Abstract
The aim of this work was to reduce the natural fermentation period of ‘idli’ from the conventional 14 h to 10 h by adding underutilized okara for the preparation of ‘idli’. Black gram was partially substituted with soy residue okara in the ratio of (1:1). After 14 h of natural fermentation, the pH and total acidity of control ‘idli’ batter was 4.51 and 0.64% and that of okara fortified ‘idli’ batter was 4.53 and 0.43%, respectively. The amount of CO2 released by the control and okara fortified batter was 19.7% and 33.6% respectively. The viable count of yeast and mold, lactics and mesophilic bacteria in control & okara batter increased with time reaching 9.00 & 10.34, 8.66 & 7.69, and 8.65 & 9.47 log10 cfu/g, respectively at the end of 10 h of natural fermentation. Okara fortified ‘idli’ was soft and spongy compared to control ‘idli’ .
Keywords: Batter, Natural fermentation time, Fortification, ‘idli’, Soy residue okara
‘idli’ is a traditional steam cooked, popular fermented breakfast food, especially in Southern parts of India. The process of fermentation of ingredients is essential which determines the quality of the end product ‘idli’. ‘idli’ is prepared by steaming the mixture of fermented rice (Oryza sativa) and black gram (Phaseolus mungo) batter in the ratio of 3:1. It makes an important contribution to the diet as a source of protein, calories and vitamins, especially B-complex vitamins, compared to the raw unfermented ingredients (Reddy et al. 1982). Table 1 gives the proximate composition of rice soji, black gram dhal and okara. During the preparation, fermentation time is an important step, which determines the sensory attributes and nutritional quality of ‘idli’ in terms of flavor and texture. ‘idli’ is famous for its soft spongy texture, desirable sour taste and characteristic aroma (Nisha et al. 2005).
Table 1.
Proximate composition of rice soji, black gram dhal and okara
| Parameter | Rice soji | Black gram dhal | okara |
|---|---|---|---|
| Moisture (%) | 13.3 | 10.9 | 8.4 |
| Protein (%) | 6.4 | 24.0 | 31.7 |
| Fats (%) | 0.4 | 1.4 | 14.7 |
| Fibre (%) | 0.2 | 0.9 | 33.6 |
| Minerals (%) | 0.7 | 3.2 | 2.0 |
| Carbohydrate (%) | 79.0 | 59.6 | 9.6 |
The biochemical changes occurring during natural fermentation include increase in non-protein nitrogen, total acids, soluble solids, methionine, cystine and a decrease in reducing sugars, pH and soluble nitrogen (Desikachar et al. 1960; Steinkraus et al. 1967). During natural fermentation water soluble B-vitamins and vitamin C increases and phytate phosphorous, which interferes with the absorption of both calcium and iron decreases significantly. There are reports that the rice and legume batter fermentation reduce phytate and tannin concentration (Hemalatha and Srinivasan 2007). In recent times fermented legume and cereal products are becoming popular in the developed countries due to their nutritive value and organoleptic characteristics (Sanjeev and Dhanwant 1990) and also considered as health foods. Fortification of popularly consumed staple foods, such as cereals, with inexpensive plant protein sources, notably legumes, has been increasingly exploited in these countries. By this way, the protein quality of staple foods is improved through a mutual complementation of their limiting amino acids (Annan et al. 2005).
The most common legumes used in India are bengal gram, red gram, green gram and black gram. Soybean, (Glycine max) although is superior to these legumes in nutritive value, its use is limited due to the presence of trypsin inhibitors and hemagglutinins (Ramakrishnan et al. 1976). Fortification of soybean in fermented foods is advantageous as increase in digestibility and nutritive value of fermented foods was reported (Rajalakshmi and Vanaja 1967). Soybean is known for its polyphenols mainly isoflavones, which are important phytoestrogens and exerts many beneficial effects, like reducing menopausal symptoms, reduction in bone loss, cancer, antioxidant activity and also has hypocholesterolemic effect (Tsangalis et al. 2002). Natural fermentation of soybean brings desirable changes in taste and texture and may result in the breakdown of some of the antinutritional factors.
