Abstract
Increasing pressure in food conservation sector to replace chemical applications has urged researchers to focus on studying new strategies of extending the postharvest life of produces. In such efforts, numerous materials have been tested for their effectiveness as well as suitability in organic consumption. In this study, effects of modified atmosphere packing (MAP) and honey solution dip on maintenance of quality of minimally processed table grape cv. Razaki were investigated. During the storage at 0 °C with relative humidity of 90%, MAP, honey dip, and their combined applications significantly retarded the weight loss of berries that retained about 2 mm of cap stem. Soluble solid contents of all berries slightly increased, while their acid amounts decreased, resulting in consecutive rises of maturity index. With respect to the sensory score, calculated as mean of ten panelists, honey treatment alone was ranked the highest while control berries had significantly lower value. Overall, MAP, honey solution dip or their combination significantly maintained the general quality of minimally processed grape by delaying quality loss and berry decay. Therefore, honey solution dip yielded promising results to use as an edible organic coating barrier to moisture and resist to water vapor diffusion during the cold storage, offering a good adherence to berry surface.
Keywords: Vitis, Table grape, Postharvest management, Fresh-cut, Quality maintenance, Edible coating, Weight loss, Berry decay
Introduction
Consumer demand for minimally processed (fresh-cut) products has increased recently due to premium product quality and convenience for fast consumption. A major challenge facing this industry is maintaining and preserving the quality of minimally processed produce because the physical damage caused by preparation accelerate the metabolism with associated increases in certain biochemical reactions responsible for quality loss. Among the limitations to storage or shelf-life of minimally processed products, microbial decay, desiccation (shriveling), discoloration or browning, bleaching, textural changes and development of off-flavor are predominating problems (Kader 2002). A low temperature regime, although insufficient alone, has been the principal way of overcoming this challenge so far (Ergun and Ergun 2009). Increasing pressure in the food conservation field to replace chemical-based applications has urged researchers to focus on studying new reliable and healthy strategies of extending the postharvest and shelf life of produces (Tharanathan 2003). Previously, a number of materials were tested for their preservative potentials and appropriateness to organic consumption during storage of produces, such as ethanol (Karabulut et al. 2004; Lichter et al. 2002; Sabir et al. 2006), mineral oils (Valero et al. 2006), chlorinated or hot water (Del Nobile et al. 2008), ethephon (Jayasena and Cameron 2009), chitosan (a natural polysaccharide) (Romanazzi et al. 2009), and ozone (Sharpe et al. 2009). In these endeavors, undesirable odors or bitterness that may confer to the produces during storage have been significant limitations in practical applications of such materials (Soliva-Fortuny and Martín-Belloso 2003; Mishra et al. 2010).
It is widely accepted that modified atmosphere packaging (MAP) helps to retard tissue senescence and consequently extends storage life of produces (Ahvenainen 1996; Soylemezoglu 2001; Lurie et al. 2006). However, reliable knowledge about the practical use of MAP on the quality of minimally processed grapes is still limited. Kader (2002) recommended the use of MAP as a supplement to avoid skin browning incident which is a significant problem occurring in storage of perishable produces like grapes. Browning inhibition (62%) in slices of apples has been already achieved by dipping in 10% honey solution for 30 min at room temperature (Oszmianski and Lee 1990). Besides, Viuda-Martos et al. (2008) indicated that unique enzymes in honey have a powerful antimicrobial and antioxidant properties when honey is diluted with water. Chemically, basic component of honey is monosaccharide which is one of the components suggested as edible coating material in minimally processing industry (Garcia and Barrett 2002).
From this perspective, we hypothetically assumed that dipping into the honey solution might protect the postharvest quality of grape berries during storage by prohibiting the water loss from berry surface and delaying browning. Thus, the objective of this study was to determine the effects of MAP, honey dip solution or their combined use on maintenance of quality of minimally processed grapes (cv. Razaki, an important white table grape cultivar in Turkey with its high quality) during storage at 0 °C. Moreover, the possibility of honey application, as an organic thin layer of protective barrier, was aimed to investigate as an alternative mean for chemical-based treatments such as SO2.
