Abstract
Banana (Musa sp var. ‘Robusta’) stored under active and passive modified atmosphere packaging (MAP) at 12 ± 1°C and 85–90% RH for 2 seasons were evaluated for fruit quality and shelf-life. A steady state of about 8.6 and 8.2% of CO2 and 2.8 and 2.6% of O2 in passive MAP and MAP+GK (Green Keeper) packages, respectively, were established after 3 weeks of storage. Passive MAP and MAP+GK treatments of banana resulted in reduction in physiological loss in weight (PLW) of 0.7 and 0.8% after 5 and 7 weeks of storage, respectively as against 5% PLW in openly kept green banana after 3 weeks. Both MAP and MAP+GK treatments delayed colour, texture, pulp to peel ratio and total soluble solids (TSS) content as compared to openly kept control banana. Results indicated that the shelf life of fruits packed under MAP and MAP+GK could be extended up to 5 and 7 weeks, respectively as compared to 3 weeks for openly kept control fruits. Sensory quality of fully ripe fruits of both passive MAP and MAP+GK treatments, 5 days after ethrel dip was very good. Thus, MAP+GK at 12 ± 1°C and 85–90% RH could be commercially used for long term storage and long distance transportation of banana with maximum shelf-life of 7 weeks.
Keywords: Banana, Modified atmosphere packaging, Ethylene absorbent, Fruit quality, Shelf-life
Introduction
India is the largest producer of banana (Musa sp.) in the world with an annual production of 16.82 million MT from an area of 0.68 million ha (Anon 2007). ‘Dwarf Cavendish and ‘Robusta’ are the most popular banana varieties commonly grown in India and are the main stays of Indian banana industry for both internal and export trades. Major constraints and problems associated with post harvest handling of fresh banana are short shelf life under tropical climate (6–10 days) and lack of appropriate post harvest technologies for storage (Anon 2007; Kudachikar et al 2007).There is a need to find appropriate storage method to extend the storage life to maintain the fruit quality for long distance transportation for domestic and export markets. Modified atmosphere packaging (MAP) is an ideal preservation technique (Mangaraj and Goswami 2009) and is known to have great potential to extending the post harvest life of fruits and vegetables (Thompson 1998). Besides maintaining a desirable high humidity around the fruit, MAP has shown that packaging in non perforated bags prolonged the preclimacteric life of ‘Mas’ banana fruits (Tan et al 1990) and ‘Sucrier’ banana (Romphophak et al 2004). However, its potential has not been completely explored so far due to its limited use by the industry (Thompson 1998).
Use of absorbents (O2, CO2 and ethylene) in MAP as active packaging of fresh fruits and vegetables could help in extending the storage life and maintain the freshness inside MA packages (Thompson 1998). In the present study, KMnO4 sachets as Green Keeper (GK) as ethylene absorbent were used in MAP to absorb endogenously produced ethylene. Information on the combined effects of MAP with or without ethylene absorbent and the low temperature (LT) storage conditions on the shelf life extension and quality of ‘Robusta’ banana is scanty. In the present study, an attempt was made to evaluate the effectiveness of MAP alone and in combination with GK as ethylene absorbent on fruit quality attributes and shelf-life extension of unripe green ‘Robusta’ banana fruits under LT conditions and also to assess their quality in full ripe condition when artificially ripened at ambient (25 ± 5°C, 60–75% RH) conditions after ethrel dip treatment following storage at 12 ± 1°C, 85–90% RH.