While manufacturing soymilk large amounts of spent residue (okara) is generated as a waste byproduct. Due to high water content, its disposal poses a severe environmental problem. During soymilk production, about 70% of soybean solids and 80% of soybean proteins are extracted and the residue solids make up okara. From 1 kg of dry soybeans about 1.0 to 1.1 kg of fresh okara is produced. A high protein content of 27–32% (on dry basis) makes okara a potential source of low cost plant origin protein for human nutrition (Chan and Ma 1999). The addition of soy residue okara to ‘idli’ batter is found to be the most appropriate and cost effective technique. In a report on Ghanaian fermented maize dough, a significant reduction in natural fermentation time was seen with the addition of okara, as a result of acceleration in acid production (Annan et al. 2005). In the present study, the effect of fortification of ‘idli’ batter with okara on natural fermentation rate, physicochemical parameters and microbial growth in ‘idli’ batter and, texture and sensory attributes of ‘idli’ were studied. This is the first report on the use of okara for ‘idli’ batter natural fermentation.
Materials and methods
Preparation of okara
Soybean was purchased from a local market. Hundred grams of cleaned soybeans were soaked in 800 ml water for 12–14 h, rinsed 2 or 3 times, dehulled manually and ground with 400 ml of water in an electrically operated blender for about 2–3 min. Additional water was added to make up the volume of Soy slurry to 800 ml (Water to bean ratio was 8:1). The soy slurry was filtered through a double layered cheese cloth to obtain soymilk and the okara was separated. The wet okara was dried in an oven at 45°C for 10–12 h and stored in airtight containers.
Preparation of control ‘idli’ batter
‘idli’ soji (oryza sativa) and black gram dhal (Phaseolus mungo) were purchased from local market in one lot for the entire study. Rice soji and black gram dhal was taken in the ratio of 3:1(Jama and Varadaraj 1999). Dehusked split black gram dhal (30 g) was washed twice, soaked in 120 ml water for 4 h at room temperature (28 ± 2°C) and ground separately with required quantity of water into a batter of desirable consisitency, using an electrically operated blender. Rice soji (90 g) was washed with water and mixed with dhal batter. The batter was dispensed into commercially available metallized polyester polyethylene pouches of 20 x 18 cm and heat sealed using a hand sealing machine (Quick Seal, Sevana, India) and allowed to ferment for 14 h at room temperature. After every 2 h of natural fermentation pH, acidity and the amount of CO2 released were measured. At the end of natural fermentation, the fermented batter was distributed into hollow depressions in steaming pans and steam cooked for 10 min.
Preparation of okara fortified ‘idli’ batter
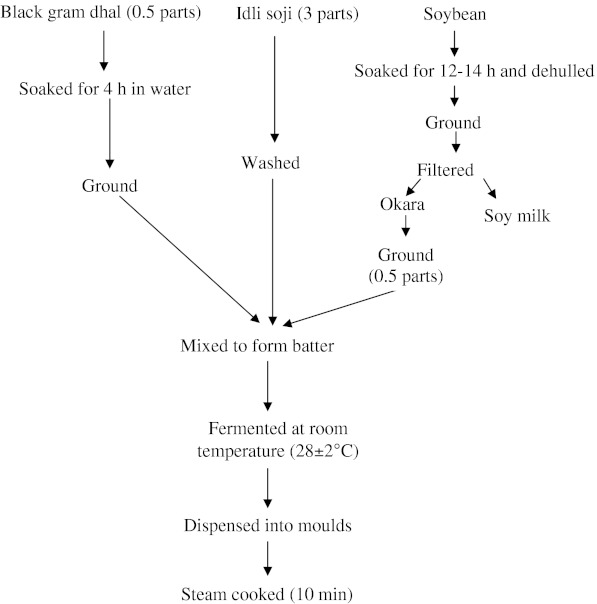
‘idli’ batter was prepared using two different proportions of rice soji, black gram and okara. To standardize ideal ratio, batters were prepared with different combinations of rice soji, black gram dhal and okara (3:0.5:0.5 and 3:1:0.5). For this 0.15 g of okara was ground to fine paste by adding little amount of water and packed in metallized polyethylene pouches and sealed. This was allowed to ferment for 10 h at room temperature. Total acidity, pH, acidity and the amount of CO2 released were measured after every 2 h of natural fermentation time. At the end of natural fermentation, the fermented batter was distributed into hollow depressions (moulds) pans and steam cooked (Fig. 1).
Fig. 1.
