Abstract
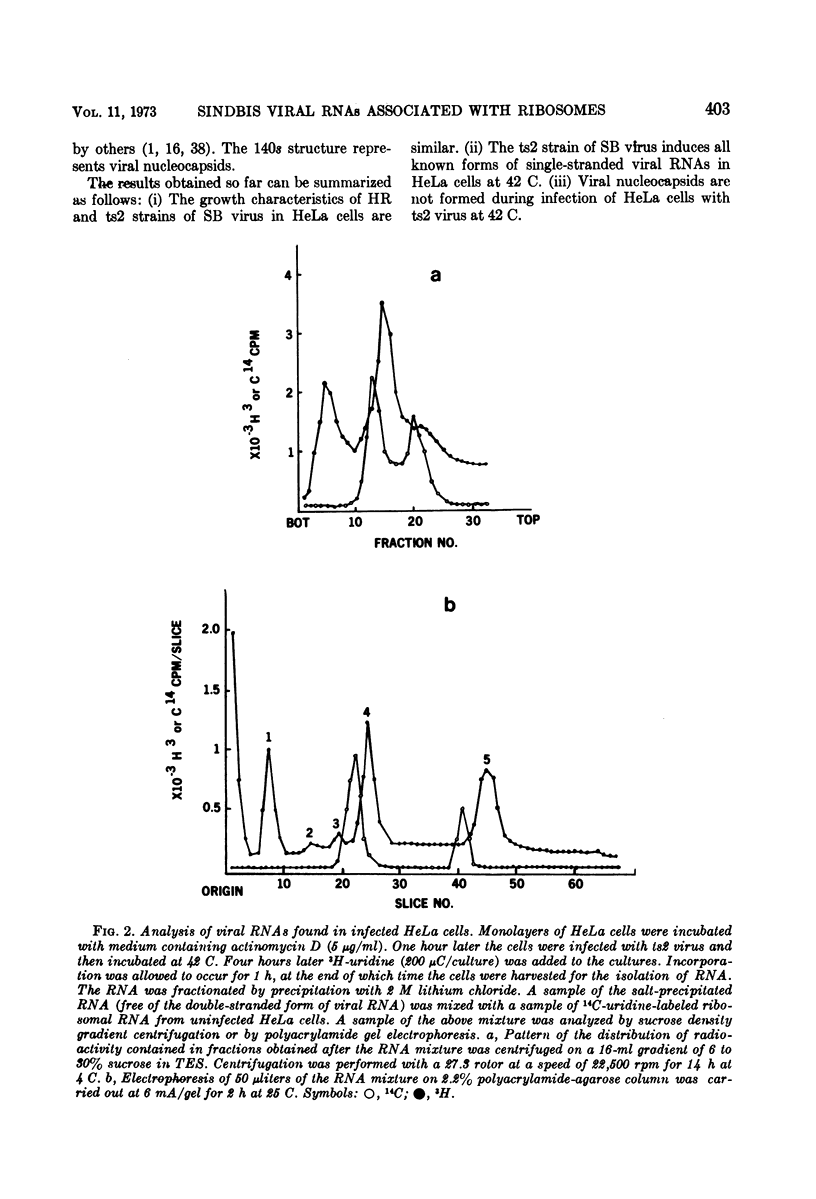
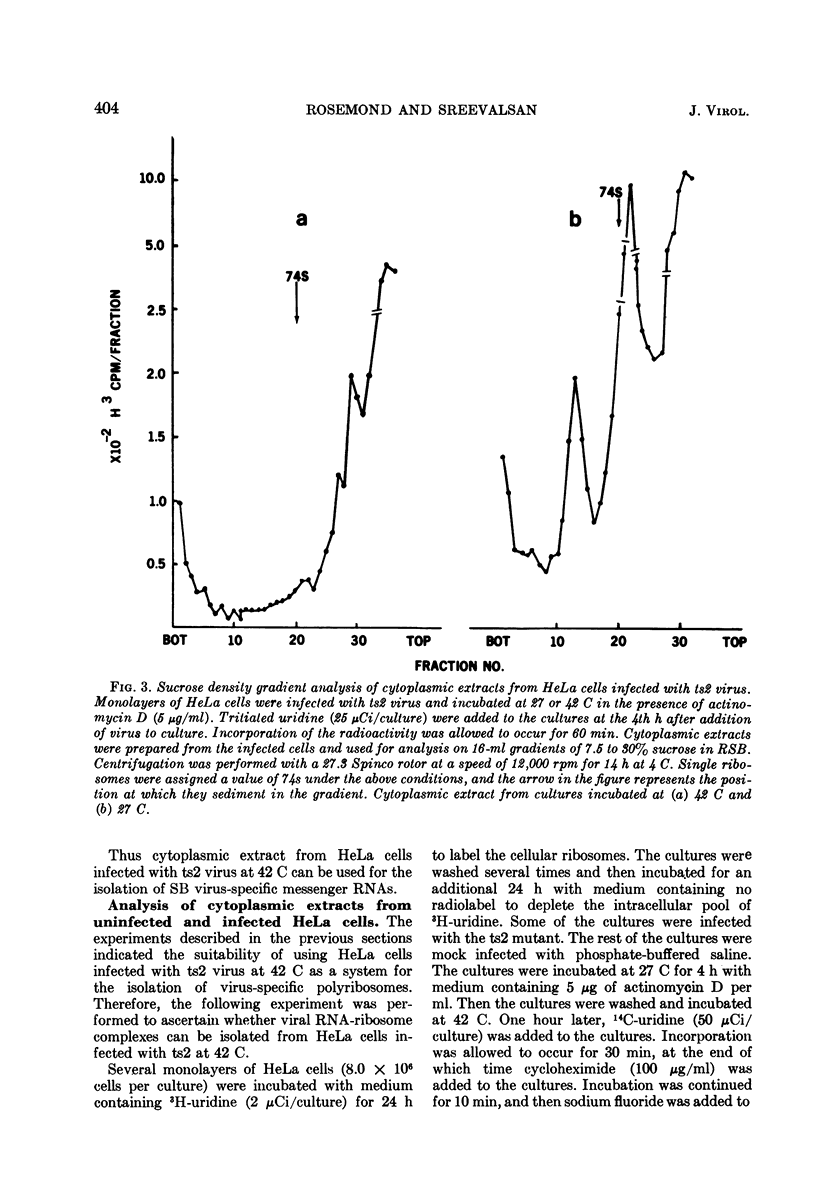
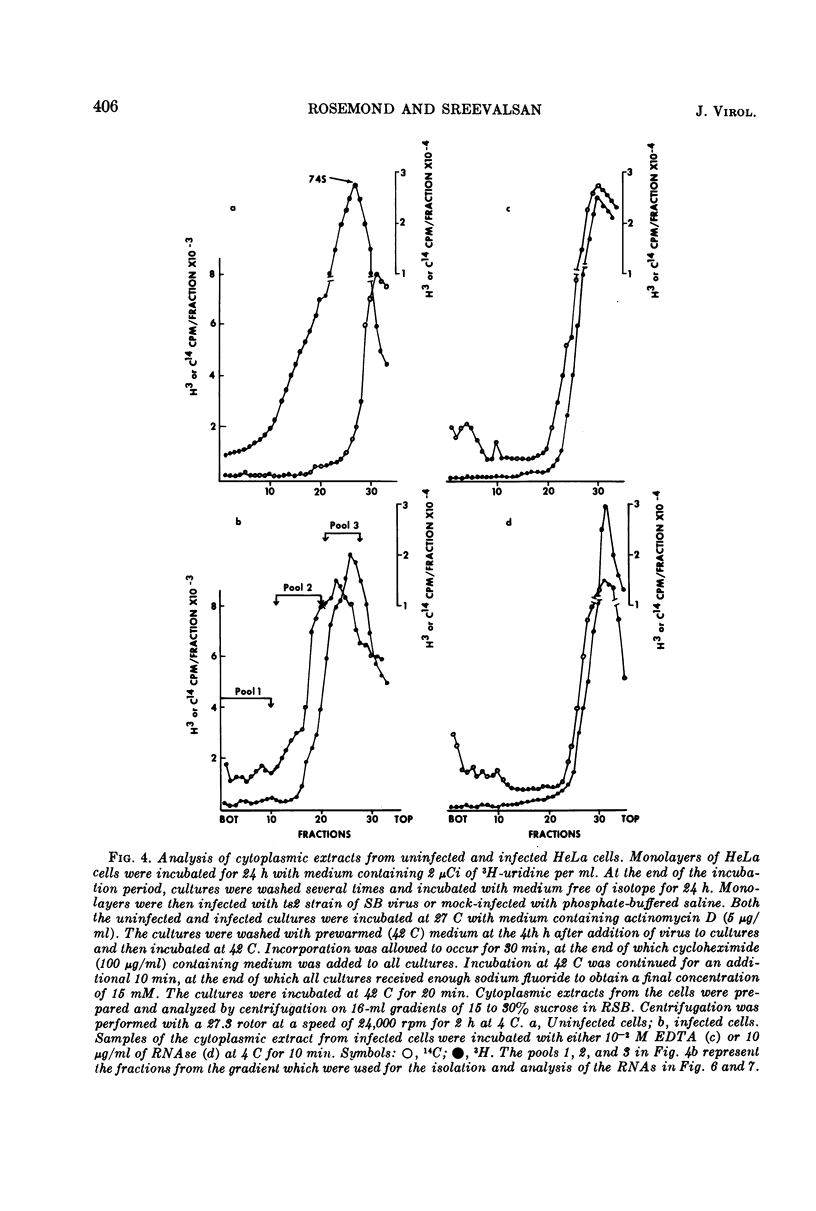
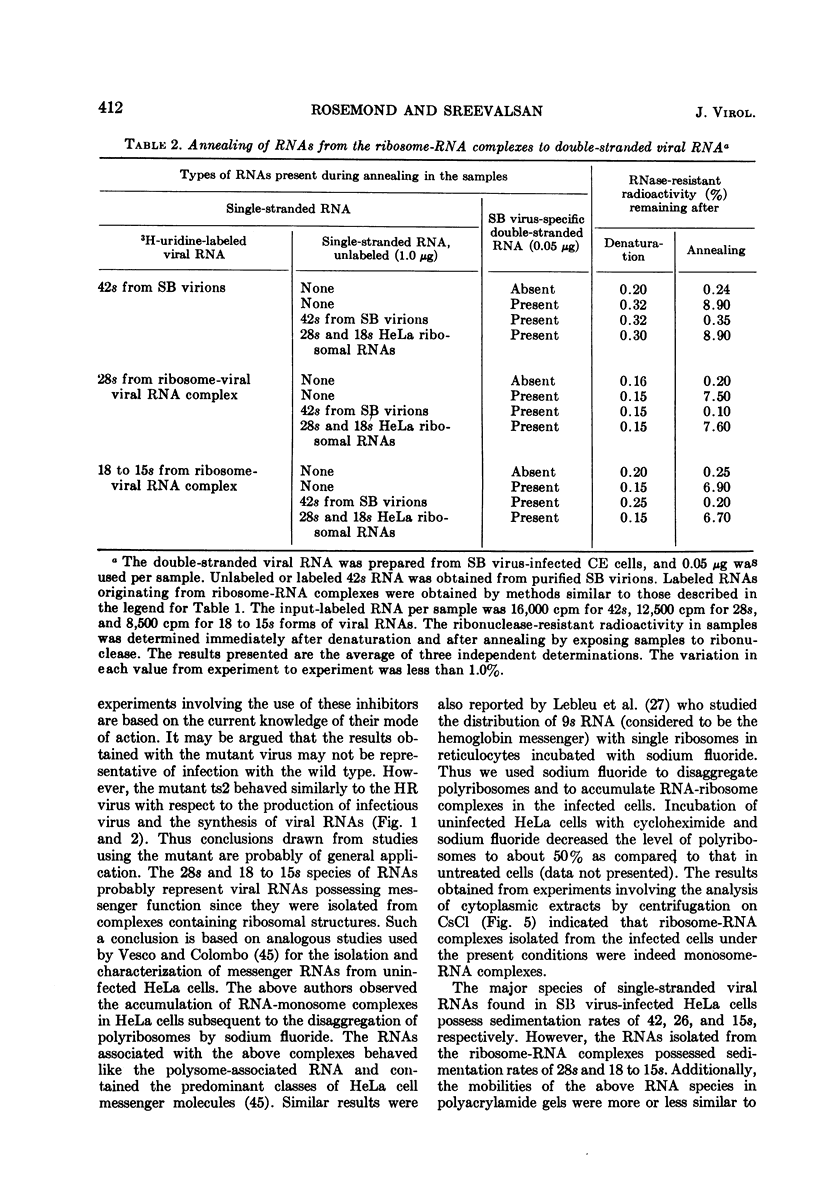
Virus specific RNA ribosome complexes were isolated by sucrose density gradient centrifugation of cytoplasmic extracts from HeLa cells infected at 42 C with an RNA+ mutant (ts2) of Sindbis virus. Viral RNA-ribosome complexes were accumulated by infected cells treated with sodium fluoride and cycloheximide. The RNA-ribosome complexes were characterized by (i) their sensitivity to the action of ribonuclease or ethylenediaminetetraacetic acid, (ii) their density in cesium chloride gradients, and (iii) presence of host ribosomes and viral RNAs. The viral RNAs were isolated and characterized. The results showed that two species of single-stranded RNAs (a 28s and 18 to 15s species) were associated with the complexes. Base composition analysis of the viral RNAs indicated that both species had a higher adenine content than the 42s or 26s forms of viral RNAs. The RNAs associated with the ribosome complexes were virus specific since they annealed with denatured double-stranded RNAs from the infected cells. Little or no 42S RNA was associated with the RNA-ribosome complexes. The results suggest that the 28s and 18 to 15s forms of RNAs may represent viral messenger RNAs.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Purification and properties of Semliki Forest virus nucleocapsids. Virology. 1970 Jun;41(2):306–320. doi: 10.1016/0042-6822(70)90083-8. [DOI] [PubMed] [Google Scholar]
- BUCKLEY S. M. APPLICABILITY OF THE HELA (GEY) STRAIN OF HUMAN MALIGNANT EPITHELIAL CELLS TO THE PROPAGATION OF ARBOVIRUSES. Proc Soc Exp Biol Med. 1964 Jun;116:354–358. doi: 10.3181/00379727-116-29246. [DOI] [PubMed] [Google Scholar]