Materials and methods
Experiments on LT storage studies of ‘Robusta’ banana were carried out for two consecutive seasons. Since the ‘Robusta’ banana was harvested from the healthy, well managed banana orchards near Mysore, wherein preharvest spray schedules for effective control of major pests and diseases were strictly followed during fruit development and maturation up to the harvest of fruits. The fruit bunches harvested after 110–115 days of inflorescence emergence were used. These bunches were dehanded, only green, firm and 75–80% matured fruit finger hands from each bunch were selected, delatexed, water washed, sorted and graded for uniform size, colour and weight and precooled at LT (12 ± 1°C, 85–90% RH) for 12 h. The individual hands were dipped in Benomyl fungicide solution (1,000 ppm) for 5 min and the fruit surface was dried under shade. The treated hands were grouped into 4 lots. One lot (12–14 fruits) was used for analysis of physico-chemical and textural characteristics. One lot (12–14 fruits) was used as unwrapped, openly kept control, Third lot was used for MAP and Fourth lot was used for MAP+GK (active) treatments. Each treatment had 12 replications with 12 kg of fruit sample per replicate. For MAP and MAP+GK treatments, LDPE films of 25 μm thickness of pouch size (120 cm × 60 cm) were used. The total surface area of each film bag was 7,200 cm2 with film permeabilities of 7,700 cc/m2/mil/day at 1 atm for CO2 and of 3,900 cc/m2/mil/day at 1 atm for O2. For MAP+GK treatment, 3 sachets containing KMnO4 (10 g/sachet) as ethylene absorbent were placed in each LDPE film pouch. All the treated fruits were stored at LT conditions.
Fruit samples were drawn periodically after 0, 3, 4, 5, 6 and 7 weeks of storage and analyzed for quality characteristics. Out of 12 replications under each treatment, 3 replicates of each treatment were used for periodical observations and for collection of data on physical characteristics such as PLW, head space gas analysis for CO2 and O2 in both MAP and MAP+GK samples and mean value of 3 replicates are reported. Out of the remaining 9 replicates, one replication per treatment was periodically drawn for observations on fruit colour, texture and pulp to peel ratio and for determining chemical characteristics of fruit pulp such as total soluble solids (TSS), total acidity, total sugars and total carotenoids and total chlorophyll pigments (from the peel portions of 6 fruits per treatment) as per the method suggested by Ranganna (1986). On each sampling day, one replicate per treatment after periodical observations, was dipped in 1,000 ppm of ethrel (2-chloro ethyl phosphonic acid) solution (Kudachikar et al 2007) and were reverted to normal ripening when stored at ambient conditions RT (25 ± 3 °C, 50–60% RH). The data on the fruit quality of the both raw green and fully ripe fruits of openly kept control, MAP and MAP+GK treatments after 3, 5 and 7 weeks of storage are presented.
Physiological loss in weight (PLW) was calculated as cumulative % loss in weight based on the initial fruit weight (before storage) and loss in weights recorded at the time of periodical sampling during LT storage. Fruit texture of both fresh and LT stored banana was measured as the maximum shear force required to cut the whole banana fruit into two halves and the shear force values are expressed in Newtons (N) by using a single blade (1 mm thickness) Warner–Bratzler shear apparatus on Instron Universal Testing machine (UTM-Instron., Model 4301, UK), with load cell of 100 Newtons (N) and cross head speed of 50 mm/min (Rushing and Huber 1983). The peel and pulp colour of banana fruit was measured by colour measured system (Model, Lab scan XE, Hunter Associates Inc., USA) and at wave length ranging from 400 to 700 nm and expressed Hunter colour values ‘L’ a and b (Hunter 1975). Mean of 3 readings is reported. The pulp and peel components of individual fruits were separated and weighed. The ratio of pulp to peel was calculated.
The O2 and CO2 levels within passive MAP and MAP+GK treatments were periodically determined by CO2/O2 analyzer (Model Checkmate 9900, PBI Dan sensor, Ringsted, Denmark). Each of these packages had a gas sampling septum. On each sampling day, gas samples were withdrawn by inserting plastic syringe into the gas sampling septa and atmospheric compositions within packages were determined.
The pulp of 6 fruits from each treatment was blended and the homogenized pulp was used for the estimation of TSS, total acidity, total sugars, total carotenoids and total chlorophyll pigments from the peel portions of 6 fruits per treatment as per the methods described by Ranganna (1986).
Sensory quality of fully ripe fruits
The green banana fruits from control, MAP and MAP+GK treatments on each sampling day(After 3, 5 and 7 weeks of LT storage) were given ethrel dip treatment and ripened at RT (25 ± 3 °C), 50–60% RH) conditions for 5 days. Fully ripe fruits in 3 replicates were tested for overall sensory quality attributes like colour and appearance, taste, flavour and overall quality were evaluated on 9-point Hedonic scale with 9 for excellent in all respects and 1 for highly disliked by a team of 12 sensory experts (Larmond 1977).The results of the sensory quality attributes of fully ripe fruits are given the Table 2.