Material and methods
Table grapes (Vitis vinifera L., cv Razaki) at commercial maturity were harvested from a commercial vineyard located in Konya Province/Turkey. No chemicals were applied to vineyard during the cultivation. Grapes harvested at commercial maturity were immediately transported to the laboratory. The rachis of the berry was removed and the cap stems (pedicels) were cut short with sanitized scissors so that the grapes retained about 2 mm of cap stem (Kou et al. 2007). Then the berries were selected to obtain homogeneous batches based on color, size, and the absence of blemishes or disease and were randomly distributed into batches. After selection (berries less than 15 mm in diameter were discarded), grapes were washed with tap water to remove residues and sorted in four groups for applications; (1) sealing with a single seal film (29.2 pmol/s/m2/Pa oxygen transmission rate, manufactured by a private company, Turkey) as control, (2) modified atmosphere packaging (MAP used was manufactured by StePac Company, Israel and having a CO2 and vapor permeability of 2203 cm3/m2.day.atm and 150 g/m2.day.atm at 23 °C, respectively), (3) honey dip treatment, and (4) honey dip treatment plus MAP. MAP packages used in this study were passive type (commodity-generated) in terms of air conditioning inside the packages.
A local type of honey (containing about 75% total sugar) known as “pine honey” (the name referring to the pine tree that grows around honey production area on the Torus Mountain located lengthwise the Mediterranean Sea) was used for honey dip treatment. This type of honey is widespread and easily available in this region. The solution was prepared by dissolving the pine honey in distilled water, with the percentage of 20% (v/v), under continuous stirring (the percentage of honey solution was adjusted according to the soluble solid content of grape at harvest). When dissolved, the berries of honey alone and honey plus MAP applications were dipped into the solution for 5 min. Care was taken to ensure that all berries were completely submerged into the honey solution. Berries of control group were dipped into distilled water for 5 min, while no application was made to MAP berries. All the berries were kept at the room temperature with a mild air movement for about 20 min in order to let the dew on berries evaporate at room temperature. Care was taken to ensure that the berries were free of any dew. Before packing, the berries were immediately cooled by transporting to storage room at 0 °C for 1 hour.
Each 200 g of berries packaged in a 12 × 15 cm rigid polypropylene cylinder cup. A total of 48 cups were used, 12 of which belonged to each treatment (apart from initial analysis). The packages were stored at 0 °C with a relative humidity of 90% for 28 day with quality evaluation performed on days 0, 7, 14, 21, and 28. For each treatment, consisted of 12 cups initially, three randomly chosen cups were used in each sampling date to analyze for weight loss, soluble solid content (SSC), pH, titratable acidity (TA), maturity index (TSS/TA), and sensory test. In addition to these assays, decay, browning, and shriveling percentages were also determined at the end of the storage for further evaluation.
Percentages of decay, browning, and shriveling were calculated separately by dividing the number of grapes in each package showing visible symptoms of relevant wastage by the total number of grapes in that package and multiplying the dividend by 100. Berry weight loss was calculated as a percentage of its fresh (harvest) weight. SSC (°Brix) was determined with a hand-held temperature-compensated refractometer (Atago 9313). TA was quantified by titrating 10 mL of the homogenized berry flesh juice (must) with 0.1 N NaOH to an endpoint of pH 8.1 and expressed as the percentage of tartaric acid. All assays were performed in triplicate.
For sensory evaluation during the storage period, a trained sensory panel consisting of 10 judges (5 male and 5 female, aged 23–30 years) evaluated the stored grapes according to the procedures described by Reitmeier and Nonnecke (1991) and Chervin et al. (2005). Panelists were initially screened for their consistency in perceiving the quality of date fruits. According to the previous studies, a grading scale for this test panel was established as follows: 1 bad, 2 not acceptable, 3 good, 4 very good and 5 excellent (exactly as the freshly harvested grapes). Three samples of each treatments were presented to panelists randomly received in individual rooms. Panelist was instructed to cleanse his or her mouth before proceeding to the next sample.
Data sets from analyzed parameters were subjected to analysis of variance (ANOVA). Sources of variation were time of storage, treatments, and their interactions. Comparisons of means were performed by Tukey’s multiple range tests at different significance levels. All analyses were preformed with SPSS software package v. 15.0 for windows.
