Abstract
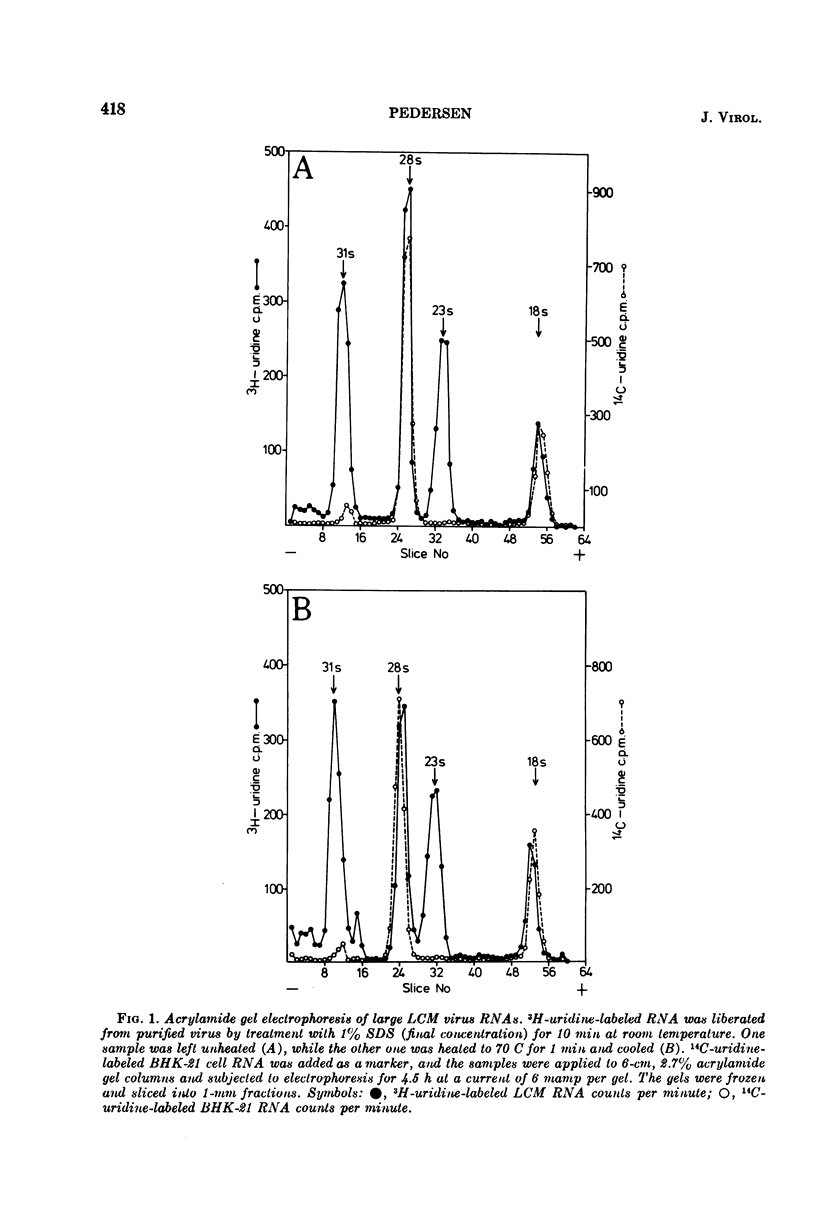
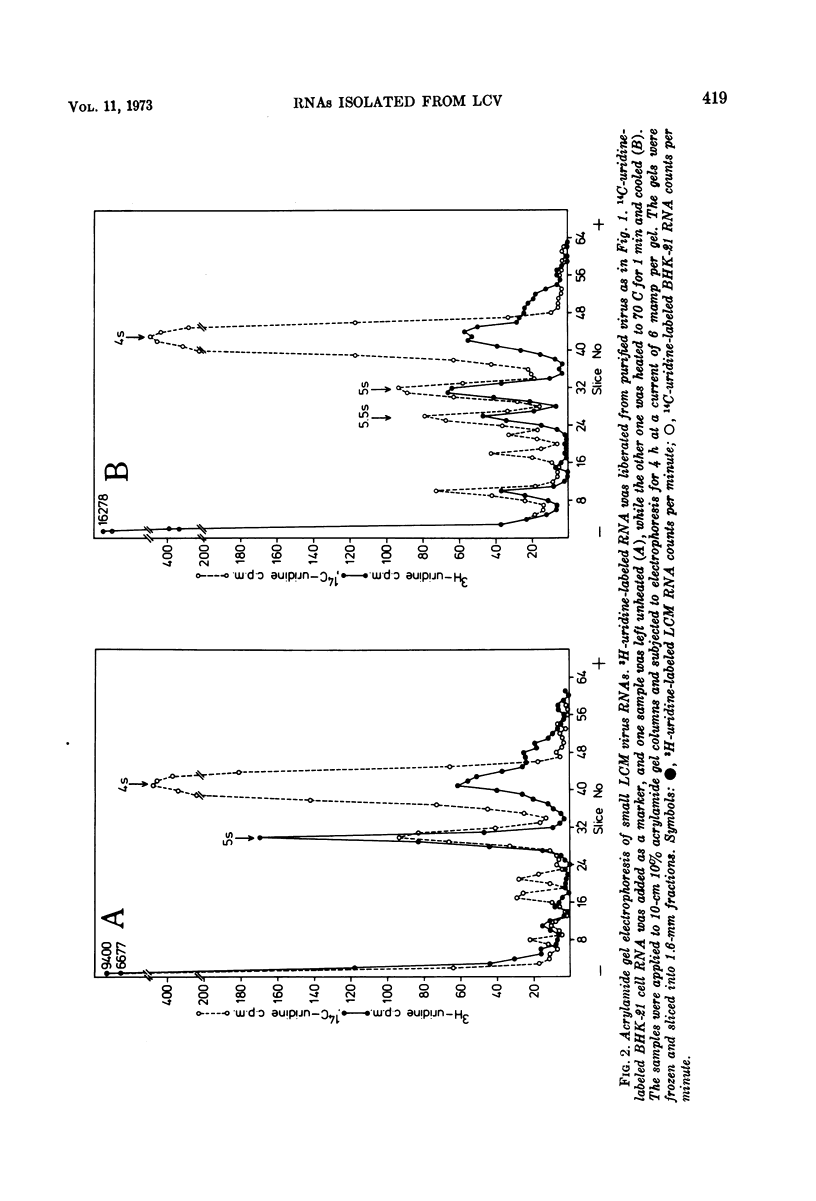
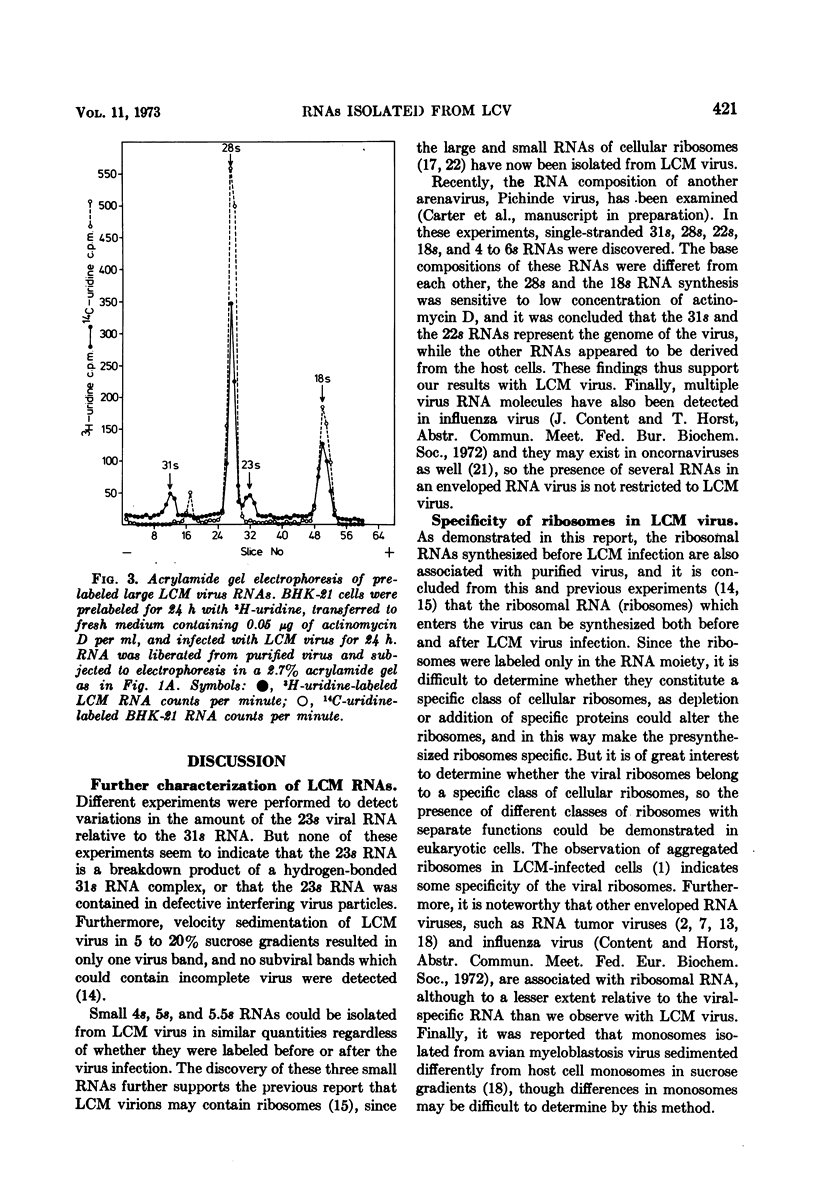
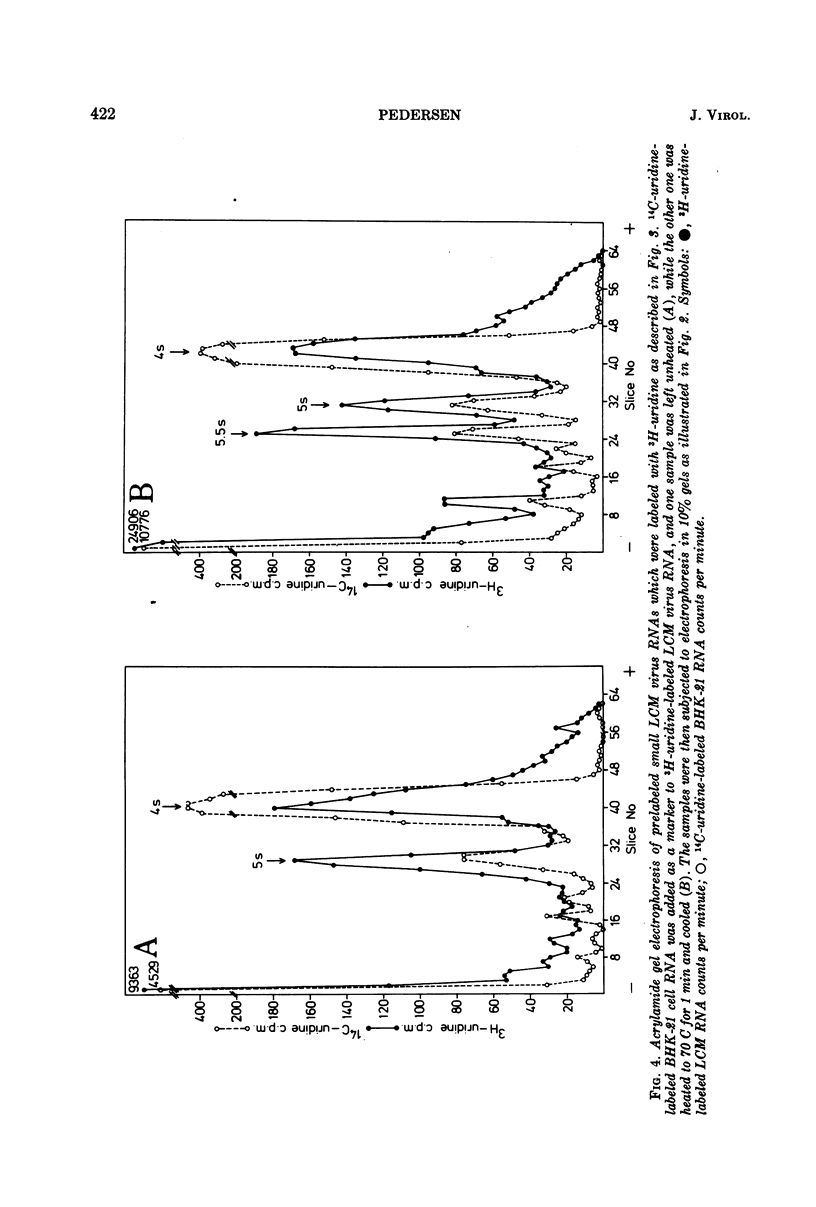
Purified preparations of lymphocytic choriomeningitis virus (LCM virus) contain three classes of RNA. The previously described 18s, 23s, 28s, and 31s RNAs, where the 23s and 31s RNAs are viral-specific, and the 18s and 28s RNAs probably are host RNAs incorporated in the virion. Now, 4s, 5s, and 5.5s RNAs can be isolated as well. Thus five RNAs which migrate by acrylamide gel electrophoresis as ribosome-derived RNA can be isolated from purified LCM virus. This observation further supports the reports that arenaviruses may contain ribosomes. The ribosome-derived RNA can be synthesized both before and after the virus infection. The viral 23s could be a hydrogen-bonded complex forming the 31s RNA, or it could be contained in defective interfering LCM virus particles; these possibilities are examined.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Smith G. H., Hoffman H. A., Rowe W. P. Use of enzyme-labeled antibody for electron microscope localization of lymphocytic choriomeningitis virus antigens in infected cell cultures. J Natl Cancer Inst. 1969 Mar;42(3):497–515. [PubMed] [Google Scholar]