Flow chart for ‘idli’ preparation with okara supplementation
Analysis of batter
Titrable acidity and pH
The pH of the samples was measured using a pH meter (Cyberscan-Eutech Instruments, India). To determine titrable acidity, 10 g of fermented batter was taken in a 100 ml conical flask to which 20 ml of distilled water was added. After adding 3–4 drops of phenolphthalein, the contents were mixed well and titrated against 0.1 N NaoH to an end point of pale pink colour and expressed as % lactic acid produced (AOAC 1984).
Measurement of CO2
Amount of CO2 released by the fermented batter was detected using CO2 Analyzer (phi Dan sensor, Denmark).
Microbiological analysis
To determine viable count of Lactic acid bacteria (LAB), mesophilic bacteria and yeast and mold of the naturally fermented ‘idli’ batter (control and okara fortified batter), 10 g of dough samples were homogenized with 90 ml of sterile diluents (0.85% NaCl) for 2 min in a shaker at normal speed. Ten fold serial dilutions were prepared and pour plated on MRS agar for the enumeration of LAB. Spread plate technique was employed to determine the counts of total mesophilic bacteria, and yeast and molds using Nutrient agar (NA) and Potato dextrose agar (PDA) respectively.
Measurement of increase in batter volume during natural fermentation
A 50 ml of batter was transferred to a sterile measuring cylinder of 250 ml capacity, covered with aluminum foil and incubated at room temperature. The increase in volume at 0 and 10 h of natural fermentation was recorded and the raise in batter volume was expressed as the % volume increase over the initial volume.
Texture analysis of the ‘idli’
The texture of ‘idli’s was analyzed using texture analyzer TA–Hdi (Stable Microsystems, Surrey, U.K.) with a cross head speed of 0.5 mm/s and with 50% compression for hardness and stickiness parameters (Bharti and Laxmi 2008). Among the several textural parameters, hardness and stickiness were selected to represent the results because of their repeatability and reasonable variations. Measurements were performed in six replicates and the average was reported in Newtons.
Sensory evaluation of the ‘idli’s
‘idli’s prepared with different batters were subjected to sensory evaluation by the method of Quantitative Descriptive Analysis (QDA) (Stone and Sidel 1998), employing a trained panel. During initial session descriptors of the product were obtained by “Free choice profiling”. Panelists were asked to describe the samples with as many spontaneous descriptive terms as they found applicable. The common descriptors chosen by more than one third of the panel was used in preparing a score card consisting of a 15 cm scale wherein 1.25 cm was anchored as low and 13.75 cm as high. The panelists were asked to quantify the perceived intensity of attributes by marking a vertical line on the respective scale and writing the code number of the sample. They were also asked to indicate the overall quality of the product on an intensity scale which was anchored at very poor, fair and very good to assess the liking or preference of the product. The scores for all the attributes were tabulated and the mean values were calculated. These mean scores represented the panel’s judgement about the sensory quality of the samples.
Statistical analysis
The data relating to the textural parameters and sensory studies were evaluated using analysis of variance and the mean values were separated by Duncan’s multiple range test.
Results and discussion
Effect of okara fortification on pH and Titrable acidity
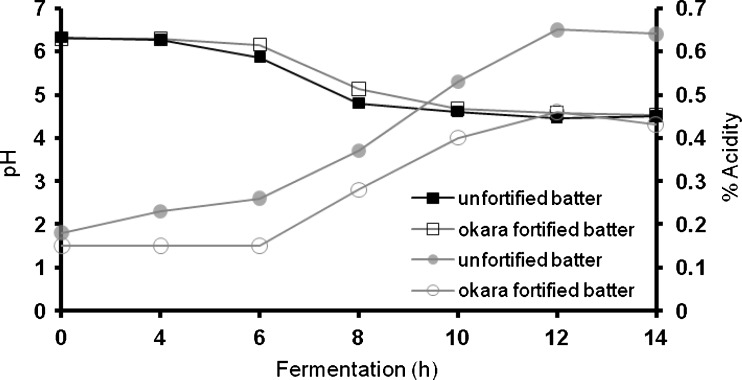
The changes in pH and acidity of okara fortified and unfortified ‘idli’ batter are represented in Fig. 2. The pH of control batter at 0 h was 6.32 which decreased to 4.51 at the end of 14 h of natural fermentation and in okara fortified batter the pH at 0 h was 6.30 and 4.53 at 14 h of natural fermentation. With the progress of natural fermentation, increase in the acid content of batter during natural fermentation was observed in both control and okara fortified ‘idli’ batter (Fig. 2). The acidity increased from 0.18 to 0.64 in control batter and from 0.15 to 0.43 in okara fortified batter over a period of 14 h of natural fermentation. Although the pH of the control batter and okara batter was almost same, the acidity was significantly different. This is due to the buffering effect caused by the higher content of soluble proteins, amino acids and also the free fatty acids of the beans (Annan et al. 2005). The increase in acidity was seen within 2 h of natural fermentation in control batter and after 6 h of natural fermentation in okara fortified batter.
