Abstract
α, α-diphenyl-β-picrylhydrazyl (DPPH) free radical scavenging method offers the first approach for evaluating the antioxidant potential of a compound, an extract or other biological sources. This is the simplest method, wherein the prospective compound or extract is mixed with DPPH solution and absorbance is recorded after a defined period. However, with the advancement and sophistication in instrumental techniques, the method has undergone various modifications to suit the requirements, even though the basic approach remains same in all of them. This article presents a critical review on various developments to the DPPH method.
Keywords: DPPH, Absorbance, Free radicals, Antioxidant, SIA
Free radicals are inevitably produced in biological systems and also encountered exogenously, and are known to cause various degenerative disorders, like mutagenesis, carcinogenesis, cardiovascular disturbances and ageing (Singh and Singh 2008). Antioxidants are the compounds, which combat the free radicals by intervening at any one of the three major steps of the free radical mediated oxidative process, viz., initiation, propagation and termination (Cui et al. 2004). These antioxidants are also produced by biological system and occur naturally in many foods and the balance between oxidants and antioxidants decides the health and vigor (Halliwell 1996).
Methods for antioxidant activity determination
Thus it is important to know the antioxidant content and their efficacy in foods, for preservation or protection against oxidative damage, to avoid deleterious changes and loss of commercial and nutritional value (Halliwell 1997). This necessitates the development of a rapid method for determining the potential antioxidant capacity in various foods.
DPPH assay
This method was developed by Blois (1958) with the viewpoint to determine the antioxidant activity in a like manner by using a stable free radical α, α-diphenyl-β-picrylhydrazyl (DPPH; C18H12N5O6, M = 394.33). The assay is based on the measurement of the scavenging capacity of antioxidants towards it. The odd electron of nitrogen atom in DPPH is reduced by receiving a hydrogen atom from antioxidants to the corresponding hydrazine (Contreras-Guzman and Srong 1982).
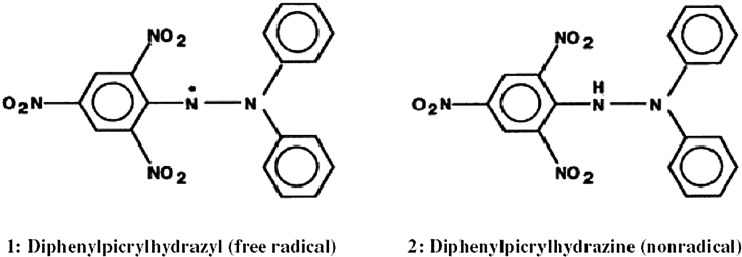
DPPH is characterized as a stable free radical by virtue of the delocalisation of the spare electron over the molecule as a whole (Fig. 1), so that the molecules do not dimerise, like most other free radicals. The delocalisation also gives rise to the deep violet colour, with an absorption in ethanol solution at around 520 nm. On mixing DPPH solution with a substance that can donate a hydrogen atom, it gives rise to the reduced form with the loss of violet colour. Representing the DPPH radical by Z• and the donor molecule by AH, the primary reaction is
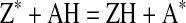 |
1 |
Where ZH is the reduced form and A• is free radical produced in the first step. The latter radical will then undergo further reactions which control the overall stoichiometry The reaction (1) is therefore intended to provide the link with the reactions taking place in an oxidising system, such as the autoxidation of a lipid or other unsaturated substance; the DPPH molecule Z• is thus intended to represent the free radicals formed in the system whose activity is to be suppressed by the substance AH.
Fig. 1.
DPPH radical and its stable form
While DPPH can accept an electron or hydrogen radical to become a stable, diamagnetic molecule, it can be oxidized only with difficulty, and then irreversibly. DPPH shows a strong absorption band at 517 nm due to its odd electron and solution appears a deep violet colour, the absorption vanishes as the electron pairs off. The resulting decolorization is stoichiometric with respect to the number of electrons taken up. The alcoholic solutions of 0.5 mM are densely colored and at this concentration, the Lambert-Beer law is obeyed over the useful range of absorption (Blois 1958).
It is a rapid, simple, inexpensive and widely used method to measure the ability of compounds to act as free radical scavengers or hydrogen donors, and to evaluate antioxidant activity of foods. It can also be used to quantify antioxidants in complex biological systems, for solid or liquid samples. This method is easy and applies to measure the overall antioxidant capacity (Prakash 2001) and the free radical scavenging activity of fruit and vegetable juices (Sendra et al. 2006). This assay has been successfully utilized for investigating antioxidant properties of wheat grain and bran, vegetables, conjugated linoleic acids, herbs, edible seed oils, and flours in several different solvent systems including ethanol, aqueous acetone, methanol, aqueous alcohol and benzene (Yu 2001; Parry et al. 2005). It is a convenient method for the antioxidant assay of cysteine, glutathione, ascorbic acid, tocopherol and polyhydroxy aromatic compounds (Masahiro et al. 2005), for olive oil, fruits, juices and wines (Sanchez-Moreno 2002).
The method is unique in carrying out the reaction of the sample with DPPH in methanol/water, which facilitates the extraction of antioxidant compounds from the sample. Determination of antioxidant activity of various types of foods using DPPH is comparable to other methods. Antioxidant analysis by other methods may be limited to those compounds soluble in the selected solvents. The advantage of this method is that DPPH is allowed to react with the whole sample and sufficient time given in the method allows DPPH to react slowly even with weak antioxidants (Prakash 2001). DPPH method may be utilized in aqueous and nonpolar organic solvents and can be used to examine both hydrophilic and lipophilic antioxidants (Prior et al. 2005).
DPPH assay is considered a valid accurate, easy and economic method to evaluate radical scavenging activity of antioxidants, since the radical compound is stable and need not be generated. Sanchez-Moreno et al. (1998, 1999a, b) proposed a new methodology for the evaluation of antiradical efficiency towards DPPH, which is advantageous over other methods. It considers not only the antioxidant concentration but also the reaction time of scavenging reaction to reach the plateau. The results are highly reproducible and comparable to other free radical scavenging methods Gil et al. 2000). The antioxidant efficiency is measured at ambient temperature so that the risk of thermal degradation of the molecules tested is eliminated (Bondet et al. 1997). In optothermal window mode, the method can also be utilized for the evaluation of antioxidant efficiency of the compounds, which form opaque solution. It can also be used for online monitoring of changes in food containing natural antioxidants.
This method is limited because DPPH radical interacts with other radicals and the time response curve to reach the steady state is not linear with different ratios of antioxidant/DPPH (Brand-Williams et al. 1995; Sanchez-Moreno et al. 1998). DPPH is sensitive to some Lewis bases and solvent types as well as oxygen (Ancerewicz et al. 1998). DPPH can only be soluble in organic solvent and the interference of absorbance from the sample compounds could be a problem for the quantitative analysis (Arnao 2000). The absorbance of DPPH in methanol and acetone decreases under light (Min 1998). DPPH method has limitations in reflecting the partitioning of antioxidants in the emulsion systems and is not useful for measuring the antioxidant activity of plasma, because proteins are precipitated in the alcoholic reaction medium.