- Bonar R. A., Sverak L., Bolognesi D. P., Langlois A. J., Beard D., Beard J. W. Ribonucleic acid components of BAI strain A (myeloblastosis) avian tumor virus. Cancer Res. 1967 Jun;27(6):1138–1157. [PubMed] [Google Scholar]
- Canaani E., Duesberg P. Role of subunits of 60 to 70S avian tumor virus ribonucleic acid in its template activity for the viral deoxyribonucleic acid polymerase. J Virol. 1972 Jul;10(1):23–31. doi: 10.1128/jvi.10.1.23-31.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton A. J., Rowe W. P., Smith G. H., Wilsnack R. E., Pugh W. E. Morphological and cytochemical studies on lymphocytic choriomeningitis virus. J Virol. 1968 Dec;2(12):1465–1478. doi: 10.1128/jvi.2.12.1465-1478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri G. L., Green H. Ribosomal RNA synthesis in human-mouse hybrid cells. J Mol Biol. 1969 Apr;41(2):253–260. doi: 10.1016/0022-2836(69)90390-8. [DOI] [PubMed] [Google Scholar]
- Erikson R. L. Studies on the RNA from avian myeloblastosis virus. Virology. 1969 Jan;37(1):124–131. doi: 10.1016/0042-6822(69)90313-4. [DOI] [PubMed] [Google Scholar]