Fig. 2.
Effect of natural fermentation period on pH and total acidity of control ‘idli’ batter and okara fortified batter
The change in pH and acid content can be attributed to the microbial growth especially of lactics producing lactic acid, which lowers the pH and leavens the batter. The role of LAB is to reduce the pH of the batter to an optimum level (4.4–4.5) for yeast activity. This accounts for fall in pH and rise in acid content of batter as the natural fermentation progresses. These findings are in accordance with the data reported by Soni and Sandhu (1990).
Measurement of increase in batter volume during natural fermentation
There was a noticeable change in batter volume during natural fermentation (Table 2). At the end of 10 h of natural fermentation, 20% raise in unfortified batter and 55% raise in okara fortified batter was seen. There was an increase of 35% raise in okara fortified batter compared to unfortified batter. The increase in volume might be due to the CO2 production by the yeast during natural fermentation and is a measure of their metabolic activity. This is also because of combined contribution of both heterofermentative lactobacilli and non LAB (Thyagaraja et al. 1992). Since both leavening and acid development are required for ‘idli’ (Susheelamma and Rao 1978), determination of the end point of the ‘idli’ fermentation becomes rather arbitrary. However, the use of different ingredients in different proportions resulted in raise in volume besides reduction of fermentation period.
Table 2.
Microbiological profile and raise in batter volume of control and okara fortified batter
| Parameter | Natural fermentation period (h) | Type of batter | |
|---|---|---|---|
| Control | okara fortified | ||
| Lactic count (log10 cfu/g)a | 0 | 5.8 ± 0.35 | 6.8 ± 0.88 |
| 10 | 8.6 ± 1.85 | 7.7 ± 1.23 | |
| Yeast and mold count ( log10 cfu/g)a | 0 | 8.0 ± 1.66 | 8.5 ± 1.56 |
| 10 | 9.0 ± 1.72 | 10.3 ± 1.82 | |
| Mesophilic bacteria count (log10 cfu/g)a | 0 | 8.3 ± 1.40 | 8.3 ± 1.36 |
| 10 | 8.6 ± 1.65 | 9.5 ± 1.22 | |
| Batter volume (ml) | 0 | 25 | 25 |
| 10 | 30 (20)* | 39 (55)* | |
aMean ± SD (n = 3). cfu/g: Colony forming units per gram, *Values in bracket indicate % raise in batter
Measurement of CO2
The amount of CO2 released by the fermented batter was analyzed from 0 to 14 h. At 10 h of natural fermentation the% of CO2 released in control was 4.0 and a tremendous increase in CO2 was seen in okara fortified batter (33.6). Further at 14 h (at the end of natural fermentation),% of CO2 was 19.7 and 35.5 in control and okara fortified batter, respectively. The increase in CO2 production was very significant in okara fortified batter within 10 h of natural fermentation. This is due to the presence of more number of yeasts present in okara fortified batter producing CO2 as a byproduct during its metabolism. It has also been reported earlier that yeasts are responsible for more than 50% of the CO2 and two fold increase in the volume of batter (Venkatasubbaiah et al. 1984).
Microbiological analysis
The microbial analysis of both the batter samples presented in Table 2 revealed that both bacteria and yeasts play an important role throughout the process of natural fermentation. The counts of mesophilic bacteria and LAB present naturally in batter showed a progressive increase in their numbers with increase in time. There was a significant increase in yeast and mold count in okara fortified batter compared to control batter. It was seen that the bacterial counts were higher in the okara fortified batter than that of control batter. There was two log increase in LAB count in control batter compared to okara fortified batter. This is due to the growth of LAB in sourdough and has been found to be enhanced by the presence of amino acid (Gobetti et al. 1994). There was only one log increase in the okara batter.
