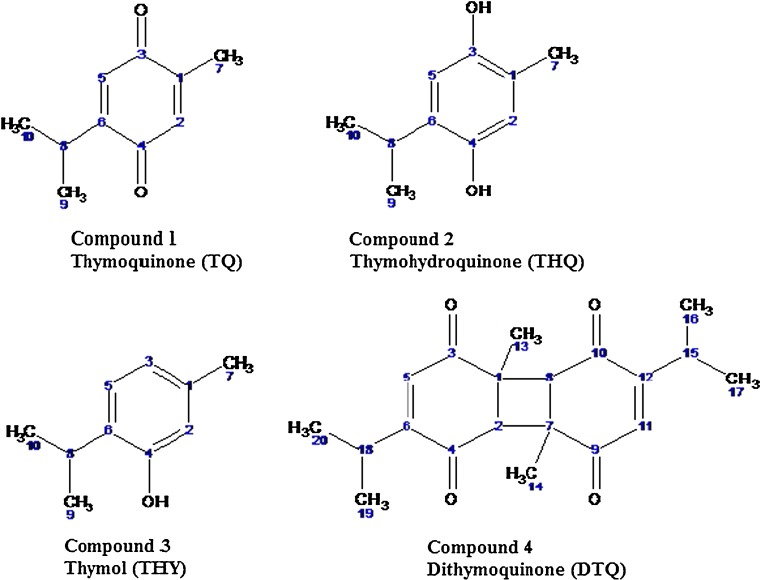
Fractions obtained from column chromatography separation, showed a mixture of four quinonic phenol compounds, all possessing the thymol skeleton through 2D HSQCT NMR spectra in different proportions.
Compound 1 showed the presence of two carbonyl groups at 187.3 ppm and 188.3 ppm. Further, two aromatic protons were detected at 6.59 ppm and 6.72 ppm. A CH3 group attached to an aromatic ring was detected at 14.9 ppm in the carbon spectrum. The presence of isopropyl group was detected by observing signals at 1.93 ppm and 1.94 ppm (doublet) and 2.12 ppm, all with the coupling constant of 5.3 Hz (Table 2). Thus, compound 1 was identified to be thymoquinone (Fig. 1).
Table 2.
1H and 13C 2D-HSQCT NMR data of compounds 1, 2 and 3a in DMSO-d6
| Carbon number | Chemical shifts (ppm) Compound 1 | Chemical shifts (ppm) Compound 2 | Chemical shifts (ppm) Compound 3 | |||
|---|---|---|---|---|---|---|
| 1H NMR (J in Hz) | 13C NMR | 1H NMR (J in Hz) | 13C NMR | 1H NMR (J in Hz) | 13C NMR | |
| 1 | ── | 145.1 | ── | 127.2 | ── | ── |
| 2 | 6.59(s) | 133.5 (i1) | 7.03 (s) | 120.4 | 6.72 (s) | 133.5 (i1) |
| 3 | ── | 188.3 (i2) | ── | ── | 7.47 (d, 8 Hz) | 127.9 (i2) |
| 4 | ── | 187.3 (i2) | ── | ── | ── | 154.1 |
| 5 | 6.72(s) | 133.3 (i1) | 5.30 (s) | 129.3 | 7.85 (d, 8 Hz) | 125.8 (i1) |
| 6 | ── | 156.4 | ── | 133.5 | ── | 127.7 (i2) |
| 7 | ── | 14.9 | ── | 14.3 | ── | 14.2 |
| 8 | 2.12 (m, 5.3 Hz) | 31.0 | 1.92 (m, 5.1 Hz) | 31.1 | 2.01 (m, 5.4 Hz) | 30.8 |
| 9 | 1.93 (d, 5.3 Hz) | 26.7 | 1.41 (d, 5.1 Hz) | 24.5 | 1.37 (d, 5.4 Hz) | 23.5 |
| 10 | 1.94 (d, 5.3 Hz) | 26.7 | 1.47 (d, 5.1 Hz) | 25.3 | 1.41 (d, 5.4 Hz) | 26.2 |
a5 mg of sample was dissolved in 0.5 ml of DMSO-d6 for recording the spectra at 20°C on Bruker Avance AQS 500 MHz NMR spectrometer. All the details are mentioned in Materials and Methods. s-singlet, d-doublet, m-multiplet and i-interchangeable
Fig. 1.
