Abstract
A study was carried out to detect the changes in colour and quality attributes of aonla juice during storage after pasteurization at different temperatures. After extracting juice from aonla cv. Chakaiya, it was pasteurized at five different temperatures and preserved with 500 ppm SO2 in PET bottles under ambient conditions. Juice was periodically analysed for colour and chemical characters up to 9 months of storage. Though the contents of ascorbic acid and polyphenols in juice decreased with increase in storage period, the effect of pasteurization temperature was not significant. High degree of browning was observed in juice heated at higher temperatures (90 and 95 °C) as compared to lower temperatures (75 and 80 °C) throughout the storage period as indicated by increase in NEB values. The degree of browning was further confirmed by higher negative numerical values of whiteness index in Hunter’s scale for intensity of colour. HPLC data indicated that content of gallic acid in juice decreased initially but increased sharply as the storage period prolonged. Higher amount of gallic acid was detected after 9 months of storage in juice pasteurized at 95 °C than in juice heated at 75 °C. The contents of kaempferol and caffeic acid decreased throughout the storage period irrespective of pasteurization temperature. Though least browning was observed in juice pasteurized at 75 °C, but microbial growth was observed after 9 months of storage. Hence, pasteurization temperature of 80 °C was found optimum for preservation of aonla juice under ambient conditions.
Keywords: Aonla juice, Pasteurization temperature, Storage, Browning, Polyphenols
Aonla (Emblica officinalis Gaertn.), also known as Indian gooseberry, belongs to family Euphorbiaceae and is widely used in pharmaceutical and processing industries because of the presence of some useful neutraceuticals like ascorbic acid and polyphenols (Jain and Khurdiya 2002). The fruits are the second richest source of vitamin C among fruits after Barbados cherry (Malpighia glabra L) (Singh et al. 2006). It is becoming an important fruit crop of 21st century due to its hardy nature, ability to grow in various agro-climatic conditions, high productivity (15–20 t ha−1) per unit area, nutritive and therapeutic value and its suitability for processing (Pandey and Misra 2007). The consumption of raw aonla fruit is good for health, but because of high astringency the fruits have little table value and so processed into various products, viz. murabba, candy, juice, pickle, powder, segments-in-syrup, etc. Among these, juice is the preferred product. Moreover, antioxidants present in aonla juice in the form of polyphenols and vitamin C have been shown to provide a cardio-protective effect (Pathak et al. 2003). Polyphenols like ferulic acid, caffeic acid and sinapic acid, found in kale (black cabbage or Brassica oleraceae var. acephala DC.) leaves and seeds, had shown to possess antimicrobial property against some bacteria, viz. Staphylococcus aureus Rosenbach, Enterococcus faecalis Schleifer and Kilpper-Balz, Bacillus subtilis Cohn and Moraxella catarrhalis Morax (Ayaz et al. 2008). Polyphenols also contribute towards the taste, colour, odour and preservation of fruits as well as processed products. Aonla juice has good potentiality for blended and spiced beverages besides direct consumption for health purposes. However, it suffers from loss of vitamin C and severe browning during storage particularly at higher temperature, which adversely affects the appearance, nutrition and overall acceptability of juice. Pasteurization of juice at high temperature is required to keep it free from microbial load, which may affect the quality of juice during storage under ambient conditions. So far very little efforts have been made to study the role of pasteurization temperature and its effect on quality of aonla juice during storage along with identification and quantification of polyphenols in juice by high performance liquid chromatography (HPLC). Therefore, the present investigation has been carried out on changes in physicochemical attributes of aonla juice after pasteurization at different temperatures and on changes in phenolic contents in juice using HPLC during storage.