Original method
The DPPH method was introduced by Marsden Blois (1958), using cysteine as model antioxidant. Representing the DPPH radical by Z• and the cysteine molecule by RSH, the initial reaction is
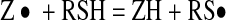 |
2 |
The free radical RS• reacts with another molecule, produced by a parallel reaction to (2)
 |
3 |
This leads to the observed reduction of two molecules of DPPH by two molecules of cysteine, that is, a 1:1 stoichiometry. However, if the molecule has two adjacent internally connected sites for hydrogen abstraction (eg., ascorbic acid), then there may be a further hydrogen abstraction reaction after the first one:
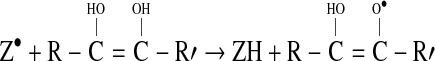 |
4 |
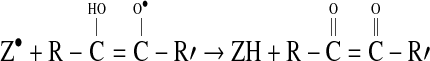 |
5 |
This leads to a 2:1 stoichiometry for DPPH and ascorbic acid. Similar pattern is shown with hydroquinone (1,4-dihydoxybenzene) forming quinine (1,4-benzoquinone) by a similar two-step mechanism. Other compounds actively participating in this reaction are glutathione, aromatic amines, α-tocopherol and polyhydroxy aromatic compounds. Inorganic ions in lower valence states (particularly Fe+2) may interfere in this reaction (Blois 1958).
The modified method introduced of Brand-Williams et al. (1995) has been extensively used (Gomez-Alonso et al. 2003; Yepez et al. 2002). This suggested the oversimplification of the interpretation by Blois (1958) and that because of the complexity of the reactions that follow the Eq. 1, the overall stoichiometry need not necessarily be a whole integer (Molyneux 2004). Furthermore, the initial step Eq. 1 may be reversible, as can be demonstrated by adding the reduced form ZH at the end of the reaction (Bondet et al. 1997).
Brand-Williams et al. (1995) and Bondet et al. (1997) used the term “EC50 (efficient concentration) for the interpretation of the results from DPPH method. This is defined as the concentration of substrate that causes 50% reduction in the DPPH colour. This parameter was subsequently used by several groups for presenting their results (Kim et al. 2002; Lebeau et al. 2000; Lu and Foo 2000). However, the parameter has the drawback that higher the antioxidant activity, lower is the value of EC50. Molyneux (2004) suggested that presentation of the results of antioxidant efficiency should involve the use of ascorbic acid as the standard due to doubts concerning the direct determination of DPPH obtained from calibration curve (Leitao et al. 2002).
This is a disadvantage particularly when results are presented graphically as a bar chart even if the same data are available in numerical form (Sanchez-Moreno et al. 1998). Brand-Williams et al. (1995) used the term ‘antiradical power’ (ARP) which is the inverse of EC50; hence, larger the ARP, the more efficient is the antioxidant. Sanchez-Moreno et al. (1998) introduced the term “antiradical efficacy” (AE), to describe the DPPH scavenging capacity, with higher AE value indicates higher antioxidant activity.
Recommended methods of measurement and interpretation
The common practice of using smaller volumes than as described reduces the accuracy of the relative volumes. The use of cheap plastic “disposable” cuvettes, which are not attacked by methanol or ethanol, are commonly used for the assay (Bondet et al. 1997). The method works equally well with methanol or ethanol. However, the use of other solvent systems, such as almost neat extracts in water or acetone, seems to give low values for the extent of reduction (Guo et al. 2001). The system should be maintained at a pH in the range 5.0 to 6.5 however; there is great uncertainty in the meaning of pH values for methanol or ethanol used as a media (Blois 1958).
The initial DPPH concentration should give absorbance values less than 1.0 (50 to 100 μM). The stock solution of DPPH slowly deteriorates; thus an automatic burette with a nitrogen atmosphere could be a choice to minimize the loss of free radical activity (Blois 1958).
For substrates of known molar mass, working in terms of molar units completely obscures the interpretation of the data on a molecular basis; it is more appropriate to use the relative molar mass for DPPH (Sanchez-Moreno et al. 1998). Use of only a single mass-in-volume concentration does not help to elucidate the structural basis of antioxidant activity, since it provides only two points on the titration curve (Yepez et al. 2002). Working in terms of numbers of free radicals necessitates the use of the Avogadro number to bring the values on to a mole basis (Schwarz et al. 2001). However, in case of complex mixtures such as plant extracts, the results should be expressed as DPPH equivalents per gram of material. Suitable standards, like ascorbic acid and α-tocopherol could also be used to check the correctness of the procedures (Kim et al. 2002; Lu and Foo 2000; Guo et al. 2001).
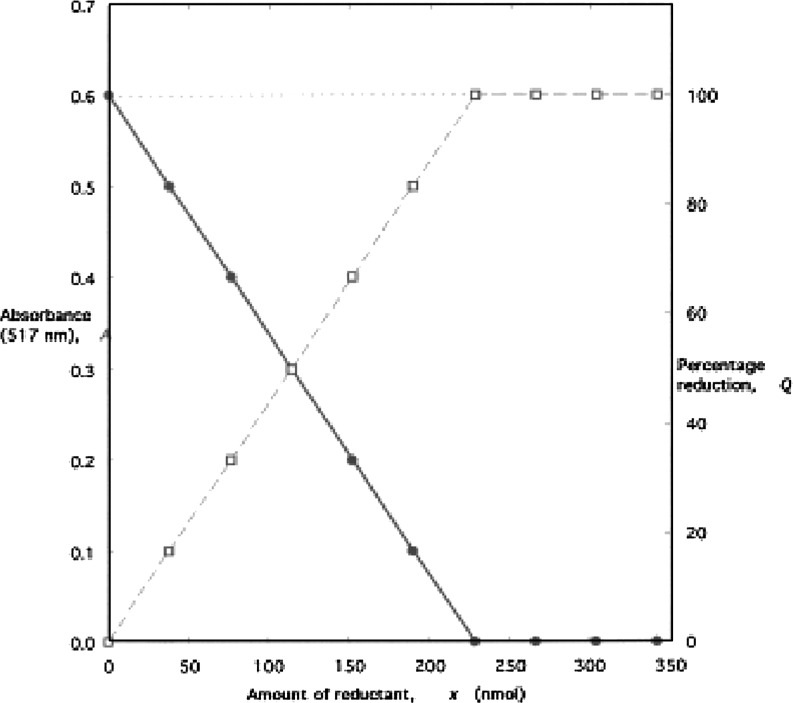
The λmax, used for the absorbance measurements are 515 nm (Gomez-Alonso et al. 2003; Lebeau et al. 2000), 516 nm (Schwarz et al. 2001), 517 nm (Lu and Foo 2000; Zhu et al. 2002), 518 nm (Leitao et al. 2002) and 520 nm (Kim et al. 2002). A reaction time of 30 min was followed by Kim et al. (2002). However, shorter reaction time of 5 and 10 min has also been reported (Lebeau et al. 2000; Schwarz et al. 2001). As the rate of reaction varies widely among substrates (Bondet et al. 1997), the best practice is to follow the reaction till completion (“plateau”, Fig. 2) (Yepez et al. 2002; Lu and Foo 2000).