- Billeter M. A., Weissmann C., Warner R. C. Replication of viral ribonucleic acid. IX. Properties of double-stranded RNA from Escherichia coli infected with bacteriophage MS2. J Mol Biol. 1966 May;17(1):145–173. doi: 10.1016/s0022-2836(66)80101-8. [DOI] [PubMed] [Google Scholar]
- Bruner R., Vinograd J. The evaluation of standard sedimentation coefficients of sodium RNA and sodium DNA from sedimentation velocity data in concentrated NaCl and CsCl solutions. Biochim Biophys Acta. 1965 Sep 6;108(1):18–29. doi: 10.1016/0005-2787(65)90104-8. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Functional defects of temperature-sensitive mutants of Sindbis virus. J Mol Biol. 1968 Jul 14;35(1):193–205. doi: 10.1016/s0022-2836(68)80047-6. [DOI] [PubMed] [Google Scholar]
- COLOMBO B., FELICETTI L., BAGLIONI C. INHIBITION OF PROTEIN SYNTHESIS BY CYCLOHEXIMIDE IN RABBIT RETICULOCYTES. Biochem Biophys Res Commun. 1965 Feb 3;18:389–395. doi: 10.1016/0006-291x(65)90719-9. [DOI] [PubMed] [Google Scholar]
- Cartwright K. L., Burke D. Virus nucleic acids formed in chick embryo cells infected with Semliki Forest virus. J Gen Virol. 1970 Feb;6(2):231–248. doi: 10.1099/0022-1317-6-2-231. [DOI] [PubMed] [Google Scholar]
- Chrambach A. Device for sectioning of polyacrylamide gel cylinders and its use in determining biological activity in the sections. Anal Biochem. 1966 Jun;15(3):544–548. doi: 10.1016/0003-2697(66)90121-7. [DOI] [PubMed] [Google Scholar]
- Colombo B., Vesco C., Baglioni C. Role of ribosomal subunits in protein synthesis in mammalian cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):651–658. doi: 10.1073/pnas.61.