Structures of thymoquinone, thymohydroquinone, thymol and dithymoquinone
Compound 2 showed methyl groups attached to an aromatic ring and an isopropyl group attached to an aromatic ring at 14.3 ppm and 31.1 ppm (1H 1.92 ppm), 24.5 ppm (1H 1.41 ppm), 25.3 ppm (1H 1.47 ppm) respectively with a characteristic coupling constant value of 5.1 Hz. Two aromatic protons were detected at 5.30 ppm and 7.03 ppm (Table 2). All these showed that compound 2 could be thymohydroquinone (Fig. 1).
Compound 3 showed the characteristic thymol 1H and 13C NMR characteristics. Two ortho coupled signals were detected at 7.47 ppm and 7.85 ppm with a characteristic ortho coupling constant of 8.0 Hz. A single aromatic peak was also detected at 6.72 ppm. Phenolic carbon was observed at 154.1 ppm. An isopropyl group attached to an aromatic ring (30.8 ppm, 23.5 ppm and 26.2 ppm) was also observed (Table 2). Thus, compound 3 clearly showed the characteristic of thymol (Fig. 1).
Two dimentional NMR characteristics of compound 4 showed clearly fusion of two thymoquinone rings with two alicyclic methyl groups (at 1.07 ppm and 1.06 ppm) along with two isopropyl groups attached to the quinone ring. Only two aromatic protons were detected at 5.4 ppm. Four keto groups were detected at 172.9 ppm and 192.7 ppm clearly indicating the fusion of two thymoquinone moieties (Table 3). Thus, compound 4 was identified to be dithymoquinone (Fig. 1).
Table 3.
1H and 13C 2D-HSQCT NMR data of compound 4a in DMSO-d6
| Carbon number | Chemical shifts (ppm) Compound 4 | |
|---|---|---|
| 1H NMR (J in Hz) | 13C NMR | |
| 1 | ── | 59.7 (i1) |
| 2 | 4.59 (s) | 59.4 (i1) |
| 3 | ── | 172.9 (i2) |
| 4 | ── | 192.7 (i2) |
| 5 | 5.40 (s) | 129.8 (i3) |
| 6 | ── | 154.1 (i4) |
| 7 | ── | 59.6 (i1) |
| 8 | 4.02 (s) | 59.4 (i1) |
| 9 | ── | 172.9 (i2) |
| 10 | ── | 192.7 (i2) |
| 11 | 5.40 (s) | 130.3 (i3) |
| 12 | ── | 153.0 (i4) |
| 13 | 1.07 (s) | 22.0 (i5) |
| 14 | 1.06 (s) | 20.9 (i5) |
| 15 | 2.27 (m, 5.3 Hz) | 33.6 |
| 16 | 1.18 (d, 5.3 Hz) | 29.2 |
| 17 | 1.17 (d, 5.3 Hz) | 29.1 |
| 18 | 2.26 (m, 5.5 Hz) | 33.4 |
| 19 | 1.16 (d, 5.5 Hz) | 28.6 |
| 20 | 1.15 (d, 5.5 Hz) | 28.5 |
a5 mg of sample was dissolved in 0.5 ml of DMSO-d6 for recording the spectra at 20°C on Bruker Avance AQS 500 MHz NMR spectrometer. All the details are mentioned in Materials and Methods. s-singlet, d-doublet, m-multiplet and i-interchangeable
This study showed that HD SFE gives an essential oil of better quality especially in terms of richness in quinones including better recovery of main compounds of thymoquinone, γ-terpinene, thymol, β-caryophyllene and thymohydroquinone than the extracts obtained by SFE alone. More than 50% of the essential oil compounds of Nigella sativa were due to thymoquinone and ρ-cymene (Machmudah et al. 2005). It is interesting to observe that ρ-cymene was not detected here because the composition of ρ-cymene in the extract was may be too low. There are some variations in the qualitative and quantitative compositions of Nigella sativa essential oil in different regions of the world. Many factors can influence the essential oil composition of seeds including genetic and phenologic stage as well as environment effects and extraction methods (Reineccius 1994; Omidbaigi 1997; Gora et al. 2002).