Table 2.
Sensory quality scores of fully ripe banana (var. Robusta) fruits after 5 days at RT (25 ± 3 °C, 50–60% RH)
| Parameters | Control | MAP | MAP+GK |
|---|---|---|---|
| *3 | *5 | *7 | |
| Ripe | Ripe | Ripe | |
| Colour | 7.0 ± 0.34b | 7.8 ± 0.36a | 7.6 ± 0.34ab |
| Flavour | 7.3 ± 0.45a | 7.2 ± 0.43a | 7.4 ± 0.26a |
| Taste | 7.4 ± 0.34a | 7.5 ± 0.52a | 7.3 ± 0.43a |
| OA | 7.2 ± 0.34a | 7.5 ± 0.70a | 7.4 ± 0.43a |
*LT Storage (25 ± 3 °C, 50–60% RH) period in weeks +5 Days RT
OA Overall acceptability (n = 12 panelists)
Means having different superscripts in the same row are significantly (p < 0.05) different
Statistical analysis
All the determinations were made in 3 replicates. The collected data were subjected to analysis of variance using the method of Snedecor and Cochran (1994). Duncan’s Multiple Range Test was employed to determine the significance (p ≤ 0.05) if any, between the treatment means (SAS 1985).
Results and discussion
PLW
PLW in MAP and MAP+GK packed green (raw) banana was lower compared to unwrapped and openly kept control fruits (Table 1). Unlike MAP and MAP+GK packed bananas, the extent of weight loss in openly kept banana increased during LT storage. However, the PLW in MAP and MAP+GK treated green banana, were considerably lower in 5 and 7 weeks LT stored samples respectively. The loss in fruit weight of fully ripe fruits among these treatments after 6 days at RT was high in MAP (7.1%), followed by MAP+GK (7.0%) treated green banana after 5 and 7 weeks of LT storage, respectively as compared to fully ripe fruits of openly kept control (12% ) after 3 weeks.
Table 1.
Changes in physical and chemical characteristics of banana (var.Robusta) stored at low temperature conditions (12 ± 1°C, 85–90% RH)
| Initial | Quality of unripe green banana after effective shelf life* (in weeks)at LT storage | Quality of ripe banana after effective shelf life* (in weeks at LT storage)+5 Days RT | |||||
|---|---|---|---|---|---|---|---|
| 0 | Control | MAP | MAP+GK | Control | MAP | MAP+GK | |
| *3 | *5 | *7 | *3 | *5 | *7 | ||
| Physical | |||||||
| PLW | – | 5.0 ± 0.34c | 0.7 ± 0.11d | 0.8 ± 0.08d | 12.0 ± 0.20a | 7.1 ± 0.26b | 7.0 ± 0.20b |
| Pulp/ Peel ratio | 1.06 ± 0.14c | 1.11 ± 0.15c | 1.54 ± 0.10b | 1.7 ± 0.13b | 1.76 ± 0.09b | 2.05 ± 0.15a | 2.15 ± 0.13a |
| Hunter colour, Peel | |||||||
| L | 44.3 ± 0.75d | 56.3 ± 3.72c | 45.3 ± 2.34d | 44.0 ± 1.73d | 82.1 ± 1.82a | 63.1 ± 2.55b | 63.7 ± 3.11b |
| a | −13.8 ± 0.70a | −7.9 ± 0.40c | −12.4 ± 0.87b | −12.6 ± 0.52b | −1.7 ± 0.04d | −0.37 ± 0.05c | 2.1 ± 0.10d |
| b | 17. 8 ± 0.34d | 23.3 ± 2.78c | 20.1 ± 1.67d | 20.2 ± 1.03d | 25.9 ± 0.98bc | 28.6 ± 1.80b | 33.8 ± 1.12a |
| Hunter colour, Pulp | |||||||
| L | 79.4 ± 0.75a | 72.9 ± 2.42b | 81.6 ± 1.22a | 70.2 ± 2.64b | 66.6 ± 2.16c | 70.7 ± 2.19b | 80.3 ± 2.02a |