Results and discussion
Weight loss
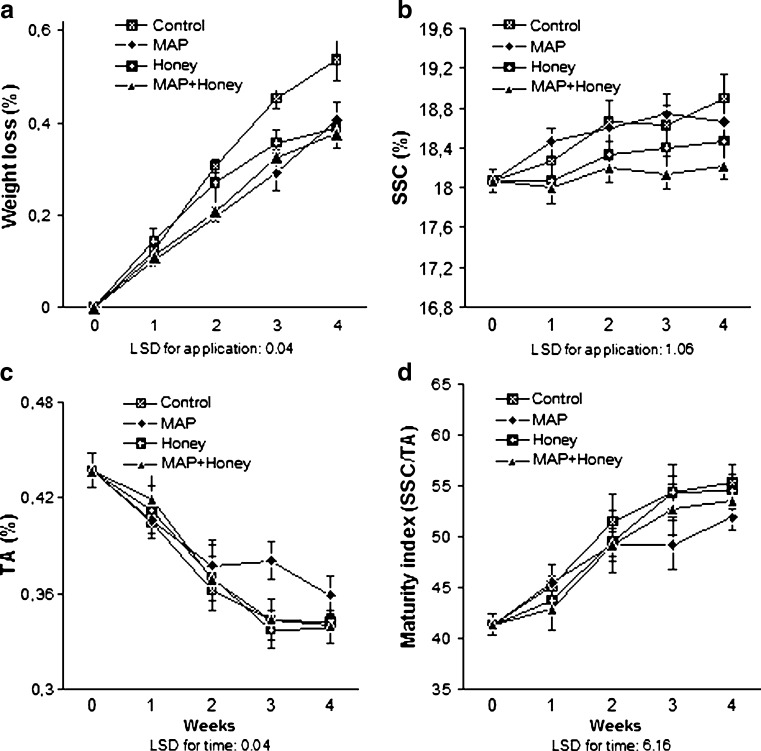
The greatest change that takes place in grapes in storage is loss of water which ultimately causes loss in weight (Kader 2002). Since the moisture content of fresh fruits, like grapes, is generally more than 80%, loss in water content during cold storage is inevitable (Sagar and Suresh Kumar 2010). Weight loss changes of grape fruit during the storage is shown in Fig. 1a. The rate of weight loss increased during the storage. Postharvest MAP, honey, and MAP plus honey treatments significantly (P < 0.0001) retarded the weight loss, compared to the control. The percentages of weight loss after 4 weeks of storage were 0.54%, 0.41%, 0.39% and 0.38% for the control, MAP, honey and MAP plus honey treatments, respectively. In a recent study in which suitability of 13 cultivars and 14 types for minimal processing was aimed to investigate, the weight loss values of stemless berries varied from 0.65% to 1.69% after 10-d storage at 4 °C (Ergun et al. 2008). In the previous studies using clusters of different cultivars without processing, weight loss values of Alphonse Lavallée and Razaki cultivars reached around 8.4% after 100 days at 0 °C (Eris et al. 1993). Similarly, Al-Bachir (1999) reported that weight loss of Baladi grape clusters after 4 weeks at 1–2 °C were between 6.3% and 7.1%, while they varied from 2.8% to 3.4% for Helwani, depending on the dose (kGy) of gamma irradiation tested. The weight loss values observed in this study are considerably lower than those of mentioned reports in which intact clusters were used, probably because of the fact that enormous quantities of water loss occur from cluster stem.
Fig. 1.
Changes in weight loss (a), soluble solid content (b), titratable acidity (c) and maturity index (d) of minimally processed grape berries during storage (n = 3)
The effect of MAP on reducing the water loss was obvious, which is considered one of the main advantages of the use of MAP in storage (Kader et al. 1989). Besides, honey dip with or without MAP showed similar impact on weight loss of stemless grape berries. As indicated by Winkler et al. (1974) and Grncarevic and Radler (1971), the surface of fresh grape berry has a waxy substance (called as bloom), occurring in amounts of about 0.1 mg/cm2, although mostly removed during handling. Ramming (2009) indicated that bloom inhibits water evaporation from berry surface. The results of present study indicate that honey coating might play a significant role, with a similar effect to that of bloom, in hindering the evaporation from the epicuticular tissue of grape berry.