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Ribonucleic acids from animal cells. Bacteriol Rev. 1968 Sep;32(3):262–290. doi: 10.1128/br.32.3.262-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P., Faulkner P. Molecular weight of Sindbis virus ribonucleic acid as measured by polyacrylamide gel electrophoresis. J Virol. 1970 Jul;6(1):145–147. doi: 10.1128/jvi.6.1.145-147.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt D. L., Robertson H. D., Zinder N. D. In vitro translation of multistranded RNA from Escherichia coli infected by bacteriophage f-2. Proc Natl Acad Sci U S A. 1968 Mar;59(3):972–979. doi: 10.1073/pnas.59.3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Berezesky I. K. Cytoplasmic fractions associated with Semliki Forest virus ribonucleic acid replication. J Virol. 1967 Apr;1(2):374–383. doi: 10.1128/jvi.1.2.374-383.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Fantes K. H., Levy H. B., Carter W. B. Interferon action on parental Semliki forest virus ribonucleic acid. J Virol. 1967 Dec;1(6):1168–1173. doi: 10.1128/jvi.1.6.1168-1173.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Protein synthesis directed by an arbovirus. J Virol. 1968 Jan;2(1):26–32. doi: 10.1128/jvi.2.1.26-32.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Replicative intermediate of an arbovirus. J Virol. 1968 Jun;2(6):547–552. doi: 10.1128/jvi.2.6.547-552.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M., Baltimore D. The effect of HeLa cell cytoplasm on the rate of sedimentation of RNA. Proc Natl Acad Sci U S A. 1966 Sep;56(3):999–1002. doi: 10.1073/pnas.56.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU A. S., BOCK R. M., HALVORSON H. O. Separation of labeled from unlabeled proteins by equilibrium density gradient sedimentation. Anal Biochem. 1962 Dec;4:489–504. doi: 10.1016/0003-2697(62)90129-x. [DOI] [PubMed] [Google Scholar]