Fig. 2.
Idealised plots of absorbance A (left hand scale and filled circles), and percentage reduction Q (right hand scale and open squares), vs. amount of reductant added, for the constant-volume colorimetric titration of DPPH with cysteine hydrochloride(5)
The simplest approach in interpreting the data is to plot absorbance against substrate concentration, extending the concentration range beyond the end-point to define the subsequent section of the plot so that the intersection point may be defined most accurately (Fig. 2); this would allow for any residual colour from the reduced DPPH, as well as any inherent absorbance from the substrate itself at the working wavelength. Alternatively, the commonly used method is to work in terms of the percentage reduction of the DPPH, Q (also referred as “inhibition” or “quenching”). The value of molar extinction coefficient for DPPH (in methanol or ethanol at 515 nm) is given as 1.09×104 (27), 1.16 × 104 (16) and 1.25 × 104 (Kim et al. 2002).
Prakash (2001) proposed the quantity of sample necessary to react with 50% DPPH to be expressed in terms of the relative amount of Trolox reacted. Antioxidants in food may be water soluble, fat soluble, insoluble being bound to cell walls and hence may not be free to react with DPPH, hence they react at different rates and follow differing kinetics, and the reaction will often not reach completion in a reasonable assay time. Therefore, the sample size that can lower the initial absorbance of DPPH solution by 50% has been chosen as the endpoint for measuring the antioxidant activity. This change is compared with the change induced by Trolox and the antioxidant activity of the sample is expressed in micromoles of Trolox equivalents TE (Molyneux 2004).
Ozcelik et al. (2003) showed that the absorbance of DPPH at 517 nm in methanol and acetone decreased by 20 and 35% for 120 min at 25 °C under light, respectively; however, it did not change significantly for 150 min in the dark. The absorbance of DPPH in pH 4.0 buffer solution in methanol, and in pH 10 buffer solution in acetone, decreased by 55 and 80%, respectively, under light for 120 min. The evaluation of antioxidant activity by the changes of DPPH absorbance should be carefully interpreted since the absorbance of DPPH at 517 nm is decreased by light, oxygen, pH, and type of solvent in addition to the antioxidant
Developments in DPPH method
The original DPPH method Blois 1958) has undergone developments and improvements (Bondet et al. 1997; Brand-Williams et al. 1995) related to operating conditions in order to adapt the DPPH• method to each kinetic case. Electrochemical methods for the determination of antioxidant activity based on the application of biosensors (Ruiz et al. 1996) to measure antioxidant properties of tert-butylhydroxyanisole and the cyclic voltammetry (Chevion et al. 1997) to study the efficiency of some low molecular weight antioxidants are reported. DPPH method has been used to evaluate the antioxidant ability of phenol compounds (Sanchez-Moreno et al. 1998). Sung-Kun et al. (2004) developed a continuous spectrophotometric assay for the reduction of DPPH by NADPH-cytochrome P450 reductase (CPR) in mixed ethanol–water solution.
Uric acid reaction with micelle-incorporated DPPH was explained by Abuin et al. (2002), while Bandoniene et al. (2002) detected the activity of radical scavenging compounds in mixed methanol–water solution using DPPH and HPLC–DPPH method. Ionita et al. (2000) used water-soluble derivate of DPPH for the study of oxidation kinetics of amino acid.
Buijnster et al. (2001) evaluated the antioxidant activity of some antioxidants using both DPPH colorimetry and optothermal window (OW) detection at 514 nm. The OW-DPPH approach for assessing antioxidant activity is based on a direct proportionality between magnitude of OW signal and the optical absorbance. The aliquots of antioxidant were added to the methanolic solution of DPPH. An aliquot of mixture was pipetted directly on the OW and the magnitude of OW signal was online monitored until the steady state is reached. The OW signal (and the absorbance) of the solution decreases depending upon the intrinsic antioxidant activity of antioxidant as well as on the speed of the reaction between DPPH and the antioxidant.
The antioxidant activity is calculated by determining the decrease in the absorbance at different concentration by using the equation.
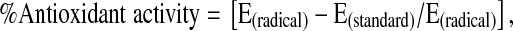 |
where E is the extinction coefficient of DPPH.
-
Inclusion of automation
Several automation in the original DPPH assay, based on flow injection analysis (FIA) (Ukeda et al. 2002; Ukeda 2004), sequential injection analysis (SIA) (Polasek et al. 2004) and HPLC-FIA (Yamaguchi et al. 1998) have been implemented in recent years. These methods were applied for screening and evaluation of scavenging capacity of several pure compounds and complex matrices, such as plant extracts and beverages.
In HPLC-FIA method, the separated analytes react postcolumn with DPPH• (Koleva et al. 2000) and the induced scavenging is detected as a negative peak. These methods have an advantage over batch methods, as bioassay-guided fractionation of natural or food samples is a time consuming and labour-intensive process and the loss of activity of antioxidants due to decomposition during the isolation and purification procedures is avoided (Luıs et al. 2006).
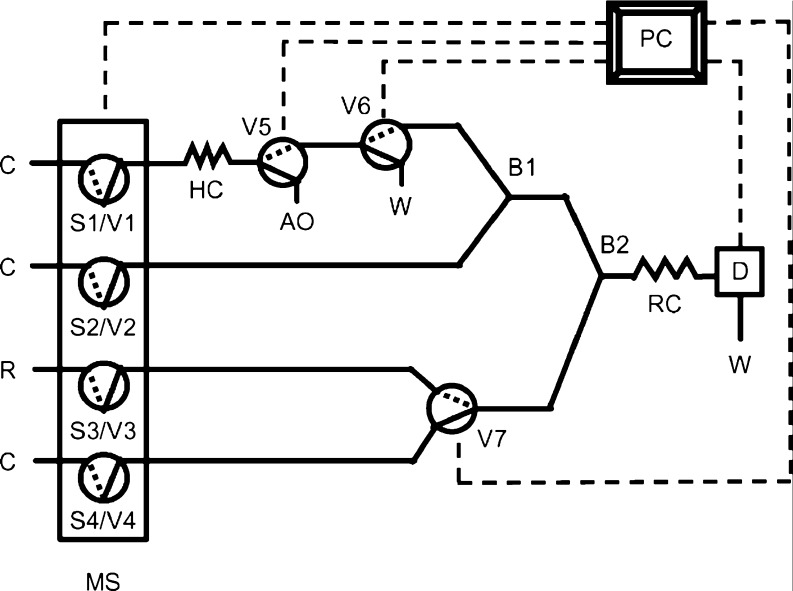
Novel computer controlled techniques, namely multi-syringe flow injection analysis (MSFIA, Fig. 3) for automatic flow analysis was reported by Cerda et al. (1999)) to combine the multi-channel operation of FIA and the flexibility of multi-commutation flow systems (Rocha et al. 2002). MSFIA was developed for the determination of total antioxidant capacity, measured as the cumulative capacity of the compounds present in the sample to scavenge free radicals, using DPPH• reaction at 517 nm. The amount of DPPH• consumed by antioxidant standards (ascorbic and caffeic acids) was shown to be independent of the initial concentration of radical, except for situations where DPPH•/antioxidant molar ratio was lower than the stoichiometric value. Furthermore, the sample dilution factor plays an important role since the exhaustion of scavenging ability of the sample should take place during the period of absorbance measurement. The proposed method was applied to several food products and the total antioxidant capacity was expressed as Vitamin C equivalent antioxidant capacity (VCEAC). The results obtained by this method were statistically comparable to those provided by batch method (Luıs et al. 2006).
