Abstract
Defatted seed flours of Lagenaria siceraria (calabash and bottle gourd) were fractionated into their major protein fractions. The amino acid composition of seed flours and their protein fractions were determined and the protein quality was evaluated. Glutamic acid (139–168 mg/g protein) was the most abundant amino acid followed by aspartic acid (89.0–116 mg/g protein) in both the seed flours and their protein fractions. The total essential amino acid ranged from 45.8 to 51.5%. The predicted protein efficiency ratio and the predicted biological value ranged from 2.4 to 2.9 and 8.7 to 44.0, respectively. Lysine and sulphur amino acids were mostly concentrated in the globulin fractions. The first and second limiting amino acids in seed flours and protein fractions were methionine and valine or threonine. The seed flours contained adequate essential amino acids required by growing school children and adults. The seed has potential as protein supplement in cereal based complementary diets or in the replacement of animal proteins in conventional foods.
Keywords: Lagenaria siceraria, Calabash, Bottle gourd Protein fractions, Amino acid composition
Introduction
Studies on the utilization of vegetable proteins continue to attract attention globally due to the increasing demand for cheap and affordable dietary proteins, particularly among the low income group. Projections based on the current trends indicate a gap between human population and protein supply (Vijayakumari et al. 1997). Hence, the need to examine unconventional legumes and oilseeds as alternative protein sources for the future (Egbe and Akinyele 1990; Onweluzo et al. 1994; Chau and Cheung 1998; Fagbemi 2007). This development has stimulated research on the utilization of Lagenaria siceraria, an indigenous underutilized oil rich seed as alternate protein source.
Lagenaria siceraria (calabash and bottle gourd) belongs to Cucurbitaceae family. The plants are annual, herbaceous, and monoecious with creeping stems. The seeds are edible and used in the preparation of local soups, fermented food product (ogiri), fried cake (robo) and pudding (igbalo or ugbaotiri). The seeds of Lagenaria siceraria are rich in dietary proteins (Fokou et al. 2004).
Researchers have worked extensively on other species of Cucurbitaceae such as Colocynthis citrullus, Citrullus vulgaris and Telfairia occidentalis (Akobundu et al. 1982; Sathe et al. 1982; Ige et al. 1984; Fagbemi and Oshodi 1991; Fagbemi et al. 2005, 2006; Fagbemi 2007). Report on Lagenaria siceraria has been limited to its proximate composition and functional properties (Fokou et al. 2004). The knowledge of its amino acid composition and amino acid profile is necessary in food product formulations. This investigation was aimed at fractionation of Lagenaria siceraria seed flour into their major protein fractions and determination of their amino acid composition to further exploit the seeds potential use in food system.
Materials and methods
Two varieties of Lagenaria siceraria (calabash seed LS1 and bottle gourd seed LS2) were bought from farmers in Irele Ekiti, Ekiti State, Nigeria. The seeds were manually shelled, washed and later dried in a hot air oven at 50 °C. The seeds were pulverized using a Brabender blender, defatted by refluxing continuously for 8 h using n-hexane. Defatted meals were dried, pulverized and sieved to pass through a 500 μm sieve.
Protein fractions were extracted according to their solubility in different solvents as described by Wisal et al. (2003). Defatted Lagenaria siceraria seed flour (3.5 g) was extracted twice with 50 ml of distilled water for 30 min at room temperature (28 ± 2 °C). The extract was centrifuged at 4,500 rpm for 20 min and the supernatant was used for the determination of water soluble albumin. The residue was then extracted successively in a similar manner with 1 M NaCl, or 1 M NaOH solution, extract was collected separately and used to estimate the salt soluble (globulin) or alkali soluble (glutelin) fractions.
The amino acid profiles of the seed flours and protein fractions were determined using ion exchange chromatography. The samples were defatted, hydrolyzed and evaporated in a rotatory evaporator and then injected into the Technicon sequential multisampling Amino Acid Analyzer (Technicon Instrument Co. Ltd., United Kingdom), (Adeyeye and Afolabi 2004). Tryptophan content of the seed flours was determined using the method of Concon (1975) as modified by Ogunsua (1988). The amino acids obtained were used to evaluate the protein quality of seed flour. Predicted biological value (BV) was calculated using the regression equation of Morup and Olesen (1976) as reported by Chavan et al. (2001).