‘idli’ fermentation is a mixed auto fermentation (Soni et al. 1985). Organisms present in the ingredients as well as the environmental contaminants determine the type of the organisms involved in the natural fermentation. Lewis et al. (1955) have reported a number of wild yeast combined with different LAB in the fermenting batters. The fact that both bacteria and yeast participate in the fermentation has been further clearly shown by Desikachar et al. (1960) using Penicillin G and chlorotetracycline as competitive inhibitors. Venkatasubbaiah et al. (1984) observed the involvement of both bacteria and yeasts in ‘idli’ batter fermentation.
From the above results it can be seen that initial natural fermentation was a ‘free for all’ with lactic and non-LAB growing together. A lag period for both lactic and non-LAB existed. The metabolic activities resulting in decrease in pH and, increase in acidity and batter volume were negligible during this period. When both lactic and non-LAB reached the end of log phase, the LAB were established as the main flora and the number of surviving non-LAB decreased, where as LAB count strangely remained constant throughout the rest of the natural fermentation. The decrease in non-LAB may be due to the antagonistic action of LAB, which is known to exert an inhibitory action by the production of lactic acid, hydrogen peroxide and bacteriocins, as well as decreasing the pH making the medium unfavorable for the growth of non-LAB (Thyagaraja et al. 1992). This is in correlation with the control batter, wherein the lactic count was more than okara fortified batter. But in okara fortified batter, the non lactic count (yeast, mold and other bacterial count) was higher than LAB. This observation is in accordance with Sarkar et al. (1994), who have reported progressive increase in the count of Bacillus subtilis and Enterococcus faecium and Candida parapsilosis in fermented soybeans. Similarly, in okara fortified batter there was an increased count of bacteria and yeast and lesser count of lactic count compared to control batter. In okara fortified batter, as there was less number of LAB, there was no antagonistic activity of LAB and increase in yeast count resulted in an increase in CO2%, resulting in soft and spongy texture of ‘idli’.
Texture analysis of ‘idli’
Table 3 shows the result of textural parameters of ‘idli’s prepared using control and okara fortified batter. ‘idli’ has a circular shape of approximately 7–10 cm diameter (depending on the mould size) flat with lower and upper surface bulging, so that the product is thick at the center (2–4 cm) and tapering towards periphery (Nisha et al. 2005). Hardness is measured as the peak force during compression in the first cycle. Hardness of traditional ‘idli’ was 33.05 Newton and ‘idli’ prepared with okara substituted batter fermented for 10 h ‘idli’ was 24.4 Newton, respectively. These values indicated that the control ‘idli’ offered more resistance to compression than that of okara fortified ‘idli’. Thus the okara substituted samples were softer and easy to bite compared to control samples (soft texture of ‘idli’s is a desirable quality). This is due to the microbes present in okara especially yeasts, which produced CO2 during natural fermentation resulting in a softer product and partial substitution of black gram with okara might have contributed for accelerated natural fermentation. On the other hand the values for stickiness in case of okara ‘idli’ were relatively low. The stickiness of traditional ‘idli’ was 0.13 Newton and okara substituted ‘idli’ was 0.14 Newton. The adhesiveness of traditional and okara substituted ‘idli’ did not have significant difference and was 0.053 and 0.050 Newtons, respectively.
Table 3.