| a | −0.73 ± 0.06c | −0.28 ± 0.03b | −1.25 ± 0.08c | −1.48 ± 0.07f | −1.0 ± 0.26d | 0.11 ± 0.01a | 0.14 ± 0.01a |
| b | 25.6 ± 0.75bc | 26.6 ± 3.29bc | 23.7 ± 0.47c | 24.9 ± 2.19bc | 26.2 ± 0.75bc | 28.2 ± 2.27ab | 31.1 ± 0.79a |
| Shear force, N | 74.1 ± 1.15a | 55.3 ± 1.55c | 62.4 ± 1.70b | 60.0 ± 2.00b | 29.2 ± 0.78d | 26.8 ± 0.98c | 26.2 ± 1.40c |
| Chemical | |||||||
| TSS,◦ Brix | 4.0 ± 0.34d | 9.0 ± 1.05b | 5.9 ± 0.21c | 9.5 ± 0.26b | 22.4 ± 1.01a | 24.0 ± 1.80a | 23.8 ± 1.12a |
| pH | 5.3 ± 0.26a | 5.3 ± 0.43a | 5.4 ± 0.23a | 5.2 ± 0.26a | 4.9 ± 0.43a | 5.0 ± 0.20a | 5.0 ± 0.20a |
| Total acidity | 0.26 ± 0.02d | 0.38 ± 0.02c | 0.25 ± 0.03d | 0.29 ± 0.02d | 0.44 ± 0.01b | 0.49 ± 0.02a | 0.51 ± 0.03a |
| Total sugars | 0.90 ± 0.05c | 5.1 ± 0.17c | 3.8 ± 0.26d | 6.6 ± 0.20b | 11.5 ± 0.43a | 12.1 ± 0.62a | 11.8 ± 0.52a |
| Total carotenoids, μg/100 g | 130 ±5.56g | 149 ± 4.35f | 208 ± 7.21c | 237 ± 3.00d | 324 ± 5.29a | 289 ± 7.93b | 270 ± 8.66c |
| Total chlorophyll in peel, mg/100 g | 4.8 ± 0.30a | 1.5 ± 0.17c | 2.1 ± 0.36b | 2.2 ± 0.01b | 0.20 ± 0.02d | 0.19 ± 0.01d | 0.27 ± 0.05d |
*Storage period in weeks; PLW-Physiological loss in weight; TSS-Total soluble solids (n = 3)
Means having different superscripts in the same row are significantly (p < 0.05) different
The reduction in PLW among MAP+GK and MA packed green banana could be due to the selective permeability characteristics of LDPE packaging films for water vapor. Thus, reduction in weight loss could be one of the reasons for extension of shelf-life and maintaining quality of green banana in MAP+GK and MA packages up to 7 and 5 weeks, respectively as compared to 3 weeks in openly kept green banana. Similar trends in changes in PLW of MAP and MAP+GK treated Plantain and ‘Poovan’ banana stored at LT conditions were also reported by Isaak et al (2006) and Kudachikar et al (2007), respectively.
Pulp to peel ratio
This ratio was 1.06 in freshly harvested green banana before LT storage which increased to 1.11 (after 3 weeks) in openly kept control banana as compared to 1.54 in MAP and 1.70 in MAP+GK green bananas after 5 and 7 weeks of storage, respectively. However, the pulp to peel ratio of fully ripe fruits after 6 days at RT of both MAP (2.05) and MAP+GK (2.15) treatments (after 7 weeks of LT storage), was higher as compared to 1.76 in fully ripe fruits of openly kept control fruits after 3 weeks of LT storage. Similar trends in ratio among fully ripe fruits of MA and MAP+GK packed and control treatments were noticed. This sudden rise in pulp to peel ratio could be due to osmotic transfer of moisture from peel to pulp during storage. Our results are similar to those reported by Isaak et al (2006) and Kudachikar et al (2007) in MAP of different varieties of banana.