- Gay F. W., Clarke J. K., Dermott E. Morphogenesis of Bittner virus. J Virol. 1970 Jun;5(6):801–806. doi: 10.1128/jvi.5.6.801-816.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A., Darlington R. W. Properties of incomplete Sendai virions and subgenomic viral RNAs. Virology. 1970 Dec;42(4):857–871. doi: 10.1016/0042-6822(70)90335-1. [DOI] [PubMed] [Google Scholar]
- Lehmann-Grube F., Slenczka W., Tees R. A persistent and inapparent infection of L cells with the virus of lymphocytic choriomeningitis. J Gen Virol. 1969 Jul;5(1):63–81. doi: 10.1099/0022-1317-5-1-63. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Webb P. A., Johnson K. M., Whitfield S. G., Chappell W. A. Arenoviruses in Vero cells: ultrastructural studies. J Virol. 1970 Oct;6(4):507–518. doi: 10.1128/jvi.6.4.507-518.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F. A., Webb P. A., Johnson K. M., Whitfield S. G. Morphological comparison of Machupo with lymphocytic choriomeningitis virus: basis for a new taxonomic group. J Virol. 1969 Oct;4(4):535–541. doi: 10.1128/jvi.4.4.535-541.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara T., Bolognesi D. P., Bauer H. Ribosomal RNA in avian leukosis virus particles. Int J Cancer. 1971 May 15;7(3):535–546. doi: 10.1002/ijc.2910070320. [DOI] [PubMed] [Google Scholar]