SSC
SSC level at harvest were 18.07 °Brix and increased gradually along with the storage at 0 °C. At the end of the storage, SSC level of control berries (18.90 °Brix) was higher than those of treated ones, although statistically insignificant (Fig. 1b). On the other hand, SSC level of the fruit treated with MAP plus honey remained quite constant, reaching to 18.22 °Brix after 28 day of storage. Similar findings for SSC were reported in Redglobe table grape stored under different controlled atmosphere (CA) conditions at 0 °C for up to 12 weeks (Crisosto et al. 2002), where SSC level after storage were almost equal to that of the harvest. In Flame table grapes, Martínez-Romero et al. (2003) determined an increase in the SSC content of about 2.5 °Brix in control after 4 days at 1 °C, which was about 1 °Brix when stored under MAP conditions. Such progressive increases in SSC, explained as the consequences of water evaporation from berry surface, were widely reported in previous studies using different cultivars (Pretel et al. 2006; Sabir et al. 2006). In terms of magnitude of SSC increases during the storage, partially divergent results of mentioned studies confirm the assertions set forth by Al-Bachir (1999), Sabir et al. (2008) and Ergun et al. (2008) who emphasized the different aptitudes of various grape cultivars/types in reactions to different treatment or storage conditions.
TA
TA at harvest was 0.437 g 100 g−1 tartaric acid equivalent. During the storage period, TA content of the grape berries significantly (P < 0.0015) decreased with the increase in storage period, although the effects of treatments were insignificant (Fig. 1c). After 4 weeks of storage, TA levels were 0.359%, 0.342%, 0.339% and 0.338% for MAP, control, MAP plus honey, and honey treatments, respectively. MAP slightly restrained the decrease in TA values after 2nd week of storage. Similarly, Sanchez-Ballesta et al. (2007) observed that CO2 enrichment inhibited the decrease in TA of Cardinal grapes stored at 0 °C.
pH
On average, treatments did not significantly affect the pH level; neither were the changes during the storage period found to be significant (data not shown).
SSC/TA
At harvest, the value of SSC/TA ratio (maturity index) was 41.31 and significantly (P < 0.0001) increased throughout the storage (Fig. 1d). During the storage, the increase was lower in berries treated with MAP than others, although the effects of treatments were insignificant. At the end of the storage, level of TSS/TA ranged from 55.24 (control) to 51.93 (MAP). The rises in SSC/TA level coincided with the decreases of TA, therefore, the lower level of maturity index in MAP grapes could be due to the lower degradation of TA occurred in the berries. This progress was more evident in berries stored under MAP conditions with or without honey dip treatment. Retentive effect of MAP observed in this study and in earlier studies (Kader 2002; Valero et al. 2006; Sabir et al. 2008) on maturity delay during storage might be attributed to the clear activity of MAP in gas permeability regulation.
Sensory quality
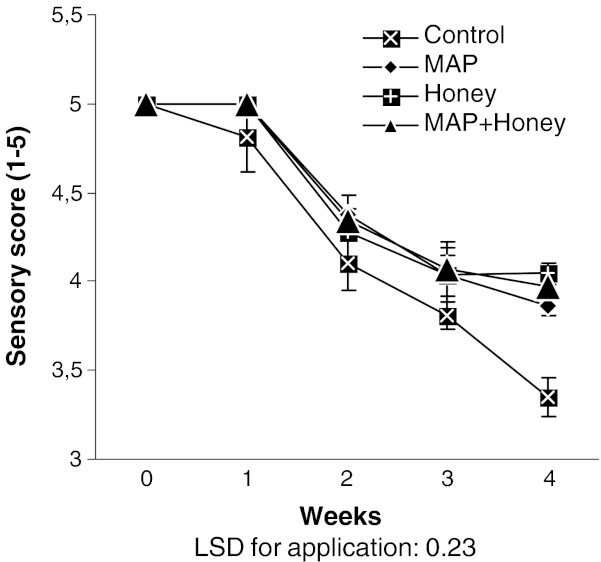
The loss of grape quality is based on weight loss, flavor change, skin browning, decay incidence, and shriveling of berry (Crisosto et al. 2002; Kader 2002). The sensory analysis, focused on firmness, color, crunch, juiciness, sweetness, flavor and appearance of the berries, was carried out weekly by a trained panel of 10 members. After 1 week storage, sensory panelists rated the stored fruits of any treatments as the freshly harvested grape while they perceived a slight decrease in sensory quality of control berries (Fig. 2). Following this analysis, the sensory quality of overall berries underwent a noticeable decline. At the end of the 28 day storage at 0 °C, significant differences (P < 0.0003) were revealed by panelists between control and treated berries. The best sensory evaluation score (4.03) was recorded for the berries stored after immersion in honey, while control fruits (3.34) declined to the critical level of acceptability, probably due to higher water loss. In a similar manner, McLellan et al. (1994) evaluated the effects of various agents (commercial sulfur dioxide gas, honey solution, sulfite, erthorbic acid, citric acid and CaCl2) on quality of Thompson Seedless raisin. Among them, honey treatment alone was ranked the highest by each of the 10 panelists, indicating the promising feature of honey dip treatment on reducing quality loss. During storage, produces lose internal moisture solely. Crispness of grape berry is highly related to turgor pressure. Therefore, slight changes in water content may have a great impact on the subsequent quality of such commodities. Providing better tasting products to consumers in convenient forms and at affordable costs would likely increase consumption of healthy foods.