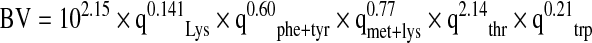 |
Where,
-
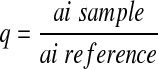 for ai sample ≤ ai reference
for ai sample ≤ ai referenceor
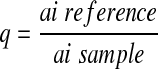 for ai sample ≥ ai reference
for ai sample ≥ ai referenceai = mg of the amino acid per g of total essential amino acids.
The predicted protein efficiency ratio (PER) was calculated using one of the equations developed by Alsmeyer et al. (1974) as stated below.
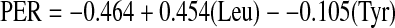 |
Isoelectric point (IP) was estimated from the amino acids using the equation of the form given by Olaofe and Akintayo (2000).
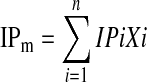 |
where, IP is the isoelectric point of the ith amino acid in the mixture, Xi is the mass or mole fraction of the ith amino acid in the mixture and IPm is the isoelectric point of the mixture.
Determinations were carried out in triplicate, along with standard deviations. Data were subjected to analysis of variance using SPSS 15 computer programme.
Results and discussion
Variety and fractionation have significant (p < 0.05) effect on the amino acid composition of Lagenaria siceraria seed flours (Table 1). Glutamic acid was the most abundant amino acid in both seed flours and all protein fractions. The values ranged from 140 to 168 mg/g protein with minimum value in LS2 globulin fraction and maximum in LS1 albumin fraction. The second most abundant amino acid in all the seed flours was aspartic acid ranging from 89.0 to 116 mg/g protein in LS2 albumin and seed flour, respectively. Mora-Escobedo et al. (1990) reported similar observation for the amino acids of the albumin and globulin fractions of amaranth. Oshodi et al. (1998) reported tryptophan to be the most concentrated amino acid in legumes. The most concentrated essential amino acid in all seed flours and their protein fractions was leucine with values ranging from 60.0 to 72.1 mg/g protein (LS2 albumin and the seed flour, respectively).
Table 1.
Amino acid composition of total seed flours and protein fractions of Lagenaria siceraria (mg/g protein) variety
| Amino acid | LS1 | LS2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Seed flour | Albumin | Globulin | Glutelin | Seed flour | Albumin | Globulin | Glutelin | |
| Cystine * | 12.3 ± 0.08c | 10.6 ± 0.08d | 13.9 ± 0.08a | 10.6 ± 0.08d | 12.5 ± 0.08b | 9.9 ± 0.08e | 12.6 ± 0.08b | 10.5 ± 0.08d |
| Methionine* | 10.4 ± 0.16c | 7.0 ± 0.08a | 11.2 ± 0.08b | 8.3 ± 0.08f | 12.5 ± 0.08a | 8.8 ± 0.08e | 12.6 ± 0.08a | 9.1 ± 0.08d |
| Aspartic acid | 109 ± 0.08d | 99.7 ± 0.16f | 112 ± 0.33c | 108 ± 0.24e | 116 ± 0.16a | 89.0 ± 0.33 g | 109 ± 0.33d | 113 ± 0.33b |
| Threonine* | 29.1 ± 0.08e | 21.6 ± 0.08 h | 25.0 ± 0.08 g | 30.1 ± 0.08d | 32.6 ± 0.16c | 25.9 ± 0.08f | 36.2 ± 0.08b | 41.1 ± 0.08a |
| Serine | 40.4 ± 0.04c | 31.7 ± 0.16 h | 39.1 ± 0.08d | 32.0 ± 0.08 g | 50.4 ± 0.08a | 32.8 ± 0.08f | 46.6 ± 0.16b | 38.0 ± 0.08e |