Texture and sensory profile of ‘idli’ prepared from control and okara fortified batter (Sample size 7 cm diameter)
| Type of ‘idli’ | ||
|---|---|---|
| Parameter | Control batter | Okara fortified batter |
| A) Texture analysis | ||
| Hardness, (N. s)◊ | 33.05a | 24.40 |
| Stickiness, (N. s) | 0.13a | 0.14a |
| Adhesiveness, (N. s) | 0.053b | 0.050b |
| B) Sensory attribute | ||
| Buff | 6.65 | 6.91 |
| Fluffiness | 7.88 | 7.98 |
| Compactness | 7.23 | 7.81 |
| Sponginess | 7.07a | 7.41a |
| Firmness | 5.96 | 6.17 |
| Sticky | 3.63a | 5.67b |
| Beany | 2.67a | 3.64b |
| Fermented | 6.98 | 6.99 |
| Salty | 5.91b | 5.00a |
| Sour | 4.28 | 4.31 |
| Bitter after taste | 2.73 | 3.18 |
Means followed by different small letters as superscripts in the same row are significantly different (p ≤ 0.05) (n = 3), n = 6 panelists, N. s◊ -Newtons
Sensory evaluation of ‘idli’s
The scores of sensory evaluation of control and okara fortified ‘idli’ batter are presented in Table 3. For sensory attributes of the final product (‘idli’), a 14 h fermented batter prepared in traditional method was taken as control, as it was reported optimum time for natural fermentation of ‘idli’ batter (Steinkraus et al. 1967; Yajurvedi 1980). It has been reported by Soni and Arora (2000) that yeast involved in the fermentation not only contribute towards gas production, which results in good texture but also towards the sensory qualities of the ‘idli’. The difference in sensory quality of control and okara containing samples was significant in some of the attributes viz sponginess, sticky, beany, salty and overall quality. Natural fermentation of okara fortified batter for 10 h resulted in ‘idli’ with more sponginess and fluffy texture when compared to the control. This is due to more yeast growth when compared to the naturally fermented batters (Bharti and Laxmi 2008).
Conclusion
The addition of soy residue okara to the ‘idli’ batter accelerated the natural fermentation rapidly and shortened the fermentation time. According to reported literature, natural fermentation time of the batter varies from 14 to 24 h with overnight natural fermentation being the most frequently practiced. Fortification of okara to ‘idli’ batter has a beneficial effect in terms of higher amount of gas production and leavening during natural fermentation. Increase in CO2 production (33.6%) resulted in soft and spongy ‘idli’ compared to control sample. Thus, the fermentation time was brought down to 10 h from the reported 14 h and with improved quality of the final product. Reduction in the fermentation time of the ‘idli’ batter is of great commercial significance for large scale ‘idli’ production (Bharti and Laxmi 2008) and this can be potentially achieved by addition of soy residue okara. Thus the under-utilized protein rich okara can be used for the preparation of value added fermented products.
Acknowledgments
First author thanks University Grants Commission, New Delhi, for giving the opportunity to pursue the research work.
References
- Annan NT, Plahar WA, Poll L, Jakobsen M. Effect of soybean fortification on Ghanaian fermented maize dough aroma. Int J Food Sci Nutr. 2005;56(5):315–326. doi: 10.1080/09637480512331390655. [DOI] [PubMed] [Google Scholar]
- Official Methods of analyis 14th edn. Washington DC: Association of Official Analytical Chemists; 1984. [Google Scholar]
- Bharti K, Laxmi A. Effect of α−amylase addition on natural fermentation of ‘idli’- A popular south Indian cereal-Legume based snack food. Lebensm Wiss Technol. 2008;41(6):1053–1059. [Google Scholar]
- Chan WM, Ma CY. Modification of proteins from soymilk residue (okara) by trypsin. J Food Sci. 1999;64(5):781–786. doi: 10.1111/j.1365-2621.1999.tb15911.x. [DOI] [Google Scholar]
- Desikachar HSR, Radhakrishna Murty R, Rama Rao G, Kadkol SB. Studies on ‘idli’ natural fermentation: part I-some accompanying changes in the batter. J Sci Ind Res. 1960;19C:168–172. [Google Scholar]
- Gobetti M, Corsetti A, Rossi J. The sour dough microflora. Interactions between lactic acid bacteria and yeasts: metabolism of amino acids. World J Microbiol Biotechnol. 1994;10:275–279. doi: 10.1007/BF00414862. [DOI] [PubMed] [Google Scholar]