The configuration of the MSFIA system was designed to allow the determination of total antioxidant capacity of several samples with stopped flow approach, as the reaction kinetics is strongly influenced by the type of antioxidant compound. The DPPH• consumption was assessed from the difference between the absorbance of DPPH• in the absence (A0) and presence of antioxidant compounds after a fixed period of time (Af). With slight modification in the system, it is possible to allow the sample exchange (standard or sample) without disturbing the content of the flow cell and make adjustments of the samples, which have intrinsic absorption at the wavelength of detection.
-
Sequential Injection Analysis (SIA)
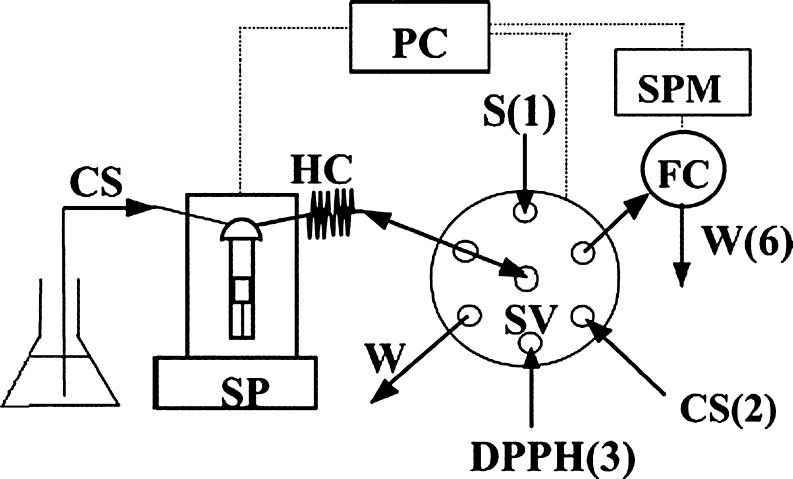
A PC-controlled sequential injection analysis (SIA) system equipped with a spectrophotometric diode array detector is used for rapid evaluation of antioxidation/radical scavenging activity of biological samples (Fig. 4) (Polasek et al. 2004). The method is based on the reaction of DPPH with antioxidants in organic or aqueous-organic media resulting in bleaching of DPPH due to its “quenching” by the interaction with the analytes and measured at 525 nm. With the optimised SIA procedure, micromolar concentrations of 45 antioxidant samples could be determined in one hour (Vít et al. 2007) which may not be possible in the batch version of the DPPH quenching method, since it is rather time consuming, the standard reaction time being 16–30 min (Guo et al. 2001). The development of an automated DPPH method, based on SIA technique was supposed to be suitable for rapid testing of anti-oxidation activity in large series of plant extracts (Polasek et al. 2004).
SIA is the modification of FIA technique to automate assays which is achieved by carrying out analyses in a flow system where a pump is used to continuously draw sample and reagent solutions into different lines or segments of plastic tubing, as well as push them forward through the system. Aliquots of the sample solution are dispensed into the carrier stream by an injection valve. Bringing solutions from different lines together, or including a reagent in the carrier stream enables seamless, automated reagent addition. Connecting a detector at the end of the sample's flow path ensures automated detection of the processed sample.
The major advantage of the SIA assay compared to the standard DPPH batch or HPLC-post column detection methods lies in its relatively high productivity and comparable reproducibility. The proposed method is suitable for rapid screening of large series of natural samples for their anti-oxidation/radical scavenging activity (Polasek et al. 2004).
-
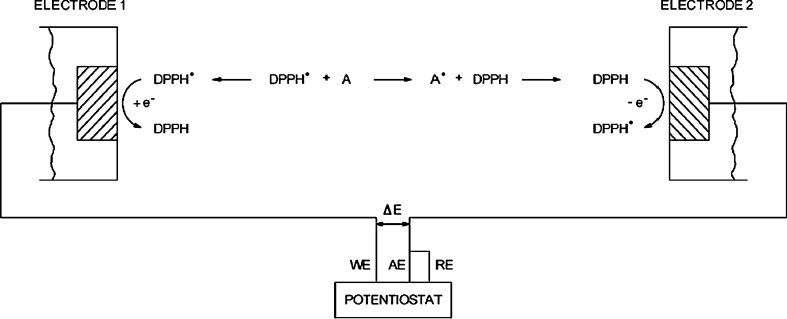
Biamperometric determination of antioxidant activity
A novel electrochemical method for selective determination of antioxidant activity based on DPPH ·/DPPH redox couple and biamperometric technique is proposed by Milardovic et al. (Fig. 5) (Milardovic et al. 2005). In this method, two identical glassy carbon disk electrodes were mounted in an electrochemical cell. The DPPH·/DPPH redox couple exhibited a high degree of reversibility. Selectivity of detector was tested by different antioxidant compounds in ethanol-phosphate buffer solution (pH 7.4). The results of antioxidant activity of pure antioxidant compounds and actual samples of beverages, and determined by biamperometric and spectroscopic measurements, were found in good correlation (R = 0.9959).
In the biamperometric method, current intensity is proportional to the residual concentration of DPPH· after reaction with the antioxidant. If the analyzed solution contains an appropriate ratio of reduced/oxidized form of indicating redox pair, interferences from the analytically undesirable oxidized or reduced species present in the solution would be minimized.
-
High Throughput RDSC
A high-throughput relative DPPH radical scavenging capacity (RDSC) assay was developed and validated by Zhihong et al. (2006). This assay is easy to perform and has acceptable accuracy, precision and reproducibility. The RDSC assay may be conducted in aqueous alcohol and acetone for hydrophilic antioxidants or in the organic solvents for lipophilic antioxidants without solubilizing agents, which makes it possible to directly compare the radical scavenging capacities of hydrophilic and lipophilic antioxidants. In addition, the high-throughput RDSC assay could be utilized for EC50 value estimation. The high-throughput assays use area under the curve (AUC) for RSC estimation, expressed as trolox equivalents (TEs) in μmol on a per sample weight basis. These approaches take into account both the kinetic and the thermodynamic measurements of the radical-antioxidant reactions and make it possible to compare data between laboratories.