Fruit firmness
Freshly harvested green bananas were firm with 74.2 N and fruit firmness decreased during storage time up to 3 and 5 weeks of LT storage in control and MAP, respectively, and further declined to 62.4 N and 60.0 N in MAP and MAP+GK samples, with storage up to 5 and 7 weeks, respectively. Changes in fruit firmness during LT storage in MAP and MAP+GK treated green bananas were inversely related to PLW of stored banana. Thus, the MAP fruits retained more firmness and greenness with reduced PLW during LT storage than the openly kept fruits. Similar observations in MAP of ‘Dwarf Cavendish’ bananas stored under ambient storage conditions and MAP of ‘Poovan’ bananas stored under LT conditions were reported by Ramana et al (1989) and Kudachikar et al (2007).
Colour characteristics of peel and pulp
During storage, the green bananas under MAP and MAP+GK packages were more firm and retained the greenness in peel region during 5 and 7 weeks of LT storage, respectively as compared to openly kept green bananas. Intensity of yellowness (+b) in the pulp of passive MAP and MAP+GK packed green banana was maximum (23.7 and 24.9) after 5 and 7 weeks of LT storage, respectively. In openly kept green banana, yellowness of pulp reached maximum (26.6) after 3 weeks of LT storage. MAP and MAP+GK bananas remained green and firm during LT storage and retained total chlorophyll in the peel than in openly kept banana (Table 1). This could be due to decreased metabolic processes responsible for chlorophyll pigments degradation and biosynthesis of carotenoids pigments in the peel portions of green banana treated with MAP and MAP+GK treatments, under elevated CO2 levels due to MAP storage conditions. Similar trends in MAP ‘Poovan banana under LT conditions and MAP plantains stored under LT conditions were reported by Kudachikar et al (2007) and Isaak et al (2006).
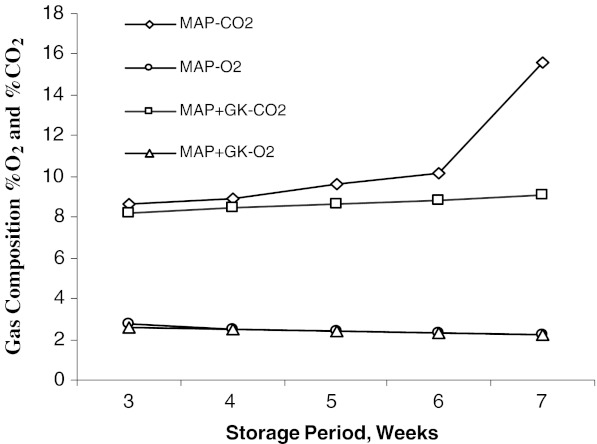
Atmosphere composition
There was a gradual increase in CO2 level and decrease in O2 level during LT storage (Fig.1). Both CO2 and O2 gas levels in MAP and MAP+GK packages reached a steady state after 3 weeks of LT storage. Thereafter, CO2 levels in MAP gradually increased to 10.2% (after 6 weeks of LT storage) and increased rapidly to 15.6% at 7 weeks after LT storage. O2 levels in MAP decreased during 7 weeks. Unlike MAP, the shift rates in CO2 and O2 levels in MAP+GK packages were progressively delayed from 8.2 to 9.1% and 2.6 to 2.2% concentration of CO2 and O2, respectively (after 7 weeks).
Fig. 1.
Gas composition of MAP and MAP+GK for banana var. ‘Robusta’ (n = 3)
LDPE films maintained comparatively lower concentration of CO2 in MAP+GK at 7 weeks than MAP at 5 weeks of LT storage. Similar pattern of changes in the gaseous atmosphere were reported (Deily and Rizvi 1981; Smith et al 1987; Nakhasi et al. 1991; Isaak et al 2006). The steady levels of CO2 and O2 in these packages could cause marked changes in the activities of specific enzymes in the respiratory metabolism and might have uncoupling effect on oxidative phosphorylation (Kader 1986). This might have led to the extension of shelf-life of ‘Robusta’ banana in MAP and MAP+GK up to 5 and 7 weeks respectively as compared to 3 weeks in openly kept control.