Materials and methods
Mature aonla fruits of cv. Chakaiya were procured from Institute’s experimental farm. Healthy fruits were washed thoroughly in running tap water for 10 min to remove adhered dust and microbes from the surface. The fruits were then crushed in a fruit mill and juice was extracted using Hydraulic press (Bajaj Machinen, New Delhi) at a pressure of 1,500 lb sq. inch−1. After filtration, juice was pasteurized at different temperatures (75, 80, 85, 90 and 95 °C) and preserved with 500 ppm SO2 as potassium metabisulphite in PET bottles (1l capacity) after cooling at room temperature. The sealed bottles were stored under ambient conditions (18–36 °C, 40–80% RH) up to 9 months. Juice was subjected to periodical analysis for various physicochemical parameters and polyphenol contents during storage.
The colour of juice was determined by ColorFlex Meter (HunterLab, USA) displaying colour in terms of L, a and b values and the whiteness index (negative value) was worked out using the formula W.I. = L (L – 5.715b)/100. Ascorbic acid in juice was estimated by titrimetric method using 2,6-dichorophenol indophenol dye solution and polyphenols content was estimated by Folin and Ciocalteu’s phenol reagent method (Ranganna 1986). Non-enzymatic browning (NEB) was determined by measuring optical density (OD) values of methanol extracted juice samples at 440 nm in UV-VIS Spectrophotometer (Labomed Inc., USA).
Identification and quantification of individual polyphenols in juice were done by Shimadzu high performance liquid chromatograph equipped with a photodiode array detector and a rheodyne injector (20 μl loop). The stationary phase consisted of reverse phase Phenomenex® Luna 5 μ C-18 stainless steel column (250 × 4.6 mm i.d.). The mobile phase was potassium dihydrogen orthophosphate solution (0.01 M) adjusted to pH 3.05 with 85% o-phosphoric acid (A) and acetonitrile : water (75 : 25, v/v) as organic phase (B). A linear gradient (A : B = 70 : 30) was carried out at a flow rate of 0.8 ml min−1 for 20 min with detector wavelength set at 280 nm. Twenty microlitre of 100 times diluted juice was injected each time. This method was carried out following the method provided by Basha et al. (2004) with slight modifications. Polyphenols, viz. gallic acid, chlorogenic acid, (+) catechin, (−) epicatechin, caffeic acid, p-coumaric acid and kaempferol were purchased from Life Technologies (India) Pvt. Ltd., Mumbai having 100% purity. Stock solutions of 1,000 μg g−1 of each polyphenol were prepared by dissolving accurately weighed 50 mg of each compound in 50 ml of HPLC grade methanol in a 50 ml volumetric flask. Working solutions of 10 and 50 μg g−1 concentrations were prepared in HPLC grade methanol by subsequent dilution. Calibration curves for standard solutions of each compound were found linear in the range of 1 to 100 μg g−1. The limit of determination of each phenolics was 1 μg g−1. Polyphenols were identified by their respective retention times. The retention times of gallic acid, chlorogenic acid, (+) catechin, (−) epicatechin, caffeic acid, p-coumaric acid and kaempferol were 4.51, 4.70, 5.27, 5.86, 6.93, 11.87 and 13.63 min, respectively. HPLC chromatograms have not been produced here, however, data have been presented in the form of graphs.