The development and application of a highthroughput RDSC assay using a microplate reader with spectrophotometric detector and 96 well plates was described and validated (Zhihong et al. 2006). This assay may be conducted in aqueous alcohol and acetone for hydrophilic antioxidants or in the organic solvents for lipophilic antioxidants without solubilizing agents. The assay for evaluating lipophilic antioxidants is in high demand. This RDSC assay is easy to perform and has acceptable accuracy, precision, and reproducibility. The high-throughput RDSC assay may be used for screening and investigating the potential natural antioxidants.
-
On-line HPLC-DPPH methods
The colorimetric method of antioxidant activity by scavenging DPPH• free radical possesses certain shortcomings, like failure to indicate antioxidant activity of certain drugs and interference from color pigments of natural products. It is also observed that activity of natural antioxidants often decreases during their isolation and purification due to decomposition (Yamaguchi et al. 1998).
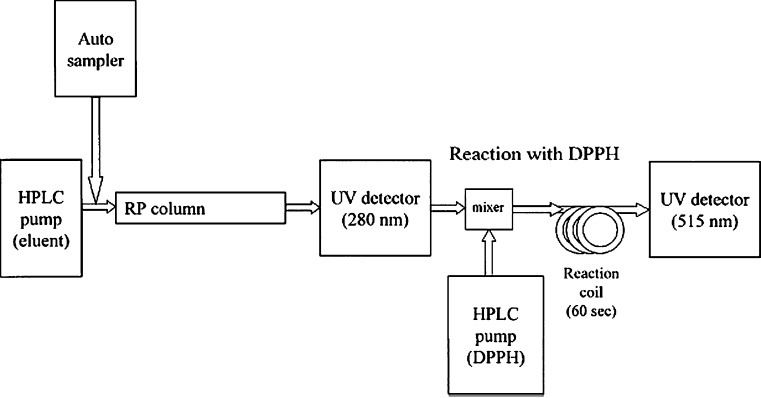
Several online HPLC methods (Fig. 6), based on online detection of antioxidants by postcolumn reaction of eluates with free radicals, have been successfully utilized to screen antioxidants in some plant extracts. Yamaguchi et al. (1998) developed a DPPH–HPLC method and compared the antioxidant activities of known standard antioxidants and commercial beverages with that of colorimetric method. Koleva et al. (2000) presented an on-line HPLC method whereby analytes separated by HPLC, were reacted post-column with DPPH. A specific HPLC method was developed by Chandrasekar et al. (2006) for evaluating the DPPH free radical scavenging activity of commercial polyherbal formulations using a LiChrospher® 100 RP-18e column. The HPLC method is sensitive and can be used as a quality control tool for the rapid determination of free radical scavenging activity of variety of products including plant extracts, foods, drugs and polyherbal formulations. The applicability of HPLC method was extended to determine the antioxidant activity of crude plant extracts and drugs (Dapkevicius et al. 1999; Karunakar et al. 2003). The HPLC method developed was specific for DPPH with an acceptable reproducibility and short run time allowing for rapid determination of radical scavenging activity of several samples (Chandrasekar et al. 2006).
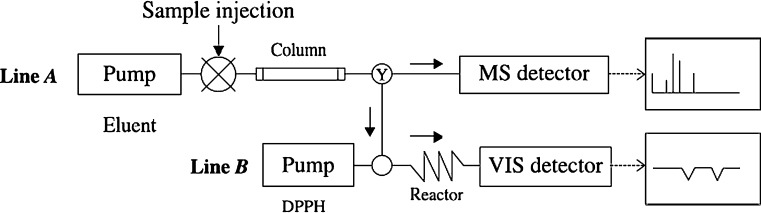
This HPLC method is often used in combination with other methods. Changqing et al. (2007) used combination of HPLC-ESI-MS (Fig. 7) and NMR method for determining antioxidant polyphenolic acids in the medicinal herb. A reversed-phase HPLC coupled on-line to a radical scavenging detection system and MS/MS was developed by Nuengchamnong et al. (2005) in order to combine separation, activity determination and structural identification of anti-oxidants in complex mixtures in one run. The sample was separated by HPLC and the eluate split into two flows. The major portion was fed into an electrospray ionisation MS/MS system, while the minor part was mixed with a free radical DPPH, and the reaction determined spectrophotometrically. The method was applied to the identification of antioxidant compounds in a fraction, obtained by solid-phase extraction, of an extract of a Thai medicinal plant.
This method has been applied to evaluate antioxidant compounds in fruit and vegetables (Pukalskas et al. 2002; Kosar et al. 2003). This technique would permit the rapid determination of antioxidant activity and provide structural identification of the antioxidant compounds involved (Nuengchamnong et al. (2005).
A rapid and simple method has been developed for the screening and identification of natural antioxidants of Flos Lonicerae Japonicae (FLJ). The hypothesis is that upon reaction with DPPH, the peak areas (PAs) of compounds with potential antioxidant effects in the HPLC chromatograms will be significantly reduced or disappeared, and the identity confirmation could be achieved by HPLC-DAD-TOF/MS hyphenated technique (Dan et al. 2008). However, these methods might not be adopted widely because the online instrumental system required was complex and not commercial.
Online HPLC method offers broad range of applicability. The compounds of over a broad range of polarity and pKa can be evaluated which exhibit different kinetic behavior towards DPPH. It can employ both isocratic and gradient runs with mobile phases of different composition and pH can be carried out (Dapkevicius et al. 1999). The major advantage of this method is that it is immediately clear which constituent possesses radical scavenging activity. Thus it is no longer necessary to purify every single constituent for off-line assays, leading to very significant reductions of cost and faster results.
-
Thin Layer Chromatography (TLC)-DPPH methods
TLC combined with DPPH radical detection (Glavind and Holmer 1967) involved TLC separation of the analyte, followed by detection of DPPH radical scavengers by spraying the TLC plates with DPPH solution. The plates were evaluated visually and a quantitative determination could be achieved by eluting the spots with DPPH reagent (Takao et al. 1994).
A method was developed to measure the radical scavenging activity of compounds separated by reversed-phase TLC (RP-TLC) using phenolic acids as model analytes. The method is simple and fast, making it suitable for automated systems in screening applications where high throughput and cost efficiency are required (Teijo et al. 2003).
Fig. 3.
MSFIA manifold used for the determination of total antioxidant capacity using DPPH• assay: MS, multi-syringe; Si, syringes; Vi, commutation valves (Solid and dotted lines represent the position on and off, respectively); B1 and B2, confluences; HC, holding coil (200 cm); RC, reaction coil (120 cm); D, detector; C, carrier (ethanol solution 50%, v/v); R, 2,2-diphenyl-1-picrylhydrazyl reagent prepared in C; AO, antioxidant standard solution or sample; PC, personal computer; W, waste
Fig. 4.