- Pedersen I. R. Density gradient centrifugation studies on lymphocytic choriomeningitis virus and on viral ribonucleic acid. J Virol. 1970 Oct;6(4):414–420. doi: 10.1128/jvi.6.4.414-420.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen I. R. Lymphocytic choriomeningitis virus RNAs. Nat New Biol. 1971 Nov 24;234(47):112–114. doi: 10.1038/newbio234112a0. [DOI] [PubMed] [Google Scholar]
- Pedersen I. R., Volkert M. Multiplication of lymphocytic choriomeningitis virus in suspension cultures of Earle's strain L cells. Acta Pathol Microbiol Scand. 1966;67(4):523–536. doi: 10.1111/apm.1966.67.4.523. [DOI] [PubMed] [Google Scholar]
- Pene J. J., Knight E., Jr, Darnell J. E., Jr Characterization of a new low molecular weight RNA in HeLa cell ribosomes. J Mol Biol. 1968 May 14;33(3):609–623. doi: 10.1016/0022-2836(68)90309-4. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Murphy F. A., Bergold G. H., Casals J., Hotchin J., Johnson K. M., Lehmann-Grube F., Mims C. A., Traub E., Webb P. A. Arenoviruses: proposed name for a newly defined virus group. J Virol. 1970 May;5(5):651–652. doi: 10.1128/jvi.5.5.651-652.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneck L. D., Trowbridge R. S., Welsh R. M., Wright E. A., Pfau C. J. Arenaviruses: cellular response to long-term in vitro infection with parana and lymphocytic choriomeningitis viruses. Infect Immun. 1972 Oct;6(4):444–450. doi: 10.1128/iai.6.4.444-450.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Mechanism of cell transformation by RNA tumor viruses. Annu Rev Microbiol. 1971;25:609–648. doi: 10.1146/annurev.mi.25.100171.003141. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Small molecular weight monodisperse nuclear RNA. J Mol Biol. 1968 Dec;38(3):289–304. doi: 10.1016/0022-2836(68)90387-2. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., O'Connell C. M., Pfau C. J. Properties of defective lymphocytic choriomeningitis virus. J Gen Virol. 1972 Dec;17(3):355–359. doi: 10.1099/0022-1317-17-3-355. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Pfau C. J. Determinants of lymphocytic choriomeningitis interference. J Gen Virol. 1972 Feb;14(2):177–187. doi: 10.1099/0022-1317-14-2-177. [DOI] [PubMed] [Google Scholar]