Fig. 2.
Sensory score of minimally processed grape berries during the storage. Each column represents the mean of three replicates (LSD for application: 0.23)
Decay, browning, and shriveling rates
The quality changes of the processed grapes were further determined by monitoring the percentages of decay, browning, and shriveling of the berries, at the end of the storage.
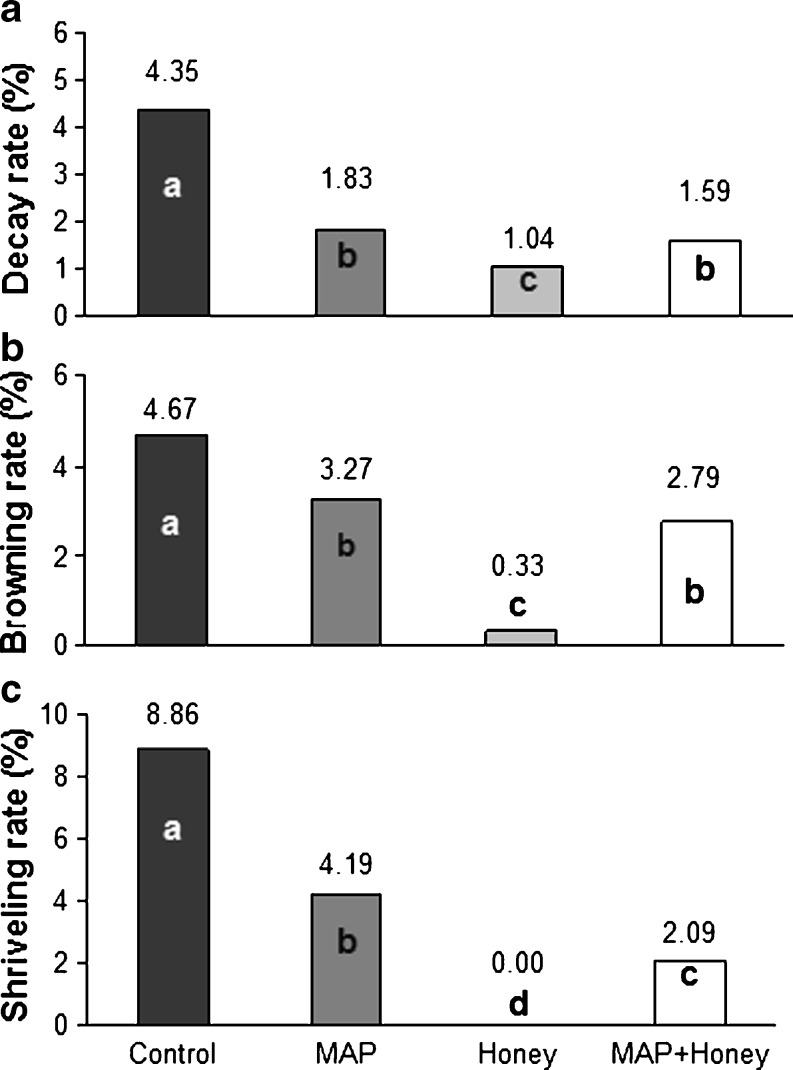
As shown in Fig. 3a, the rate of berry decay was significantly (P < 0.005) higher in the control berries than those treated with MAP and/or honey. Immersion of grapes in 20% honey solution markedly decreased decay incidence, confirming the results of a recent study in which honey solution dip treatments significantly inhibited microbial developments in minimally processed pomegranate arils (Ergun and Ergun 2009). As previously indicated by Viuda-Martos et al. (2008), unique enzymes in honey have a powerful antimicrobial and antioxidant properties. When honey is diluted with water, a chemical reaction between glucose, water, and oxygen produces small amounts of hydrogen peroxide. The slow release of hydrogen peroxide makes honey a mild protective barrier against decay-causing microorganisms. However, the effect of MAP on restriction of berry decay was lower than that of honey in terms of magnitude. This is most likely because grapes are nonclimacteric fruits with low respiration intensity, and thus the concentration of CO2 inside MAP were not high enough to act as fungicide (Guillén et al. 2007).