Chemical characteristics
Green banana had initially TSS of 4.0% and 0.90% of total sugars in the fruit pulp (Table 1). The TSS increased to 9.0 and 5.9% and total sugars increased to 5.1 and 3.8% in openly kept banana and MAP banana on 3 and 5 weeks of LT storage, respectively. Elevated CO2 levels (>8.2%) in MAP and MAP+GK green banana resulted in marked reduction in rate of conversion of starch to sugars as compared to openly kept control. As storage of fruits at elevated CO2 levels (>5.2%) could alter the activities of specific enzymes of the respiratory metabolism that might have led to uncoupling effect on oxidative phosphorylation (Kader 1986). Similar response of MAP plantains under different storage conditions was also reported (Isaak et al 2006). Similarly, Goodenough and Thomas (1980) also observed a decrease in starch pool in controlled atmosphere stored tomatoes kept in 5% O2 and 5% CO2. It is also reported that elevated CO2 inhibited glycolysis, Krebs cycle intermediates and succinic dehydrogenase activity leading to accumulation of only succinate, thereby subsequently reducing the formation of citrate/isocitrate and 2-ketoglutarate (Monning 1983).
Total acidity of green bananas increased from 0.26 to 0.38% in openly kept control after 3 weeks and to 0.29% in MAP+GK bananas after 7 weeks of storage at LT (Table 1). Elevated CO2 levels under MAP+GK green banana reduced the losses in total acidity as compared to MAP and openly kept control fruits. Therefore, only MAP+GK green banana after extended LT storage period of 7 weeks maintained a higher total acidity (0.51%) in fully ripe fruits than the openly kept control (0.44%) and MAP packed green banana (0.49%). Our results are in agreement with Kudachikar et al (2007) in MAP+GK green ‘Poovan’ bananas stored under LT conditions.
Both green and fully ripe fruits of openly kept and MAP treated had higher levels of total carotenoids (TC) after 3 and 5 weeks of LT storage, respectively as compared to TC in green and fully ripe fruits of MAP+GK green banana after 7 weeks of LT storage (Table 1). This could be due to inhibition of metabolic processes linked to biosynthesis of carotenoid pigments in the fruit pulp under elevated CO2 and reduced O2 concentrations. Similar observations were also reported by Smock (1979), Dalal and Nagaraja (1989) and Kudachikar et al (2007).
‘Robusta’ banana initially had higher levels of chlorophyll pigment (4.8 mg/100 g) which decreased progressively with storage in control fruits. However, it slightly increased in MAP and MAP+GK green bananas up to 5 and 7 weeks of LT storage, respectively. This could be due to prevention of rapid loss in chlorophylls by delayed biosynthesis of carotenoids in MAP and MAP+GK green bananas. Goodenough and Thomas (1980) also observed that the chlorophyll degradation and biosynthesis of carotenoids and anthocyanins were slowed down in fruits and vegetables kept under MAP and controlled atmosphere storage conditions.
Sensory quality of fully ripe fruits
Sensory quality of fully ripe fruits of MAP+GK and MAP treated banana after 5 days at RT conditions was very good (7.5, 7.4) and could be compared with the sensory quality of fully ripened fruits of openly kept control treatment (7.2) (Table 2).
Conclusion
Optimally matured (75–80%) ‘Robusta’ banana packed in MAP with LDPE films alone and MAP with LDPE film in combination with green keeper as ‘ethylene absorbent’ under low temperature (12 ± 1°C, 85–90% RH) conditions extended shelf-life up to 5 and 7 weeks, respectively, with minimum losses in fruit weight, fruit texture and fruit composition during LT storage as compared to 3 weeks in openly kept control fruits stored under similar conditions.
Acknowledgement
Authors are grateful to Prakash V, Director, CFTRI, Mysore for his keen interest, constant encouragement and support during the course of this investigation. Authors extend their sincere thanks to Rajarathnam S, Head, and Ramana KVR, Former Head, Department of Fruit and Vegetable Technology for their constant encouragement and guidance during the course of investigation.