| Glutamic acid | 166 ± 0.24b | 168 ± 0.24a | 152 ± 0.41d | 161 ± 0.41c | 143 ± 0.16f | 149 ± 0.16e | 140 ± 0.41 g | 148 ± 0.24e |
| Proline | 37.5 ± 0.08a | 30.8 ± 0.08e | 34.2 ± 0.08b | 32.0 ± 0.08d | 32.5 ± 0.08c | 29.0 ± 0.08 g | 30.1 ± 0.08f | 28.0 ± 0.08 h |
| Glycine | 31.7 ± 0.08 g | 40.6 ± 0.16b | 40.1 ± 0.08c | 38.9 ± 0.08d | 32.4 ± 0.04f | 40.3 ± 0.08c | 35.0 ± 0.08e | 47.0 ± 0.16a |
| Alanine | 40.6 ± 0.08ab | 40.1 ± 0.08b | 36.3 ± 0.08e | 37.2 ± 0.08d | 32.6 ± 0.08 g | 34.7 ± 0.08f | 40.9 ± 0.08a | 37.9 ± 0.08c |
| Valine* | 40.6 ± 0.08b | 30.2 ± 0.08 g | 43.6 ± 0.08a | 39.2 ± 0.16d | 40.0 ± 0.08c | 31.1 ± 0.08f | 40.2 ± 0.08c | 32.5 ± 0.08e |
| Isoleucine* | 33.0 ± 0.08d | 35.1 ± 0.08b | 35.5 ± 0.08a | 30.4 ± 0.08f | 35.6 ± 0.08a | 28.2 ± 0.08 g | 31.4 ± 0.08e | 34.5 ± 0.08c |
| Phenylalanine* | 46.3 ± 0.08b | 32.9 ± 0.08 g | 41.4 ± 0.16d | 36.3 ± 0.08f | 48.8 ± 0.08a | 37.2 ± 0.08e | 45.6 ± 0.16c | 37.2 ± 0.08e |
| Lysine* | 56.2 ± 0.16c | 42.4 ± 0.16 g | 60.1 ± 0.24a | 57.2 ± 0.016b | 50.8 ± 0.16e | 37.5 ± 0.08 h | 52.6 ± 0.16d | 48.9 ± 0.16f |
| Arginine* | 58.6 ± 0.16d | 50.2 ± 0.16 g | 62.9 ± 0.24a | 60.3 ± 0.24b | 55.2 ± 0.16e | 49.4 ± 0.08 h | 54.4 ± 0.16f | 59.6 ± 0.16c |
| Histidine* | 26.1 ± 0.08a | 16.3 ± 0.08 g | 24.4 ± 0.08b | 22.4 ± 0.08e | 23.0 ± 0.08e | 16.9 ± 0.08f | 22.6 ± 0.08d | 24.5 ± 0.08b |
| Leucine* | 65.8 ± 0.016e | 65.0 ± 0.24f | 71.1 ± 0.16b | 66.2 ± 0.16d | 72.1 ± 0.16a | 60.0 ± 0.16 h | 68.3 ± 0.08c | 61.5 ± 0.21 g |
| Tyrosine* | 34.9 ± 0.08b | 22.5 ± 0.08 g | 35.6 ± 0.08a | 22.5 ± 0.08 g | 30.1 ± 0.04d | 27.4 ± 0.08f | 30.5 ± 0.08c | 29.0 ± 0.08e |
| Tryptophan* | 11.9 ± 0.16c | 13.0 ± 0.16b | 13.9 ± 0.24a | 10.6 ± 0.08d | 9.9 ± 0.08e | 8.1 ± 0.08f | 10.8 ± 0.16d | 10.7 ± 0.16d |
| Cal. Isoelectric point | 5.1 | 4.5 | 5.1 | 4.9 | 4.9 | 4.3 | 4.9 | 4.9 |
*Essential amino acids; values followed by different letters in the same row are significantly different (p < 0.05); LS1: Calabash seed flours; LS2: Bottle gourd seed flours
The lysine content of Lagenaria siceraria seed flours and their protein fractions ranged between 37.5 and 60.1 mg/g protein, this is similar to the lysine content of fluted pumpkin (37.5–66.6 mg/g cp) reported by Fagbemi (2007). The lysine content of seed flour is comparable with that of reference egg protein (63 mg/g crude protein, FAO/WHO/UNU 1985). Hence, Lagenaria siceraria seed flours and their protein fractions could be mixed with cereals like maize in weaning food formulation (Chavan et al. 2001). Globulins were however, richer in lysine than the water-soluble albumin or alkali soluble glutelin. This indicates that globulin fraction may be a better supplement in cereal based diet preparation. Tryptophan content of seed flours ranged from 8.1 to 13.9 mg/g protein. The calculated isoelectric point varied from 4.3 to 5.1 in LS2 albumin and LS1 seed flour respectively. This will serve as useful guide in quick precipitation of proteins from biological samples (Olaofe and Akintayo 2000).