Conclusion
Sixteen compounds were identified for the first time in hydrodistillate of SFE and supercritical CO2 extracts of Nigella sativa. To our knowledge, this is the first report on the chemical composition of essential oil obtained by hydrodistillation of SC CO2 extract of Nigella sativa seeds. This study has clearly brought out the possibilities to obtain higher percentage of valuable volatiles such as thymoquinone through the SC CO2 technology and proved its superiority to earlier reports in the literature.
Electronic supplementary material
(DOC 465 kb)
Acknowledgement
The first author acknowledges the management and Dr. H. Muhamed Mubarack, Advisor, School of Life Sciences of the RVS College of Arts and Science, Coimbatore, Tamil Nadu, India for sponsoring as a Teacher Research Fellow at CFTRI, Mysore. We thank the Director of the Central Food Technological Research Institute, (CSIR), Mysore, for providing all the facilities.
References
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. 4. Carol Stream, and IL: Allured; 2007. [Google Scholar]
- Akhondian J, Parsa A, Rakhshande H. The effect of Nigella sativa L. (black cumin seed) on intractable pediatric seizures. Med Sci Monit. 2007;13:555–559. [PubMed] [Google Scholar]
- Badary OA, Al-Shabanah OA, Nagi MN, Al-Rikabi AC, Almazar MMA. Inhibition of benzo (a) pyrene-induced forestomach carcinogenesis in mice by thymoquinone. Eur J Cancer Prev. 1999;8:435–440. doi: 10.1097/00008469-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Badary OA, Gamal El-Din AM. Inhibitory effects of thymoquinone against 20-methyl cholanthrene-induced fibrosarcoma tumorigenesis. Cancer Detec Prev. 2001;25:362–368. [PubMed] [Google Scholar]
- Badary OA, Taha RA, Gamal El-Din AM, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003;26:87–98. doi: 10.1081/DCT-120020404. [DOI] [PubMed] [Google Scholar]
- Badary OA, Abd-Ellah MF, El-Mahdy MA, Salama SA, Hamada FM. Anticlastogenic activity of thymoquinone against benzo (a) pyrene in mice. Food Chem Toxicol. 2007;45:88–92. doi: 10.1016/j.fct.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Benkaci-Ali F, Baaliouamer A, Meklati BY, Chemat F. Chemical composition of seed essential oils from Algerian Nigella sativa extracted by microwave and hydrodistillation. Flavour Fragr J. 2007;22:148–153. doi: 10.1002/ffj.1773. [DOI] [Google Scholar]
- Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–328. doi: 10.1002/1099-1573(200008)14:5<323::AID-PTR621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A, Emerson MR, LeVine SM. Heme oxygenase 1 in SJL mice with experimental allergic encephalomyelitis. Mult Scler. 2003;9:372–381. doi: 10.1191/1352458503ms928oa. [DOI] [PubMed] [Google Scholar]
- El-Dakhakhny M. Studies on the chemical constitution of Egyptian Nigella sativa L. seeds II the essential oil. Planta Med. 1963;11:465–470. doi: 10.1055/s-0028-1100266. [DOI] [Google Scholar]
- El-Ghorab AH. Supercritical fluid extraction of the Egyptian rosemary (Rosmarinus officinalis) leaves and Nigella sativa L. seeds volatile oils and their antioxidant activities. J Essent Oil Bear Plants. 2003;6:67–77. [Google Scholar]