References
- Anon 2007. Scenario of banana production, utilization and trade. In: Proc National Conference on production and utilization of banana for economic livelihood and nutritional security, National Research Centre for Banana, Tiruchinapalli, India, 25–28th Oct. 2007, Organized by Association for Improvement in Production and Utilization of Banana, New Delhi, Indian Council of Agricultural Research, New Delhi
- Dalal VB, Nagaraja N. Treatments, packaging and storage of some commercial fruits of India. Beverage Food World. 1989;16(3):17–23. [Google Scholar]
- Deily KR, Rizvi SS. Optimization of parameters for packaging of fresh peaches in polymeric films. J Food Process Eng. 1981;5:23–31. doi: 10.1111/j.1745-4530.1982.tb00258.x. [DOI] [Google Scholar]
- Goodenough PW, Thomas TH. Comparative physiology of field—grown tomatoes during ripening on the plant or retarded ripening in controlled atmospheres. Ann Appl Biol. 1980;94:445–449. doi: 10.1111/j.1744-7348.1980.tb03960.x. [DOI] [Google Scholar]
- Hunter S. The measurement of appearance. New York: Wiley; 1975. pp. 304–305. [Google Scholar]
- Isaak PG, Kudachikar VB, Kulkarni SG, Vasantha MS, Keshava Prakash MN, Ramana KVR. Shelf life and quality of modified atmosphere packed plantains during low temperature storage. J Food Sci Technol. 2006;43:671–676. [Google Scholar]
- Kader AA. Biochemical and physiological basis for effects of controlled and modified atmospheres on fruits and vegetables. Food Technol. 1986;40(5):99–104. [Google Scholar]
- Kudachikar VB, Kulkarni SG, Vasantha MS, Aravinda Prasad B, Aradhya SM. Effect of modified atmosphere packaging on shelf life and fruit quality of banana stored at low temperature. J Food Sci Technol. 2007;44:74–78. [Google Scholar]
- Larmond E. Laboratory methods for sensory evaluation of food. Ottawa: Canada Dept of Agric Publ; 1977. [Google Scholar]
- Mangaraj S, Goswami TK. Modified atmosphere packaging-An ideal food preservation technique. J Food Sci Technol. 2009;46:399–410. [Google Scholar]
- Monning A. Studies on the reaction of Krebs cycle enzymes for apple tissue (cv. Cox orange) to increased levels of CO2. Acta Hortic. 1983;138:113–115. [Google Scholar]
- Nakhasi S, Schlimme D, Solomos T. Storage potential of tomatoes harvested at the breaker stage using modified atmosphere packaging. J Food Sci. 1991;56:55–59. doi: 10.1111/j.1365-2621.1991.tb07974.x. [DOI] [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruit and vegetable products. 2. New Delhi: McGraw Hill Publ Co. Ltd; 1986. pp. 12–16. [Google Scholar]
- Ramana SV, Mohan Kumar BL, Jayaraman KS. Effect of postharvest treatments and modified atmosphere on the storage life of fresh banana and guava under ambient temperature. Indian Food Pack. 1989;43(1):29–35. [Google Scholar]
- Romphophak T, Siriphanich J, Promdang S, Yoshinoriueda S. Effect of modified atmosphere storage on the shelf life of banana ‘Sucrier’. J Hort Sci Biotechnol. 2004;79:659–663. [Google Scholar]
- Rushing JW, Huber DJ. Colour and firmness of selected Florida grown tomato cultivars. Proc Fla Sta Hort Soc. 1983;96:107–109. [Google Scholar]
- SAS Users Guide: statistics version. 5. Cary: SAS Institute; 1985. [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. 8. Iowa: Iowa State University Press; 1994. pp. 156–160. [Google Scholar]
- Smith SM, Gieeson JD, Browne M, Genge PM, Everson HP. Modified atmosphere retail packaging of discovery apples. J Sci Food Agric. 1987;40:165–173. doi: 10.1002/jsfa.2740400209. [DOI] [Google Scholar]
- Smock RM. Controlled atmosphere storage of fruits. Hort Rev. 1979;1:301–327. [Google Scholar]
- Tan SC, Mohamed AA, Tan SC. The effect of CO2 on phenolic compounds during the storage of ‘Mas’ banana in polybags. Acta Hortic. 1990;269:389. [Google Scholar]
- Thompson AK. Controlled atmosphere storage of fruits and vegetables. New York: CAB International; 1998. [Google Scholar]