The total amino acid content ranged from 715 to 851 mg/g protein (LS2 albumin and LS1 seed flour, Table 2). The total essential amino acid of Lagenaria siceraria flours ranged between 340 and 439 mg/g protein. This is lower than 566 mg/g protein reported for egg reference protein (Paul et al. 1980). It is, however comparable with values (190–503 mg/g protein) reported for some oilseeds such as Colocynthis citrullus, peanut meal and soybean flours (Lusas 1979; Akobundu et al. 1982; Sosulski 1983; Kuri et al. 1991). The range of percentage total essential amino acid (45.8–51.5) obtained for Lagenaria siceraria seed flour and their protein fractions is well above 36%, which is considered adequate for an ideal protein (FAO/WHO 1973). This suggests that seed flours and their protein fractions may find use as a food supplement. Globulin fraction had the highest percentage of total essential amino acid (51.0 and 51.5%) in all the protein fractions. The total sulphur amino acid content ranged from 17.6 to 25.2 mg/g protein with cystine ranging from 50.0 to 60.2%. The range of total neutral, acidic and basic amino acids were 49.1–53.8%, 30.4–35.4% and 14.4–17.4%, respectively, which showed that protein in seed flours and their protein fractions may be acidic in nature. Similar observation was reported by Aremu et al. (2006) for some Nigerian underutilized oilseeds.
Table 2.
Summary of amino acid composition of the total seed flour and protein fractions of Lagenaria siceraria (mg/g protein) variety
| LS1 | LS2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Seed flour | Albumin | Globulin | Glutelin | Seed flour | Albumin | Globulin | Glutelin | |
| Total amino acids (TAA) | 851 | 758 | 852 | 803 | 830 | 715 | 819 | 811 |
| Total essential amino acids (TEAA) | 426 | 347 | 439 | 394 | 423 | 340 | 418 | 399 |
| TEAA/TAA (%) | 49.9 | 45.8 | 51.5 | 49.1 | 51.0 | 47.6 | 51.0 | 49.2 |
| Total non essential amino acids (TNEAA) | 425 | 411 | 413 | 409 | 407 | 375 | 402 | 412 |
| Total sulphur amino acids (TSAA) | 22.7 | 17.6 | 25.1 | 18.9 | 25.0 | 18.7 | 25.2 | 19.6 |
| Cystine (%) in TSAA | 54.2 | 60.2 | 55.4 | 56.1 | 50.0 | 52.9 | 50.0 | 53.6 |
| Total aromatic essential amino acids phe.+tyr. (ArEAA) | 81.2 | 54.4 | 77.0 | 58.8 | 78.9 | 64.6 | 76.1 | 66.2 |
| Total acidic amino acids (TAAA)% Glu. + Asp. | 32.4 | 35.4 | 30.9 | 33.5 | 31.2 | 33.3 | 30.4 | 32.2 |
| Total basic amino acids (TBAA)% Lys. + Arg. + His. | 16.6 | 14.4 | 17.3 | 17.4 | 15.5 | 14.5 | 15.8 | 16.4 |
| Total neutral amino acids (TNAA)% | 50.0 | 50.2 | 51.8 | 49.1 | 53.3 | 52.2 | 53.8 | 51.4 |
| Ratio of TEAA:TNEAA | 1.0 | 0.8 | 1.1 | 1.0 | 1.0 | 0.9 | 1.0 | 1.0 |
| Predicted protein efficiency ratio (PER) | 2.5 | 2.7 | 2.8 | 2.7 | 2.9 | 2.4 | 2.7 | 2.4 |
| Predicted biological value (BV) | 27.1 | 8.7 | 21.0 | 23.4 | 31.4 | 12.3 | 40.2 | 44.0 |
LS1 Calabash seed flours; LS2 Bottle gourd seed flours
The predicted PER of Lagenaria siceraria seed flours and their protein fractions ranged from 2.4 (LS2 albumin and glutelin) to 2.9 (LS2 seed flour). The predicted PER values were higher than seed proteins of cowpea (1.21), pigeon pea (1.82) and L. sativus (negative value to 0.03) (Salunkhe and Kadam 1989). These values are also higher than the ranges of 0.66–1.24 and 0.63–2.21 for fluted pumpkin seed flours and cotton seed, respectively (Fagbemi 2007). The predicted BV of Lagenaria siceraria protein ranged between 8.7 and 44.0. Fractionation affected BV. Globulin and glutelin fractions have higher BV than their albumin fraction. The predicted BV of Lagenaria siceraria compared well with the range of 36.5–40.13 reported for beach pea protein isolates (Chavan et al. 2001)
The chemical score ranged from 21.9 (LS1 albumin) to 39.4 (LS2 globulin) (Table 3). The first limiting amino acid in both raw seed flours and their protein fractions was methionine (7.0–12.6 mg/g protein) followed by valine (30.2–43.6 mg/g protein) except in LS1 globulin fraction, where threonine (25.0 mg/g protein) was the second limiting amino acid. The recommended sulphur amino acid for infants, growing preschool children and growing school children are 42, 25 and 22 mg/g crude protein respectively (FAO/WHO/UNU 1985). The seed flours on this basis provide 41.9–60%; 70.4–100.8% and 80—over 100% of the recommended sulphur amino acid for infant, a growing preschool child and growing school child, respectively. Salt soluble proteins are found to be more concentrated in sulphur amino acid. In general, Lagenaria siceraria seed flours and their protein fractions contained adequate amounts of most of the essential amino acids required by preschool children and all amino acids essential for school children and adults.