Fig. 3.
Decay (a), browning (b) and shriveling (c) rates of minimally processed grape berries after 28 day storage. Columns (each treatment) with unlike letters differ significantly (LSD values for decay, browning and shriveling rates are 0.52, 0.71 and 1.29, respectively)
Browning is one of the major post-harvest problems resulting in reduced commercial value of berry fruits (Neog and Saiki 2010). At the end of the storage, browning rate was substantially lower in berries immersed in honey solution (Fig. 3b). Similarly, MAP had also significant (P < 0.0001) effect on prevention of browning, although not as effective as that of honey. As previously indicated by Martínez-Romero et al. (2003) and Sabir and Agar (2008), MAP with low O2 and elevated CO2 slows down the respiration rate of produces during cold storage. This decrease, subsequently, restricts the enzymes and substrates participating in decay-causing reactions. Nevertheless, MAP alone was proven as insufficient for preventing the browning of minimally processed commodities (Kader 2002; Soliva-Fortuny and Martín-Belloso 2003). In this study, significant effect of honey on inhibition of the browning incidence verifies the mentioned reports (Viuda-Martos et al. 2008).
The water loss in table grapes that occurs during postharvest handling can lead to berry shriveling. At the end of storage, no shriveling was found in honey treated samples while a few shriveled berries (2.09%) were observed in honey plus MAP treatment (Fig. 3c). However might it be surprising, honey dip treatment has higher weigh loss than MAP plus honey dip. This case might be probably due to loss of berry water from the wound occured when detaching the pedicel. The percentage of shriveled berries in the control samples increased significantly (P < 0.0001) up to 8.86%. MAP alone was also effective to prohibit the shriveling.
To generalize, MAP, honey solution dip or their combination significantly maintained the overall quality of minimally processed grape (cv. Razaki) by delaying quality loss and decay development. The use of MAP in a number of minimally processed produces is likely to continue, because it maintains quality and safety of fresh commodities. Maintaining the quality of commodities for a period provides higher income and therefore MAP technology could be proven as an economical application. On the other hand, honey offered good adherence to berry surface along the storage. Therefore, it could be used as an edible coating barrier to moisture and resist to water vapor diffusion. The present study pays specific attention to minimally processed grapes, by adjusting a potentially safe organic protection means, the utilization of honey solution.
References
- Ahvenainen R. New approaches in improving the shelf life of minimally processed fruits and vegetables. Trends in Food Sci Tech. 1996;7:179–186. doi: 10.1016/0924-2244(96)10022-4. [DOI] [Google Scholar]
- Al-Bachir M. Effects of gamma irradiation on storability of two cultivars of Syrian grapes (Vitis vinifera L.) Radiat Phys Chem. 1999;55:81–85. doi: 10.1016/S0969-806X(98)00295-3. [DOI] [Google Scholar]
- Chervin C, Westercamp P, Monteils G. Ethanol vapours limit Botrytis development over the postharvest life of table grapes. Postharvest Biol Tech. 2005;36:319–322. [Google Scholar]
- Crisosto CH, Garner D, Crisosto G. Carbon dioxide-enriched atmospheres during cold storage limit losses from Botrytis but accelerate rachis browning of ‘Redglobe’ table grapes. Postharvest Biol Tech. 2002;26:181–189. doi: 10.1016/S0925-5214(02)00013-3. [DOI] [Google Scholar]
- Del Nobile MA, Sinigaglia M, Conte A, Speranza B, Scrocco C, Brescia I, Bevilacqua A, Laverse J, La Notte E, Antonacci D. Influence of postharvest treatments and film permeability on quality decay kinetics of minimally processed grapes. Postharvest Biol Tech. 2008;47:389–396. doi: 10.1016/j.postharvbio.2007.07.004. [DOI] [Google Scholar]