- Hogan B. L. he effect of inhibitors of protein synthesis on the level of ribosomal subunits in ascites cells. Biochim Biophys Acta. 1969 May 20;182(1):264–266. doi: 10.1016/0005-2787(69)90546-2. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Balitmore D. Initiation of polyribosome formation in poliovirus-infected HeLa cells. J Mol Biol. 1970 Feb 14;47(3):275–291. doi: 10.1016/0022-2836(70)90302-5. [DOI] [PubMed] [Google Scholar]
- Johnston R. E., Bose H. R. An adenylate-rich segment in the virion RNA of Sindbis virus. Biochem Biophys Res Commun. 1972 Jan 31;46(2):712–718. doi: 10.1016/s0006-291x(72)80198-0. [DOI] [PubMed] [Google Scholar]
- KATZ S., COMB D. G. A NEW METHOD FOR THE DETERMINATION OF THE BASE COMPOSITION OF RIBONUCLEIC ACID. J Biol Chem. 1963 Sep;238:3065–3067. [PubMed] [Google Scholar]
- Kennedy S. I. Isolation and identification of the virus-specified RNA species found on membrane-bound polyribosomes of chick embryo cells infected with Semliki Forest virus. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1254–1258. doi: 10.1016/0006-291x(72)90846-7. [DOI] [PubMed] [Google Scholar]
- Lebleu B., Huez G., Burny A., Marbaix G. Evidence for a naF-resistant association between mRNA and ribosomes in rabbit reticulocytes. Biochim Biophys Acta. 1967 Mar 29;138(1):186–188. doi: 10.1016/0005-2787(67)90599-0. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Friedman R. M. Analysis of arbovirus ribonucleic acid forms by polyacrylamide gel electrophoresis. J Virol. 1971 Apr;7(4):504–514. doi: 10.1128/jvi.7.4.504-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Burka E. R., Conconi F. M., Perl W., Rifkind R. A. Polyribosome dissociation and formation in intact reticulocytes with conservation of messenger ribonucleic acid. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1437–1443. doi: 10.1073/pnas.53.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble J., Kass S. J., Levintow L. Analysis of poliovirus-specific RNA in infected HeLa cells by polyacrylamide gel electrophoresis. Virology. 1969 Apr;37(4):535–544. doi: 10.1016/0042-6822(69)90271-2. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Buoyant densities of cytoplasmic ribonucleoprotein particles of mammalian cells: distinctive character of ribosome subunits and the rapidly labeled components. J Mol Biol. 1966 Apr;16(2):255–268. doi: 10.1016/s0022-2836(66)80171-7. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Virus-specific proteins synthesized in cells infected with RNA+ temperature-sensitive mutants of Sindbis virus. J Virol. 1970 Mar;5(3):329–337. doi: 10.1128/jvi.5.3.329-337.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin A. S., Belitsina N. V., Lerman M. I. Use of formaldehyde fixation for studies of ribonucleoprotein particles by caesium chloride density-gradient centrifugation. J Mol Biol. 1965 Dec;14(2):611–615. doi: 10.1016/s0022-2836(65)80213-3. [DOI] [PubMed] [Google Scholar]
- Sreevalsan T., Allen P. T. Replication of Western equine encephalomyelitis virus. II. Cytoplasmic structure involved in the synthesis and development of the virions. J Virol. 1968 Oct;2(10):1038–1046. doi: 10.1128/jvi.2.10.1038-1046.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevalsan T. Association of viral ribonucleic acid with cellular membranes in chick embryo cells infected with Sindbis virus. J Virol. 1970 Oct;6(4):438–444. doi: 10.1128/jvi.6.4.438-444.