Table 3.
Amino acid scores of the total seed flour and protein fractions of Lagenaria siceraria variety
| LS1 | LS2 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Essential amino acid | Referencea | Seed flour | Albumin | Globulin | Glutelin | Seed flour | Albumin | Globulin | Glutelin | ||||||||
| EAAC | AAS(%) | EAAC | AAS(%) | EAAC | AAS(%) | EAAC | AAS(%) | EAAC | AAS(%) | EAAC | AAS(%) | EAAC | AAS(%) | EAAC | AAS(%) | ||
| Cys. | 18.0 | 12.3 | 68.3 | 10.6 | 58.9 | 13.9 | 77.2 | 10.6 | 58.9 | 12.5 | 69.4 | 9.9 | 55.0 | 12.6 | 70.0 | 10.5 | 58.3 |
| Met. | 32.0 | 10.4 | 32.5 | 7.0 | 21.9 | 11.2 | 35.0 | 8.3 | 25.9 | 12.5 | 39.1 | 8.8 | 27.5 | 12.6 | 39.4 | 9.1 | 28.4 |
| Thre. | 51.0 | 29.1 | 57.1 | 21.6 | 42.4 | 25.0 | 49.0 | 30.1 | 59.0 | 32.6 | 63.9 | 25.9 | 50.8 | 36.2 | 70.9 | 41.1 | 80.6 |
| Val. | 76.0 | 40.6 | 53.4 | 30.2 | 39.7 | 43.6 | 57.4 | 39.2 | 51.6 | 40.0 | 52.6 | 31.1 | 40.9 | 40.2 | 52.9 | 32.5 | 42.8 |
| Iso. | 56.0 | 33.0 | 58.9 | 35.1 | 62.7 | 35.5 | 63.4 | 30.4 | 54.3 | 35.6 | 63.6 | 28.2 | 50.4 | 31.4 | 56.1 | 34.5 | 61.6 |
| Phe. | 51.0 | 46.3 | 90.8 | 32.9 | 64.5 | 41.4 | 81.2 | 36.3 | 71.2 | 48.8 | 95.7 | 37.2 | 72.9 | 45.6 | 89.4 | 37.2 | 72.9 |
| Lys. | 63.0 | 56.2 | 89.2 | 42.4 | 67.3 | 60.1 | 95.4 | 57.2 | 90.8 | 50.8 | 80.6 | 37.5 | 59.5 | 52.6 | 83.5 | 48.9 | 77.6 |
| Arg. | 61.0 | 58.6 | 96.1 | 50.2 | 82.3 | 62.9 | 103 | 60.3 | 98.9 | 55.2 | 90.5 | 49.4 | 80.9 | 54.4 | 89.2 | 59.6 | 97.7 |
| His. | 24.0 | 26.1 | 109 | 16.3 | 67.9 | 24.4 | 102 | 22.4 | 93.3 | 23.0 | 95.8 | 16.9 | 70.4 | 22.6 | 94.2 | 24.5 | 102 |
| Leu. | 83.0 | 65.8 | 79.3 | 65.0 | 78.3 | 71.1 | 85.7 | 66.2 | 79.8 | 72.1 | 86.9 | 60.0 | 72.3 | 68.3 | 82.3 | 61.5 | 74.1 |
| Tyr. | 40.0 | 34.9 | 87.3 | 22.5 | 56.3 | 35.6 | 89.0 | 22.5 | 56.3 | 30.1 | 75.3 | 27.4 | 68.5 | 30.5 | 76.3 | 29.0 | 72.5 |
| Trp. | 18.0 | 11.9 | 66.1 | 13.0 | 72.2 | 13.9 | 77.2 | 10.6 | 58.9 | 9.9 | 55.0 | 8.1 | 45.0 | 10.8 | 60.0 | 10.7 | 59.4 |
| Chemical score | 32.5 | 21.9 | 35.0 | 25.0 | 39.1 | 27.5 | 39.4 | 28.4 | |||||||||
| 1st limiting a. a | Methionine | Methionine | Methionine | Methionine | Methionine | Methionine | Methionine | Methionine | |||||||||
| 2nd limiting a. a | Valine | Valine | Threonine | Valine | Valine | Valine | Valine | Valine | |||||||||
aProvisional amino acid pattern egg as reference (FAO/WHO/UNU 1985). EAAC Essential amino acid composition (mg/g protein)
AAS Amino acid score; a.a amino acid; LS1 Calabash seed flours; LS2 Bottle gourd seed flours
Conclusion
Amino acid profile of Lagenaria siceraria seed flours and their water, salt and alkali (albumin, globulin and glutelin, respectively) soluble protein fractions are of high quality and very high in lysine. The percentage total essential amino acids in all flour samples were well above the recommended values. Lagenaria siceraria seed flours and their protein fractions may be incorporated into cereals for the formulation of a wide range of cereal based weaning foods and other complementary diets because of their high lysine content.