- Ergun M, Ergun N. Maintaining quality of minimally processed pomegranate arils by honey treatments. Br Food J. 2009;111:396–406. doi: 10.1108/00070700910951524. [DOI] [Google Scholar]
- Ergun M, Akkaya O, Ergun N. Suitability of some mid-season table grape cultivars and types for minimally processed produce. J Int Sci Vigne Vin. 2008;42:99–106. [Google Scholar]
- Eris A, Turkben C, Ozer MH (1993) A research on CA-storage of grape cultivars ‘Alphonse Lavallée’ and ‘Razaki’. In: Proceedings of the Sixth International CA Research Conference (June 15-17, 1993) ‘NRAES-71’. Cornell University, Ithaca, pp 705–710
- Garcia E, Barrett DM. Preservative treatments for fresh-cut fruits and vegetables. In: Lamikanra O, editor. Fresh-cut fruits and vegetables. Boca Raton: CRC Press; 2002. pp. 267–303. [Google Scholar]
- Grncarevic M, Radler F. A review of the surface lipids of grapes and their importance in the drying process. Am J Enol Vitic. 1971;22:80–86. [Google Scholar]
- Guillén F, Zapata PJ, Martínez-Romero D, Castillo S, Serrano M, Valero D. Improvement of the overall quality of table grapes stored under modified atmosphere packaging in combination with natural antimicrobial compounds. J Food Sci. 2007;72:185–190. doi: 10.1111/j.1750-3841.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- Jayasena V, Cameron I. The effect of ethephon and clone on physical characteristics and sensory quality of Crimson Seedless table grapes after 1 month storage. Int J Food Sci Tech. 2009;44:409–414. doi: 10.1111/j.1365-2621.2008.01787.x. [DOI] [Google Scholar]
- Kader AA (2002) Postharvest technology of horticultural crops, 3rd edn. University of California, Agriculture and Natural Resources, UC Publication 3311, Oakland, CA, 535p
- Kader AA, Zagory D, Kerbel EL. Modified atmosphere packaging of fruits and vegetables. CRC Crit Rev Food Sci Nut. 1989;28:1–30. doi: 10.1080/10408398909527490. [DOI] [PubMed] [Google Scholar]
- Karabulut OA, Gabler FM, Mansour M, Smilanick JL. Postharvest ethanol and hot water treatments of table grapes to control gray mold. Postharvest Biol Tech. 2004;34:169–177. doi: 10.1016/j.postharvbio.2004.05.003. [DOI] [Google Scholar]
- Kou L, Luo Y, Wu D, Liu X. Effects of mild heat treatment on microbial growth and product quality of packaged fresh-cut table grapes. J Food Sci. 2007;72:567–573. doi: 10.1111/j.1750-3841.2007.00503.x. [DOI] [PubMed] [Google Scholar]
- Lichter A, Zutkhy Y, Sonego L, Dvir O, Kaplunov T, Sarig P, Ben-Arie R. Ethanol controls postharvest decay of table grapes. Postharvest Biol Tech. 2002;24:301–308. doi: 10.1016/S0925-5214(01)00141-7. [DOI] [Google Scholar]
- Lurie S, Pesis E, Gadiyeva O, Feygenberg O, Ben-Arie R, Kaplunov T, Zutahy Y, Lichter A. Modified ethanol atmosphere to control decay of table grapes during storage. Postharvest Biol Tech. 2006;42:222–227. doi: 10.1016/j.postharvbio.2006.06.011. [DOI] [Google Scholar]
- Martínez-Romero D, Guillén F, Castillo S, Valero D, Serrano M. Modified atmosphere packaging maintains quality of table grapes. J Food Sci. 2003;68:1838–1843. doi: 10.1111/j.1365-2621.2003.tb12339.x. [DOI] [PubMed] [Google Scholar]
- McLellan MR, Kime RW, Lee CY, Long TM. Effect of honey as an antibrowning agent in light raisin processing. J Food Process Pres. 1994;19:1–8. doi: 10.1111/j.1745-4549.1995.tb00273.x. [DOI] [Google Scholar]
- Mishra B, Khatkar BS, Garg MK, Wilson LA. Permeability of edible coatings. J Food Sci Technol. 2010;47(1):109–113. doi: 10.1007/s13197-010-0003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neog M, Saiki L. Control of post-harvest pericarp browning of litchi (Litchi chinensis Sonn) J Food Sci Technol. 2010;47(1):100–104. doi: 10.1007/s13197-010-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oszmianski J, Lee CY. Inhibition of polyphenol activity and browning by honey. J Agric Food Chem. 1990;38:1892. doi: 10.1021/jf00100a002. [DOI] [Google Scholar]