Schematic view of SIA set-up for the assay of antioxidants based on DPPH radical bleaching. SP syringe pump, SV selector valve, FC flow cell, SPM spectrophotometer, HC holding coil, CS(2) carrier ethanol-water 1:1 (v/v), S(1) sample, DPPH(3) 0.1 mM DPPH in ethanol-water 1:1, W(6) waste from detector. The numbers in parentheses refer to valve port numbers indicated in Table 1 extract in 10 mL of aqueous 50% (v/v) ethanol under 10-min sonication in Sonorex Super 10P (Bandelin) ultrasound bath (sonication level 10); the same solvent was used for appropriate dilution of the extract stock solutions. Ethanol-water 1:1 served also as the SIA carrier. All solvents used for the dissolution of standards, extract samples and dilution of stock solutions, DPPH reagent, and SIA carrier stream were degassed by 10-min sonication in Sonorex Super 10P (Bandelin) ultrasound bath (sonication level 10)
Fig. 5.
Scheme of biamperometric measurement.WE: Working electrode, AE: Auxillary electrode, RE: Reference electrode, ∆E: Applied voltage
Fig. 6.
Schematic view of online HPLC-DPPH method(53)
Fig. 7.
Diagrammatic scheme of an HPLC coupled to an ESI-MS together with an online DPPH-based antioxidant assay. (Y represents a Y connecter)
TLC method for free radical scavenging activity by video scanning technology
TLC separation combined with the detection of antioxidants in situ by postchromatographic derivatization, was introduced by Olga et al. (2007). However, the separation ability of TLC was much lower than that of conventional HPLC as several compounds might be co-eluted as single spot in TLC. Moreover, TLC could not be interfaced with MS analysis (Dan et al. 2008).
Li et al. (2005) developed a reversed phase TLC method combined with video scanning detection for quantitative evaluation of free radical scavenging activity of antioxidative fractions from rapeseed meal by DPPH method. The activity was evaluated by measuring the area of bright yellow bands against the purple background by a CCD video camera after dipping the plate in DPPH solution. Comparison of the results showed good correlation between the activities measured by TLC-DPPH and by the conventional spectrophotometric assay. Also, no sample purification is needed and both separation and the activity measurement can be done in the same TLC-DPPH plate simultaneously.
-
Hyphenated High Speed Counter Current Chromatography (HSCCC)–DPPH Method
A method for rapid preparative isolation and screening of antioxidants has been developed by combining preparative High Speed Counter Current Chromatography (HSCCC) with on-line radical scavenging detection by use of DPPH. radical. HSCCC is a liquid–liquid chromatographic technique with no solid support matrix; therefore eliminates the irreversible adsorption of samples (Yoichiro 1981). This method has been successfully used to separate and isolate many natural products (David et al. 2007; Gutzeit et al. 2007.
Following preparative isolation and purification by HSCCC, the activity of the collected fractions has been evaluated by use of off-line methods which is a time-consuming and labor-intensive process (Pukalskas and van Beek 2005; Perez-Bonilla et al. 2006). Therefore, HSCCC has been coupled on-line with radical scavenging detection (HSCCC–DPPH) for isolation and screening of antioxidants (Shuyun et al. 2008).
Conclusion
There are various methods for the determination of antioxidant potential of different biological samples. However, a single method is not suitable for all and there is no shortcut approach to determine antioxidant activity. Amongst all the available methods, DPPH method has been widely applied for estimating antioxidant activity, however, its applications should to be carried out bearing in mind the basis of the method, and the need wherever possible to establish the stoichiometry for the quenching reaction, so that the antioxidant activity may be related to the structure of the substrate molecule. The method offers advantages of being rapid, simple and inexpensive and provides first hand information on the overall antioxidant capacity of the test system. The trend in antioxidant activity obtained by using the DPPH method is comparable to trends found using other methods. For a better understanding of the mechanisms involving the DPPH radical and potential antioxidants, it would be interesting to characterize the reaction intermediates and products. To do this, it is necessary to separate these compounds by chromatography and to identify them. It would also be very useful to build a plausible kinetic model and determine the order of the different reactions and their constants. Various modifications in DPPH method are discussed for a wide range of applications based on the requirement and more importantly, the affordable inputs.
References
- Abuin E, Lissi E, Ortiz P, Henriquez C. Uric acid reaction with DPPH radicals at the micellar interface. Bol Soc Chil Quim. 2002;47:145–149. [Google Scholar]
- Ancerewicz J, Miglavaca E, Carrupt PA, Testa B, Bree F, Zinin R, Tillement JP, Labidelle P, Goyot SD, Chauvet-Monges AM, Crevent A, Le Ridant A. Structure property relationship of trimetadizine derivatives and model compounds as potential antioxidants. Free Rad Biol Med. 1998;25(1):113–120. doi: 10.1016/S0891-5849(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Arnao MB. Some methodological problems in the determination of antioxidant activity using chromogen radicals:a practice case. Tr Food Sc Tehnol. 2000;11(11):419–421. doi: 10.1016/S0924-2244(01)00027-9. [DOI] [Google Scholar]
- Bandoniene D, Murkovic M, Pfannhauser W, Venskutonis PR, Gruzdiene D. Detection and activity evaluation of radical scavenging compounds by using DPPH free radical and on-line HPLC– DPPH methods. Euro Food Res Technol. 2002;214(2):143–147. doi: 10.1007/s00217-001-0430-9. [DOI] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Bondet V, Brand-Williams W, Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. Lebensmitt Wissenschaft Technologie Food Sci Technol. 1997;30:609–615. doi: 10.1006/fstl.1997.0240. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci Technol. 1995;28:25–30. [Google Scholar]