Contributor Information
Moriyike Esther Ogunbusola, Email: riikebusola@yahoo.com.
Tayo Nathaniel Fagbemi, Email: tnfagbemi55@yahoo.co.uk.
Oluwatooyin Faramade Osundahunsi, Email: tosundahunsi@yahoo.com.
References
- Adeyeye EI, Afolabi EO. Amino acid composition of three different types of land snails consumed in Nigeria. Food Chem. 2004;85:471–478. doi: 10.1016/S0308-8146(03)00247-4. [DOI] [Google Scholar]
- Akobundu ENT, Cherry JP, Simmons JG. Chemical, functional and nutritional properties of Egusi (Colocynthis citrullus L.) seed protein products. J Food Sci. 1982;47:829–835. doi: 10.1111/j.1365-2621.1982.tb12725.x. [DOI] [Google Scholar]
- Alsmeyer RH, Cunningham AE, Happich ML. Equations predict PER from amino acid analysis. Food Technol. 1974;28(7):34–38. [Google Scholar]
- Aremu MO, Olaofe O, Akintayo ET. Compositional evaluation of cowpea (Vigna unguiculata) and scarlet runner bean (Phaseolus coccineus) varieties grown in Nigeria. J Food Agric Environ. 2006;4:39–43. [Google Scholar]
- Chau CF, Cheung PCk. Functional properties of flours prepared from three Chinese indigenous legume seed. Food Chem. 1998;61:429–433. doi: 10.1016/S0308-8146(97)00091-5. [DOI] [PubMed] [Google Scholar]
- Chavan UD, Mckenzie DB, Shahidi F. Functional properties of proteins isolates from beach pea (Lathyrus maritimus L.) Food Chem. 2001;74:177–187. doi: 10.1016/S0308-8146(01)00123-6. [DOI] [Google Scholar]
- Concon JM. Rapid and simple method for the determination of tryptophan in cereal grains. Anal Biochem. 1975;67:206–219. doi: 10.1016/0003-2697(75)90288-2. [DOI] [PubMed] [Google Scholar]
- Egbe IA, Akinyele IO. Effect of cooking on the antinutritional factors of limabeans (Phaseolus lunatus) Food Chem. 1990;35:81–88. doi: 10.1016/0308-8146(90)90022-V. [DOI] [Google Scholar]
- Fagbemi TN. Effects of processing on the nutritional composition of fluted pumpkin (Telfairia occidentalis) seed flour. Nig Food J. 2007;25:1–22. [Google Scholar]
- Fagbemi TN, Oshodi AA. Chemical composition and functional properties of full fat and defatted fluted pumpkin seed flour (Telfairia occidentalis) Nig Food J. 1991;9:26–32. [Google Scholar]
- Fagbemi TN, Oshodi AA, Ipinmoroti KO. Processing effects on some antinutritional factors and in vitro multienzyme protein digestibility, (IVPD) of three tropical seeds. breadnut (Artocarpus altilis), cashewnut (Anacardium occidentale), fluted pumpkin (Telfairia occidentalis) Pakistan J Nutr. 2005;4:250–256. doi: 10.3923/pjn.2005.250.256. [DOI] [Google Scholar]
- Fagbemi TN, Oshodi AA, Ipinmoroti KO. Effects of processing on the functional properties of full fat and defatted fluted pumpkin (Telfairia accidentalis) seed flours. J Food Technol. 2006;4:70–79. [Google Scholar]
- FAO/WHO (1973) Energy and protein requirements. Report of FAO Nutritional meeting series, No 52. Rome, Italy
- FAO/WHO/UNU (1985) Energy and protein requirements. Report of a Joint FAO/WHO/UNU Expect consultation. WHO Technical Report series 742, WHO, Geneva