- Gopalan C, Rama sastri BV, Balsubranamian SC. Nutritive value of Indian foods. ICMR, Hyderabad: National Institute of Nutrition; 1971. [Google Scholar]
- Hemalatha SP, Srinivasan K. Influence of germination and natural fermentation on bioaccessibility of zinc and iron from food grains. Eur J Clin Nutr. 2007;61(3):342–348. doi: 10.1038/sj.ejcn.1602524. [DOI] [PubMed] [Google Scholar]
- Jama YH, Varadaraj MC. Antibacterial effect of plantaricin LP84 on foodborne pathogenic bacteria occurring as contaminants during ‘idli’ batter natural fermentation. World J Microbiol Biotechnol. 1999;15:27–32. doi: 10.1023/A:1008887201516. [DOI] [Google Scholar]
- Katayama M, Wilson LA. Utilization of okara, a byproduct from soymilk production, through the development of soy-Based snack Food. J Food Sci. 2008;73(3):S152. doi: 10.1111/j.1750-3841.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- Lewis YS, Johar DS, Subrahmanyan V (1955) Studies on process simplification in the preparation of a fermented type of foodstuff—‘idli’. Central Food Technological Research Institute, Bull 4, Mysore, India, 257
- Nisha P, Laxmi A, Rekha S. Effect of stabilizers on stabilization of ‘idli’ (traditional south Indian food) batter during storage. Food Hydrocoll. 2005;19:179–186. doi: 10.1016/j.foodhyd.2004.03.007. [DOI] [Google Scholar]
- Rajalakshmi R, Vanaja K. Chemical and biological evolution of the effects of natural fermentation on the nutritive value of foods prepared from rice and gram. Br J Nutr. 1967;21:467–473. doi: 10.1079/BJN19670048. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan CV, Parekh LJ, Akolkar PN, Rao GS. Studies on soy’idli’ natural fermentation. Plant Foods for Man. 1976;2:15–33. [Google Scholar]
- Reddy NR, Sathe SK, Pierson MD, Salunkhe DK. ‘idli’, an Indian fermented food: a review. J Food Qual. 1982;5(2):89–101. doi: 10.1111/j.1745-4557.1982.tb00736.x. [DOI] [Google Scholar]
- Sanjeev KI, Dhanwant K. Indian fermented foods: microbiological and biochemical aspects. Indian J Microbiol. 1990;30(2):135–157. [Google Scholar]
- Sarkar PK, Tamang JP, Cook PE, Owens JD. Kinema—a traditional soybean fermented food: proximate compostion and microflora. Food Microbiol. 1994;11:47–55. doi: 10.1006/fmic.1994.1007. [DOI] [Google Scholar]
- Soni SK, Arora JK. Indian fermented foods: Biotechnological approaches. Food processing: Biotechnological Application. New Delhi: Asiatech Publishers Pvt Ltd; 2000. [Google Scholar]
- Soni SK, Sandhu DK. Indian fermented foods: microbiological and biochemical aspects. Indian J Microbiol. 1990;30(2):135–157. [Google Scholar]
- Soni SK, Sandhu DK, Vikhu KS, Karma N. Studies in dosa—An indigenous Indian fermented food: some biochemistry and changes occurring during natural fermentation. Food Microbiol. 1985;3(1):45. doi: 10.1016/S0740-0020(86)80025-9. [DOI] [Google Scholar]
- Soni SK, Sandhu DK, Vikhu KS, Karma N. Microbiological studies on dosa natural fermentation. Food Microbiol. 1986;3:45–53. doi: 10.1016/S0740-0020(86)80025-9. [DOI] [Google Scholar]
- Steinkraus KH, Van Veen AG, Thiebeau DB. Studies on ‘idli’—An Indian fermented Black gram-rice food. Food Technol. 1967;21:916–91. [Google Scholar]
- Stone H, Sidel JL. Quantitative descriptive analysis: developments, applications and the future. Food Technol J. 1998;52:48–52. [Google Scholar]
- Susheelamma NS, Rao MVL. Isolation and characterization of arabino galactan from black gram (Phaseolus mungo) J Agric Food Chem. 1978;26(6):1434–1437. doi: 10.1021/jf60220a048. [DOI] [PubMed] [Google Scholar]
- Thyagaraja N, Otani H, Hosono A. Studies on microbiological changes during the natural fermentation of ‘Idly’. Lebensm Wiss Technol. 1992;25:77–79. [Google Scholar]
- Tsangalis D, Ashton JE, Mcgill AEJ, Shah NP. Enzymic transformation of isoflavone phytoestrogens in soymilk by β – glucosidase producing Bifidobacteria. J Food Sci. 2002;67:3104–13. doi: 10.1111/j.1365-2621.2002.tb08866.x. [DOI] [Google Scholar]
- Venkatasubbaiah P, Dwarkanath CT, Sreenivasamurthy V. Microbiological and physico chemical changes in ‘idli’ batter during natural fermentation. J Food Sci Technol. 1984;21:59–63. [Google Scholar]
- Yajurvedi RP (1980) Microbiology of ‘idli’ Natural fermentation. Indian Food Pack pp 33–38