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevalsan T., Lockart R. Z., Jr, Dodson M. L., Jr, Hartman K. A. Replication of Western equine encephalomyelitis virus. I. Some chemical and physical characteristics of viral ribonucleic acid. J Virol. 1968 Jun;2(6):558–566. doi: 10.1128/jvi.2.6.558-566.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevalsan T., Lockart R. Z., Jr Heterogeneous RNA's occurring during the replication of Western equine encephalomyelitis virus. Proc Natl Acad Sci U S A. 1966 Apr;55(4):974–981. doi: 10.1073/pnas.55.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevalsan T., Yin F. H. Sindbis virus-induced viral ribonucleic acid polymerase. J Virol. 1969 Jun;3(6):599–604. doi: 10.1128/jvi.3.6.599-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Levintow L. Constitution and function of polyribosomes of poliovirus-infected HeLa cells. Virology. 1965 Sep;27(1):44–53. doi: 10.1016/0042-6822(65)90142-x. [DOI] [PubMed] [Google Scholar]
- Vesco C., Colombo B. Effect of sodium fluoride on protein synthesis in HeLa cells: inhibition of ribosome dissociation. J Mol Biol. 1970 Feb 14;47(3):335–352. doi: 10.1016/0022-2836(70)90306-2. [DOI] [PubMed] [Google Scholar]
- WECKER E., SCHONNE E. Inhibition of viral RNA synthesis by parafluorophenylalanine. Proc Natl Acad Sci U S A. 1961 Mar 15;47:278–282. doi: 10.1073/pnas.47.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A. R., Askonas B. A. Biosynthesis of immunoglobulins: the separate classes of polyribosomes synthesizing heavy and light chains. J Mol Biol. 1967 Jan 28;23(2):201–216. doi: 10.1016/s0022-2836(67)80027-5. [DOI] [PubMed] [Google Scholar]
- Yin F. H., Lockart R. Z., Jr Maturation defects in temperature-sensitive mutants of Sindbis virus. J Virol. 1968 Jul;2(7):728–737. doi: 10.1128/jvi.2.7.728-737.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