- Buijnster M, Bicanic D, Chirtoc M, Nicoli MC, Min-Kucience Y. Evaluation of antioxidative activity of some antioxidants by means of combined opthothermal window and DPPH free radical colorimetry. Anal Sci. 2001;17:544–546. [Google Scholar]
- Cerda V, Estela JM, Forteza R, Cladera A, Becerra E, Altimira P, Sitjar P. An environmental friendly method for the automatic determination of hypochlorite in commercial products using multisyringe flow injection analysis. Talanta. 1999;50:695. doi: 10.1016/S0039-9140(99)00196-4. [DOI] [PubMed] [Google Scholar]
- Chandrasekar D, Madhusudhana K, Ramakrishna S, Diwan PV. Determination of DPPH free radical scavenging activity by reversed-phase HPLC: A sensitive screening method for polyherbal formulations. J Pharm Biomed Anal. 2006;40(2):460–464. doi: 10.1016/j.jpba.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Changqing W, Feng C, Xi W, Yonnie W, Meidui D, Guoqing H, Ronald DG, Lilin H, Guohui H. Identification of Antioxidant Phenolic Compounds in Feverfew (Tanacetum parthenium) by HPLC-ESI-MS/MS and NMR. Phytochem Anal. 2007;18:401–410. doi: 10.1002/pca.995. [DOI] [PubMed] [Google Scholar]
- Chevion S, Berry EM, Kitrossky NK, Kohen N. Evaluation of plasma low molecular weight antioxidant capacity by cyclic voltammetry. Free Radic Biol Med. 1997;22:411–421. doi: 10.1016/S0891-5849(96)00337-1. [DOI] [PubMed] [Google Scholar]
- Contreras-Guzman ES, Strong FC. Determination of tocopherols (Vitamin E) by reduction of cupric ion. JAOAC. 1982;65:1215–1222. [Google Scholar]
- Cui K, Luo X, Murthy MRV. Role of oxidative stress in neurodegeneration: recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Prog Neuropsychopharmacol Biol Psych. 2004;28:771–799. doi: 10.1016/j.pnpbp.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Dan T, Hui-Jun L, Jun C, Chao-Wei G, Ping L. Rapid and simple method for screening of natural antioxidants from Chinese herb Flos Lonicerae Japonicae by DPPH-HPLC-DAD-TOF/MS. J Sep Sci. 2008;31(20):3519–3526. doi: 10.1002/jssc.200800173. [DOI] [PubMed] [Google Scholar]
- Dapkevicius A, van Beek TA, Niederlander HAG, de Groot AE. Evaluation and comparison of two improved techniques for the on-line detection of antioxidants in liquid chromatography eluates. Anal Chem. 1999;71:736–740. doi: 10.1021/ac9805908. [DOI] [PubMed] [Google Scholar]
- David MR, Christopher O, Slavko K, Georgie F, Alexander P, Peter IA, Yoichiro I, Ilya R. Preparative isolation and identification of tyrosinase inhibitors from the seeds of Garcinia kola by high-speed counter-current chromatography. J Chromato A. 2007;1151(1–2):45–50. doi: 10.1016/j.chroma.2007.02.085. [DOI] [PubMed] [Google Scholar]
- Gil MI, Thomas Barberan FA, Hess-Pierce B, Hplcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relation ship with phenolic composition and processing. J Agri Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Glavind J, Holmer G. Thin-Layer Chromatographic Determination of Antioxidants by the Stable Free Radical Diphenyl-picrylhydrazyl. Ibid. 1967;44:539–542. [Google Scholar]
- Gomez-Alonso S, Fregapane G, Salvador MD, Gordon MH. Changes in phenolic composition and antioxidant activity of virgin olive oil during frying. J Agri Food Chem. 2003;51:667–672. doi: 10.1021/jf025932w. [DOI] [PubMed] [Google Scholar]
- Guo JT, Lee HL, Chiang SH, Lin FI, Chang CY. Antioxidant properties of the extracts from different parts of broccoli in Taiwan. J Food Drug Anal. 2001;9(2):96–101. [Google Scholar]
- Gutzeit D, Wray VP, Winterhalter P, Jerz G. Preparative Isolation and Purification of Flavonoids and Protocatechuic Acid from Sea Buckthorn Juice Concentrate (Hippophaë rhamnoides L. sp. rhamnoides) by High-Speed Counter-Current Chromatography. Chromatographia. 2007;65(1–2):1–7. [Google Scholar]
- Halliwell B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Rad Res. 1996;25(1):57–74. doi: 10.3109/10715769609145656. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Antioxidants and human disease: a general introduction. Nutr Rev. 1997;5:544–552. doi: 10.1111/j.1753-4887.1997.tb06100.x. [DOI] [PubMed] [Google Scholar]
- Ionita G, Em Sahini V, Semenescu G, Ionita P. Kinetics of oxidation of amino acids by some free stable hydrazyl radicals. Act Chim Slov. 2000;47:111–119. [Google Scholar]
- Karunakar V, Prabhakar MC, Krishna V. Determination of antioxidant activity of some drugs using high-pressure liquid chromatography. Arzneim Forsch Drug Res. 2003;53:254–259. doi: 10.1055/s-0031-1297105. [DOI] [PubMed] [Google Scholar]
- Kim JK, Noh JH, Lee S, Choi JS, Suh H, Chung HY, Song YO, Choi WC. The first total synthesis of 2, 3, 6-tribromo-4, 5-dihydroxybenzyl methyl ether (TDB) and its antioxidant activity. Bull Korean Chem Soc. 2002;23(5):661–662. doi: 10.5012/bkcs.2002.23.5.661. [DOI] [Google Scholar]
- Koleva II, Niederlander HAG, van Beek TA. An on-line HPLC method for detection of radical scavenging compounds in complex mixtures. Anal Chem. 2000;72:2323–2328. doi: 10.1021/ac9912451. [DOI] [PubMed] [Google Scholar]
- Kosar M, Dorman HJD, Bachmayer O, Baser KHC, Hiltunen R. An improved on-line HPLC-DPPH method for the screening of free radical scavenging compounds in water extracts of Lamiaceae plants. Plant Chem Nat Comp. 2003;39:161–166. doi: 10.1023/A:1024853628326. [DOI] [Google Scholar]
- Lebeau J, Furman C, Bernier JL, Duriez P, Teissier E, Cotelle N. Antioxidant properties of di-tert-butylhydroxylated flavonoids. Free Radic Biol Med. 2000;29(9):900–912. doi: 10.1016/S0891-5849(00)00390-7. [DOI] [PubMed] [Google Scholar]
- Leitao GG, Leitao SG, Vilegas W. Quick preparative separation of natural naphthoquinones with antioxidant activity by high-speed counter-current chromatography. Z Naturforsch. 2002;57:1051–1055. doi: 10.1515/znc-2002-11-1217. [DOI] [PubMed] [Google Scholar]
- Li P, Anu H, Jari S, Teijo Y, Heikki V (2005). TLC method for evaluation of free radical scavenging activity of rapeseed meal by video scanning technology, http://www.regional.org.au, 2005 (Accessed on September 2008).