Results and discussion
Changes in some quality attributes in aonla juice during storage
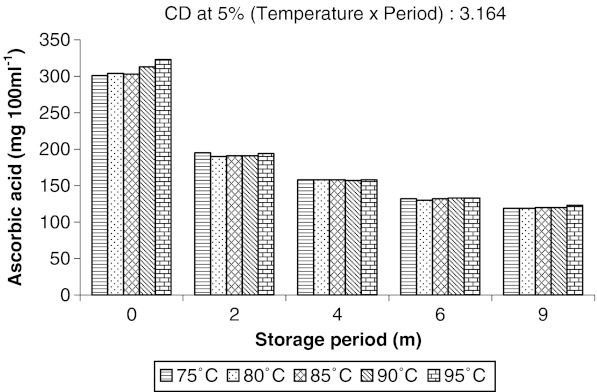
The most significant change in chemical composition of juice was observed in ascorbic acid content and in physical appearance in terms of browning, which was measured as NEB. Ascorbic acid content rapidly decreased from around 300 to 120 mg 100 ml−1 after 9 months of storage in all juice pasteurized at different temperatures resulting in a massive loss of 60.00% (Fig. 1). Surprisingly it was found that pasteurization temperature had very little effect on ascorbic acid content in aonla juice as compared to storage period. The amount of ascorbic acid was almost same in juice pasteurized at different temperatures and stored for different periods, but it decreased invariably with increase in storage time. The loss of ascorbic acid during storage might be attributed to the increase in ambient temperature with the increase in storage period involving change of weather from winter to summer season, which led to the conclusion that temperature might play an important role in retention of ascorbic acid in aonla juice during storage. Pasteurization of aonla juice at 90 °C followed by addition of 350 ppm SO2 and low temperature (4 ± 1 °C) storage was found one of the best treatment to minimize the loss of vitamin C as well as non-enzymatic browning even after 6 months of storage in glass bottles (Jain and Khurdiya 2009). Aonla juice stored in glass bottles at ambient conditions could retain maximum vitamin-C (298.4 mg 100 g−1) up to 6 months of storage than other aonla products (Damame et al. 2002), which might be due to the better solubility of vitamin C in high content of water in juice. Mehta and Rathore (1976) also observed that vitamin C in aonla juice decreased with increase in temperature during storage. Suresh Kumar and Sagar (2009) found that storage of osmo-vac dehydrated aonla segments at low temperature (7 ± 1 °C) could retain maximum vitamin C up to 6 months.
Fig. 1.
Effect of pasteurization temperature on retention of ascorbic acid in aonla juice during storage
The data on polyphenols content, non-enzymatic browning (NEB) and whiteness index of aonla juice are presented in Table 1. Though the polyphenols content in juice (in terms of tannins) were also not affected by pasteurization temperature and decreased with increase in storage period, but some individual phenolic compounds changed significantly as revealed by HPLC analysis. The juice pasteurized at higher temperatures exhibited higher degree of browning as indicated by increase in NEB values from 0.045 (90 °C) and 0.047 (95 °C) OD at the time of juice preparation to 0.310 and 0.340 OD after 9 months of storage (Table 1). The changes in NEB values were minimum at lower pasteurization temperature (OD from 0.030 to 0.202 at 75 °C and OD from 0.039 to 0.260 at 80 °C) and moderate at juice pasteurized at medium temperature (OD from 0.041 to 0.276 at 85 °C). The increase in NEB values in juice pasteurized at higher temperatures was faster during storage than in juice pasteurized at lower temperatures. Jain and Khurdiya (2009) reported that aonla juice treated with SO2 alone was least brown as compared to pasteurization at 90 °C + SO2 or pasteurization alone after 6 months of storage. They concluded that Maillard reaction (reaction between sugars and amino acids) might contribute to browning. Shinoda et al. (2005) reported that ascorbic acid was essential for browning of orange juice during storage and proposed that furfural might be an indicator for browning of orange juice. Kacem et al. (1987) concluded that reaction between dehydroascorbic acid and α–amino acids might be a factor for browning of orange drinks. Ibarz et al. (2008) observed that presence of D-galacturonic acid increased non-enzymatic browning in apple, pear and peach juices. The instance of browning in aonla juice was further confirmed by negative numerical values of whiteness index in Hunter’s scale for intensity of colour. The negative values of whiteness index increased during storage, which indicated the increase in browning, after initial decrease (up to 2 months) in all juice irrespective of pasteurization temperature (Table 1). But in case of juice pasteurized at higher temperatures the negative value increased at a faster rate than in juice pasteurized at lower temperatures.
Table 1.