- Gali-Muhtasib H, Diab-Assaf M, Boltze C, Al-Hmaira J, Hartig R, Roessner A. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int J Oncol. 2004;25:857–866. [PubMed] [Google Scholar]
- Gora J, Lis A, Kula J, Staniszewska M, Woloszyn A (2002) Chemical composition variability of essential oils in the ontogenesis of some plants. Flav Fragr J 445–451
- Hajhashemi V, Ghannadi A, Jafarabadi H. Black cumin seed essential oil, as a potent analgesic and anti-inflammatory drug. Phytother Res. 2004;18:195–199. doi: 10.1002/ptr.1390. [DOI] [PubMed] [Google Scholar]
- Haq A, Lobo PI, Al-Tulfail M, Rama NR, Al-Sedairy ST. Immunomodulatory effect of Nigella sativa proteins fractionated by ion exchange chromatography. Int J Immunopharmacol. 1999;21:283–295. doi: 10.1016/S0192-0561(99)00010-7. [DOI] [PubMed] [Google Scholar]
- Joulain D, Konig WA. The Atlas of spectral data of sesquiterpene hydrocarbons. Hamburg: E.B.-Verlag; 1998. [Google Scholar]
- Kanter M. Effects of Nigella sativa and its major constituent, thymoquinone on sciatic nerves in experimental diabetic neuropathy. Neurochem Res. 2008;33:87–96. doi: 10.1007/s11064-007-9419-5. [DOI] [PubMed] [Google Scholar]
- Machmudah S, Shiramizu Y, Goto M, Sasaki M, Hirose T. Extraction of Nigella sativa L. using supercritical CO2: a study of antioxidant activity of the extract. Sep Sci Technol. 2005;40:1267–1275. doi: 10.1081/SS-200053005. [DOI] [Google Scholar]
- Mansour MA, Nagi MN, El-Khatib AS, Al-Bekairi AM. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: a possible mechanism of action. Cell Biochem Funct. 2002;20:143–151. doi: 10.1002/cbf.968. [DOI] [PubMed] [Google Scholar]
- Morikawa T, Xu F, Kashima Y, Matsuda H, Ninomiya K, Yoshikawa M. Novel dolabellane-type diterpene alkaloids with lipid metabolism promoting activities from the seeds of Nigella sativa. Org Lett. 2004;6:869–872. doi: 10.1021/ol036239c. [DOI] [PubMed] [Google Scholar]
- Mutabagani A, El-Mehdy SAM. A study of the antiinflammatory activity of Nigella sativa and thymoquinone in rats. Saudi Pharm J. 1997;5:110–113. [Google Scholar]
- Omidbaigi R (1997) Approaches to production and processing of medicinal plants, vol. 2. Tarrahane Nashr Public, Tehran, p 14, 70–78, 166–184
- Ravindran J, Nair HB, Sung B, Prasad S, Tekmal RR, Aggarwal BB. Thymoquinone poly (lactide-co-glycolide) nanoparticles exhibit enhanced anti-proliferative, anti-inflammatory, and chemosensitization potential. Biochem Pharmacol. 2010;79:1640–1647. doi: 10.1016/j.bcp.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Reineccius G (1994) Source Book of Flavors. Chapman & Hall. London, p73
- Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Topozada HH, Masloum H, El-Dakhakhany M. The anti-bacterial properties of Nigella sativa seeds: active principle with some clinical application. J Egypt Med Assoc. 1965;48(suppl):187–202. [PubMed] [Google Scholar]
- Udaya Sankar K. Studies on physico-chemical characteristic of essential oil of pepper (Pipernigrum L.) obtained by supercritical carbon dioxide. J Sci Food Agric. 1989;48:105–112. [Google Scholar]
- Worthen DR, Ghosheh OA, Crooks PA. The in vitro anti-tumor activity of some crude and purified components of black seed, Nigella sativa. Anticancer Res. 1998;18:1527–1532. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 465 kb)