- Fokou E, Achu MB, Techounguel FM. Preliminary nutritional evaluation of five species of egusi seed in Cameroon. African J Food Agric Nut Dev Rural Outr Prog. 2004;4(1):1–7. [Google Scholar]
- Ige MM, Ogunsua AO, Oke OI. Functional properties of the protein of some Nigeria oil seeds: Conophor seeds and three varieties of melon seeds. J Agric Food Chem. 1984;32:822–825. doi: 10.1021/jf00124a031. [DOI] [Google Scholar]
- Kuri YE, Sunday RK, Kahuwi C, Jones GP, Rivett DE. Chemical composition of Mnerdics charantis L. fruits. J Agric Food Chem. 1991;39:1702–1703. [Google Scholar]
- Lusas EW. Food uses of peanut protein. J Am Oil Chem Soc. 1979;56:242–256. doi: 10.1007/BF02671530. [DOI] [PubMed] [Google Scholar]
- Mora-Escobedo R, Paredez-Lopez O, Ordorica-Falomir C. Characterization of albumins and globulins from amaranth. Lebensm Technol. 1990;23:484–487. [Google Scholar]
- Morup IK, Olesen ES. New method for prediction of protein value from essential amino acid pattern. Nutr. 1976;12:355–365. [Google Scholar]
- Ogunsua AO. Amino acid determination in conophor nut by gas—liquid chromatography. Food Chem. 1988;28:287–298. doi: 10.1016/0308-8146(88)90104-5. [DOI] [Google Scholar]
- Olaofe O, Akintayo ET. Prediction of isoeletric points of legume and oil seed proteins from amino acid composition. J Technol Sci. 2000;4:49–53. [Google Scholar]
- Onweluzo SC, Obanu ZA, Onuoha KC. Functional properties of some lesser known tropical legumes. J Food Sci Technol. 1994;31:302–306. doi: 10.1007/BF01089200. [DOI] [PubMed] [Google Scholar]
- Oshodi AA, Esuoso KO, Akintayo ET. Proximate and amino acid composition of some underutilized Nigerian legume flour and protein concentrates. La Riv Taliana Sostanze Grass. 1998;75:409–412. [Google Scholar]
- Paul AA, Southgate DAT, Russell J 1980. First supplement to McCance and Widdowsen’s the composition of foods. HMSO London, Elsevier, New York
- Salunkhe DK, Kadam SS. Handbook of world food legumes, nutritional chemistry, processing technology and utilization. Boca Raton: CRC; 1989. [Google Scholar]
- Sathe SK, Desphande SS, Salunhke DK. Functional properties of winged bean (Psophocarpus tetragonolobus L.) proteins. J Food Sci. 1982;47:483–508. [Google Scholar]
- Sosulski FW. Rapeseed proteins for food use. In: Hudson BJF, editor. Development in food protein, vol 2. London: Applied Science Publ; 1983. [Google Scholar]
- Vijayakumari K, Siddhuraju P, Janadhanan K. Chemical composition amino acid content and protein quality of the little known legume Bauhinia purpurea L. J Sci Food Agric. 1997;73:279–286. doi: 10.1002/(SICI)1097-0010(199703)73:3<279::AID-JSFA713>3.0.CO;2-H. [DOI] [Google Scholar]
- Wisal HI, Elfadil EB, Abdulahi HE. Fractionation, solubility and functional properties of wheat bran proteins as influenced by pH and/or salt concentration. Nahrung Food. 2003;47:425–429. doi: 10.1002/food.200390094. [DOI] [PubMed] [Google Scholar]