Chemical attributes in aonla (cv. Chakaiya) juice during storage as affected by pasteurization temperature
| Parameter | Pasteurization temperature (°C) | Storage period (month) | ||||
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 9 | ||
| Polyphenols (%) | 75 | 3.87 | 2.76 | 2.99 | 2.61 | 2.26 |
| 80 | 3.83 | 2.78 | 2.95 | 2.64 | 2.33 | |
| 85 | 3.71 | 2.96 | 2.88 | 2.64 | 2.47 | |
| 90 | 3.62 | 2.83 | 2.88 | 2.81 | 2.58 | |
| 95 | 3.79 | 2.83 | 2.98 | 3.10 | 2.75 | |
| CD at 5% | Temperature (T) | Period (P) | T x P | |||
| 0.087 | 0.087 | 0.195 | ||||
| Non-enzymatic browning (OD at 440 nm) | 75 | 0.030 | 0.041 | 0.099 | 0.153 | 0.202 |
| 80 | 0.039 | 0.042 | 0.116 | 0.173 | 0.260 | |
| 85 | 0.041 | 0.043 | 0.122 | 0.179 | 0.276 | |
| 90 | 0.045 | 0.043 | 0.150 | 0.201 | 0.310 | |
| 95 | 0.047 | 0.045 | 0.154 | 0.217 | 0.340 | |
| CD at 5% | Temperature (T) | Period (P) | T x P | |||
| 0.003 | 0.003 | 0.007 | ||||
| Whiteness index (–ve value) | 75 | 121 | 88 | 156 | 201 | 218 |
| 80 | 120 | 108 | 157 | 171 | 216 | |
| 85 | 121 | 108 | 170 | 196 | 234 | |
| 90 | 124 | 113 | 174 | 208 | 260 | |
| 95 | 131 | 119 | 184 | 230 | 252 | |
| CD at 5% | Temperature (T) | Period (P) | T x P | |||
| NS | 18.293 | NS | ||||
NS Non-significant
Effect of pasteurization temperature and storage time on changes in individual polyphenols
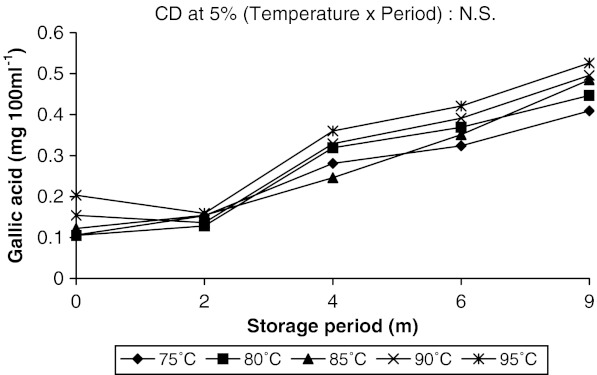
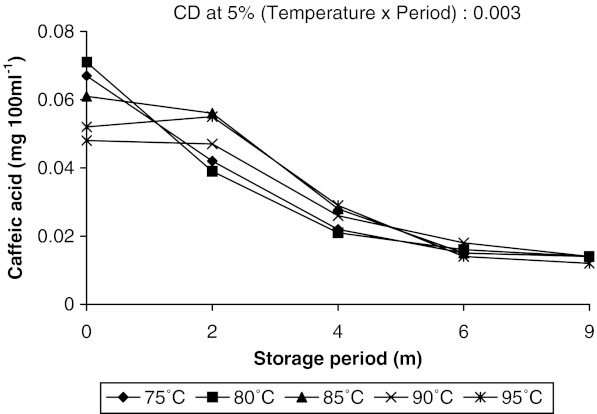
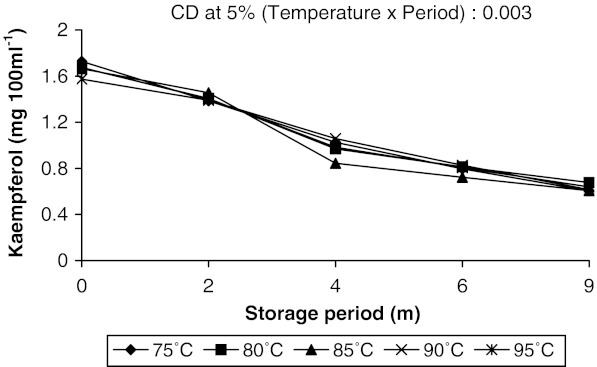
The changes in the contents of gallic acid, kaempferol and caffeic acid in aonla juice were more prominent. Along with these three some other phenolic compounds like chlorogenic acid, catechin, epicatechin and p-coumaric acid were also identified in aonla juice but their changes were not so significant during storage. Guyot et al. (2003) estimated polyphenolic composition of French cider apple fruits and juices by reversed phase HPLC using UV-VIS detector. Some flavonoid and nonflavonoid phenols in Hungarian wines were identified by Bonerz et al. (2008) using reverse-phase HPLC with a C-18 column, PDA detector and acidified water—acetonitrile as mobile phase, where detection limit for phenolics ranged from 0.1 to 1.0 mg l−1. The amount of gallic acid decreased slightly at initial stages in juice pasteurized at higher temperatures and increased subsequently with increase in storage period. But in juice pasteurized at lower temperatures, it increased throughout the storage (Fig. 2). Higher amount of gallic acid was recovered in juice heated at 95 °C than in juice heated at 75 °C throughout the storage period. It is to be mentioned here that as the storage period increased the temperature of storage also increased from 18 to 36 °C due to change of season from winter to summer. Increase in gallic acid content with the rise of temperature during storage proved that temperature played a major role on changes of gallic acid concentration in aonla