- Pretel MT, Martinez-Madrid MC, Martinez JR, Carreno JC, Romojaro F. Prolonged storage of ‘Aledo’ table grapes in a slightly CO2 enriched atmosphere in combination with generators of SO2. LWT Food Sci Tech. 2006;39:1109–1116. doi: 10.1016/j.lwt.2005.07.022. [DOI] [Google Scholar]
- Ramming DW. Water loss from fresh berries of raisin cultivars under controlled drying conditions. Am J Enol Vitic. 2009;60:208–214. [Google Scholar]
- Reitmeier CA, Nonnecke GR. Objective sensory evaluation of fresh day-neutral strawberry fruit. HortSci. 1991;26:843–845. [Google Scholar]
- Romanazzi G, Gabler FM, Margosan D, Mackey BE, Smilanick JL. Effect of chitosan dissolved in different acids on its ability to control postharvest gray mold of table grape. Phytopat. 2009;99:1028–1036. doi: 10.1094/PHYTO-99-9-1028. [DOI] [PubMed] [Google Scholar]
- Sabir FK, Agar IT (2008) Effects of different types of modified atmosphere packages on storage period and quality of sweet cherry cv. 0900 Ziraat. IV. Turkish National Symp on Postharvest Physiol Mark Hort Prod, 8-11 Oct, Antalya, Turkey, 44-51
- Sabir A, Sabir FK, Tangolar S, Agar IT. Comparison of the effects of SO2 generators with different concentrations of ethanol application on cold storage of grape cv. Alphonse Lavallée. J Cukurova Uni Agr Fac. 2006;21:45–50. [Google Scholar]
- Sabir A, Sabir FK, Tangolar S, Agar IT (2008) Effects of ethanol and sulphurdioxide applications on storage period and quality characteristics in some grape cultivars. IV. Turkish National Symp on Postharvest Physiol Mark Hort Prod, 8-11 Oct, Antalya, Turkey 441-448
- Sagar VR, Suresh Kumar P. Recent advances in drying and dehidration of fruits and vegetables: a review. J Food Sci Technol. 2010;47(1):15–26. doi: 10.1007/s13197-010-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ballesta MT, Romero I, Bernardo-Jiménez J, Orea JM, González-Ureña A, Escribano MI, Merodio C. Involvement of phenylpropanoid pathway in the response of table grapes to low temperature and high CO2 levels. Postharvest Biol Tech. 2007;46:29–35. doi: 10.1016/j.postharvbio.2007.04.001. [DOI] [Google Scholar]
- Sharpe D, Fan L, McRae K, Walker B, MacKay R, Doucette C. Effects of ozone treatment on Botrytis and Sclerotinia sclerotiorum in relation to horticultural product quality. J Food Sci. 2009;74:250–257. doi: 10.1111/j.1750-3841.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- Soliva-Fortuny RC, Martín-Belloso O. New advances in extending the shelf-life of fresh-cut fruits: a review. Trends Food Sci Tech. 2003;14:341–353. doi: 10.1016/S0924-2244(03)00054-2. [DOI] [Google Scholar]
- Soylemezoglu G. Storage of table grapes. Ankara: Ankara University, Faculty of Agriculture, Department of Horticulture, Ankara University Press; 2001. [Google Scholar]
- Tharanathan RN. Biodegradable films and composite coatings: past, present and future. Trends Food Sci Tech. 2003;14:71–78. doi: 10.1016/S0924-2244(02)00280-7. [DOI] [Google Scholar]
- Valero D, Valverde JM, Martínez-Romero D, Guillén F, Castillo S, Serrano M. The combination of modified atmosphere packaging with eugenol or thymol to maintain quality, safety and functional properties of table grapes. Postharvest Biol Tech. 2006;41:317–327. doi: 10.1016/j.postharvbio.2006.04.011. [DOI] [Google Scholar]
- Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Alvarez JA. Functional properties of honey, propolis, and royal jelly. J Food Sci. 2008;73:117–124. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- Winkler AJ, Cook JA, Kliewer WM, Lider LA. General viticulture. Berkeley: Uni Cal Press; 1974. p. 639. [Google Scholar]