juice. It was reported earlier that gallic acid could be formed in a biochemical pathway either from caffeic acid, where β–oxidation occurred at caffeic acid to give protocatechuic acid which was further hydroxylated to form gallic acid, or from shikimic acid, which came from glucose (Haslam 1981). Present findings also indicated that with the increase in temperature caffeic acid content in juice decreased and gallic acid content increased. Therefore, it may be opined that gallic acid might have formed in juice through metabolism of caffeic acid however, further study in this regard is required to confirm the increase in gallic acid with the rise in temperature. Kaempferol and caffeic acid contents were decreased throughout the storage period irrespective of pasteurization temperature. But changes in caffeic acid content was sharp during the initial period of storage (up to 4 months) in juice pasteurized at lower temperatures (75 and 80 °C) as compared to juice pasteurized at higher temperatures (90 and 95 °C) (Fig. 3). Thereafter, the decrease was slow and almost same amount of caffeic acid had been recovered from all juice samples after 9 months of storage (0.014 mg 100 ml−1). Even in juice pasteurized at 95 °C caffeic acid increased slightly after 2 months and decreased sharply thereafter during rest of the storage period. Whereas, kaempferol content in juice gradually decreased up to 9 months of storage at all the pasteurization temperatures (Fig. 4). Gliszezynska and Tyrakowska (2003) also reported a decrease in the contents of phenolic acids (5–21%) and flavonoids (8–19%) in apple juice up to 11 months of storage under ambient conditions. They concluded that Trolox equivalent antioxidant capacity (TEAC) value, directly related to polyphenols content, may serve as a useful parameter to assess the quality of apple juice at any stage of its shelf life.
Fig. 2.
Changes in gallic acid content in aonla juice as affected by pasteurization temperature while storing under ambient conditions
Fig. 3.
Effect of pasteurization temperature on caffeic acid content in aonla juice during storage
Fig. 4.
Concentration of kaempferol in aonla juice as affected by pasteurization temperature and storage period
Though juice pasteurized at 75 and 80 °C were of best quality in terms of least browning and maximum retention of ascorbic acid during 9 months of storage, but juice pasteurized at 75 °C was found contaminated with microbes after 9 months of storage. Hence, it can be concluded from the study that pasteurization temperature of 80 °C was optimum for preservation of aonla juice under ambient conditions. Also HPLC data on polyphenols indicated that gallic acid might play a significant role in browning of aonla juice during storage.
Acknowledgment
The authors are grateful to the Director and the Head, Division of Post Harvest Management, CISH for providing necessary facilities to carry out the research work.
References
- Ayaz FA, Hayirlioglu-Ayaz S, Alpay-Karaoglu S, Gruz J, Valentova K, Ulrichova J, Straad M. Phenolic acid contents of kale (Brassica oleraceae var. acephala DC.) extracts and their antioxidant and antibacterial activities. Food Chem. 2008;107:19–25. doi: 10.1016/j.foodchem.2007.07.003. [DOI] [Google Scholar]
- Basha SM, Musingo M, Colova VS. Compositional differences in the phenolics compounds of muscadine and bunch grape wines. Afr J Biotechnol. 2004;3:523–528. [Google Scholar]
- Bonerz DPM, Nikfardjam MSP, Creasy GL. A new RP-HPLC method for analysis of polyphenols, anthocyanins and indole-3-acetic acid in wines. Am J Enol Viticul. 2008;59:106–109. [Google Scholar]
- Damame SV, Gaikwad RS, Patil SR, Masalkar SD. Vitamin C content of various aonla products during storage. Orissa J Hort. 2002;30:19–22. [Google Scholar]
- Gliszezynska SA, Tyrakowska B. Quality of commercial apple juices evaluated on the basis of the polyphenols contents and the TEAC antioxidant activity. J Food Sci. 2003;68:1844–1849. doi: 10.1111/j.1365-2621.2003.tb12340.x. [DOI] [Google Scholar]
- Guyot S, Marnet N, Sanoner P, Drilleau JF. Variability of the polyphenolic composition of Cider Apple (Malus domestica) fruits and juices. J Agric Food Chem. 2003;51:6240–6247. doi: 10.1021/jf0301798. [DOI] [PubMed] [Google Scholar]
- Haslam E. Vegetable tannins. In: Conn EE, editor. The biochemistry of plants—a comprehensive treatise. Secondary plant products. New York: Academic Press; 1981. pp. 527–556. [Google Scholar]
- Ibarz A, Garza S, Pagan J. Nonenzymatic browning of selected fruit juices affected by D-galacturonic acid. Int J Food Sci Tech. 2008;43:908–914. doi: 10.1111/j.1365-2621.2007.01541.x. [DOI] [Google Scholar]
- Jain SK, Khurdiya DS. Physico-chemical characteristics and post-harvest technology of aonla (Phyllanthus emblica L.)—a resume. Indian Food Packer. 2002;47:46–49. [Google Scholar]
- Jain SK, Khurdiya DS. Ascorbic acid content and non-enzymatic browning in stored Indian gooseberry juice as affected by sulphitation and storage. J Food Sci Technol. 2009;46:500–501. [Google Scholar]
- Kacem B, Cornell JA, Marshall MR, Shireman RB, Matthews RF. Non-enzymatic browning in aseptically packaged orange drinks: effect of ascorbic acid, amino acids and oxygen. J Food Sci. 1987;52:1668–1672. doi: 10.1111/j.1365-2621.1987.tb05902.x. [DOI] [Google Scholar]
- Mehta U, Rathore H. Storage studies of pressed juice from aonla (Phyllanthus emblica L.) Indian Food Packer. 1976;30:9–11. [Google Scholar]
- Pandey D, Misra AK 2007. Scientific Cultivation of Aonla. Extension Folder No. 2007 (3), CISH, Lucknow
- Pathak RK, Pandey D, Misra AK, Mishra M (2003) Aonla for Health and Prosperity. Extension Literature 18, CISH, Lucknow
- Ranganna S. Handbook of analysis and quality control for fruit and vegetable products. 2. New Delhi: Tata McGraw-Hill Publishing Company Ltd.; 1986. [Google Scholar]
- Shinoda Y, Komura H, Homma S, Murata M. Browning of model orange juice solution: factors affecting the formation of decomposition products. Biosci Biotechnol Biochem. 2005;69:2129–2137. doi: 10.1271/bbb.69.2129. [DOI] [PubMed] [Google Scholar]
- Singh S, Singh AK, Joshi HK. Standardization of maturity indices in Indian gooseberry (Emblica officinalis Gaertn) under semi-arid conditions of Gujarat. Indian J Agr Sci. 2006;76:591–595. [Google Scholar]
- Suresh Kumar P, Sagar VR. Influence of packaging materials and storage temperature on quality of osmo-vac dehydrated aonla segments. J Food Sci Technol. 2009;46:259–262. [Google Scholar]