- Lu Y, Foo LY. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000;68:81–85. doi: 10.1016/S0308-8146(99)00167-3. [DOI] [Google Scholar]
- Luıs MM, Marcela AS, Salette R, Lima JLFC. Automatic method for determination of total antioxidant capacity using 2, 2-diphenyl-1-picrylhydrazyl assay. Anal Chim Acta. 2006;558:310–318. doi: 10.1016/j.aca.2005.11.013. [DOI] [Google Scholar]
- Masahiro N, Masahiro K, Minemitsu N, Akio K, Yoshimi N. Non-reductive Scavenging of 1, 1-Diphenyl-2-picrylhydrazyl (DPPH) by Peroxyradical: A Useful Method for Quantitative Analysis of Peroxyradical. Chem Pharm Bull. 2005;53(6):714–716. doi: 10.1248/cpb.53.714. [DOI] [PubMed] [Google Scholar]
- Milardovic S, Ivekovic D, Rumenjak V, Bozidar SG. Use of DPPH./DPPH redox couple for biamperometric determination of antioxidant activity. Electroanalysis. 2005;17(20):1847–1853. doi: 10.1002/elan.200503312. [DOI] [Google Scholar]
- Min DB. Lipid oxidation of edible oil. In: Akoh K, Min DB, editors. Food Lipids: Chemistry, Nutrition and Biotechnology. New York: Marcel Dekkar; 1998. pp. 283–296. [Google Scholar]
- Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004;26(2):211–219. [Google Scholar]
- Nuengchamnong N, de Jong CF, Bruyneel B, Niessen WMA, Irth H, Ingkanina K. HPLC Coupled On-line to ESI-MS and a DPPH-based assay for the rapid identification of antioxidants in Butea superba. Phytochem Anal. 2005;16:422–428. doi: 10.1002/pca.865. [DOI] [PubMed] [Google Scholar]
- Olga NP, Svetlana AI, Alexander NS, Valery GM. Separation and evaluation of free radical-scavenging activity of phenol components of Emblica officinalis extract by using an HPTLC-DPPH method. J Sep Sci. 2007;30(9):1250–1254. doi: 10.1002/jssc.200600532. [DOI] [PubMed] [Google Scholar]
- Ozcelik B, Lee JH, Min DB. Effects of light, oxygen, and pH on the absorbance of 2, 2-Diphenyl-1-picrylhydrazyl. J Food Sci. 2003;68(2):487–490. doi: 10.1111/j.1365-2621.2003.tb05699.x. [DOI] [Google Scholar]
- Parry J, Su L, Luther M, Zhou KQ, Yurawecz MP, Whittaker P, Yu LL. Fatty acid composition and antioxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils. J Agric Food Chem. 2005;53:566–573. doi: 10.1021/jf048615t. [DOI] [PubMed] [Google Scholar]
- Perez-Bonilla M, Salido S, van Beek TA, Linares-Palomino PJ, Altarejos J, Nogueras M, Sanchez A. Isolation and identification of radical scavengers in olive tree (Olea europaea) wood. J Chromato A. 2006;1112(1–2):311–318. doi: 10.1016/j.chroma.2005.12.055. [DOI] [PubMed] [Google Scholar]
- Polasek M, Skala P, Opletal L, Jahodar L. Rapid automated assay of anti-oxidation/radical-scavenging activity of natural substances by sequential injection technique (SIA) using spectrophotometric detection. Anal Bioanal Chem. 2004;379:754–758. doi: 10.1007/s00216-004-2559-4. [DOI] [PubMed] [Google Scholar]
- Prakash A. Antioxidant activity. Med Lab Anal Prog. 2001;19(2):1–6. [Google Scholar]
- Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agri Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Pukalskas A, van Beek TA. Development of a triple hyphenated HPLC–radical scavenging detection–DAD–SPE–NMR system for the rapid identification of antioxidants in complex plant extracts. J Chromato A. 2005;1074(1–2):81–88. doi: 10.1016/j.chroma.2005.03.089. [DOI] [PubMed] [Google Scholar]
- Pukalskas A, van Beek TA, Venskutonis RP, Linssen JPH, van Veldhuizen A, de Groot A. Identification of radical scavengers in sweet grass (Hierochloe odorata) J Agric Food Chem. 2002;50:2914–2919. doi: 10.1021/jf011016r. [DOI] [PubMed] [Google Scholar]
- Rocha FRP, Reis BF, Zagatto EAG, Lima JLFC, Lapa RAS, Lapa SJLM. Multicommutation in flow analysis: concepts, applications and trends. Anal Chim Acta. 2002;468:119. doi: 10.1016/S0003-2670(02)00628-1. [DOI] [Google Scholar]
- Ruiz MA, Reviejo AJ, Parrado C, Pingarron JM. Development of an amperometric enzyme biosensor for the determination of the antioxidant tert-butylhydroxyanisole in a medium of reversed micelles. Electroanalysis. 1996;8(6):529–533. doi: 10.1002/elan.1140080606. [DOI] [Google Scholar]
- Sanchez-Moreno C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci Technol Int. 2002;8(3):121–137. [Google Scholar]
- Sanchez-Moreno C, Larrauri JA, Saura-Calixto F. A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agri. 1998;79:270–276. doi: 10.1002/(SICI)1097-0010(199802)76:2<270::AID-JSFA945>3.0.CO;2-9. [DOI] [Google Scholar]
- Sanchez-Moreno C, Larrauri JA, Saura-Calixto F. Free radical scavenging capacityand inhibition of wines, grape juices and related polyphenolic constituents. Food Res Int. 1999;32:407–412. doi: 10.1016/S0963-9969(99)00097-6. [DOI] [Google Scholar]
- Sanchez-Moreno C, Larrauri JA, Saura-Calixto F. Free radical scavenging capacity of selected red, rose and white wines. J Sci Food Agri. 1999;79:1301–1304. doi: 10.1002/(SICI)1097-0010(19990715)79:10<1301::AID-JSFA367>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Schwarz K, Bertelsen G, Nissen LR, Gardner PT, Heinonen MI, Hopia A, Huynh-Ba T, Lambelet P, McPhail D, Skibsted LH, Tijburg L. Investigation of plant extracts for the protection of processed foods against lipid oxidation. Comparison of antioxidant assays based on radical scavenging, lipid oxidation and analysis of the principal antioxidant compounds. Eur Food Res Technol. 2001;212:319–328. doi: 10.1007/s002170000256. [DOI] [Google Scholar]
- Sendra JM, Sentandreu E, Navarro JL. Reduction kinetics of the free stable radical 2, 2-diphenyl-1-picrylhydrazyl (DPPH•) for determination of the antiradical activity of citrus juices. Eur Food Res Technol. 2006;223:615–624. doi: 10.1007/s00217-005-0243-3. [DOI] [Google Scholar]
- Shuyun S, Honghao Z, Yuping Z, Kelong H. Hyphenated HSCCC–DPPH for rapid preparative isolation and screening of antioxidants from Selaginella moellendorffii. Chromatographia. 2008;68:173–178. doi: 10.1365/s10337-008-0716-1. [DOI] [Google Scholar]
- Singh S, Singh RP. In vitro methods of assay of antioxidants: An overview. Food Rev Int. 2008;24(4):392–415. doi: 10.1080/87559120802304269. [DOI] [Google Scholar]
- Sung-Kun Y, Su-Jung Y, Chul-Ho Y. A continuous spectrophotometric assay for NADPH-cytochrome P450 reductase activity using 1, 1-diphenyl-2-picrylhydrazyl. J Biochem Mol Biol. 2004;37:629–633. doi: 10.5483/BMBRep.2004.37.5.629. [DOI] [PubMed] [Google Scholar]
- Takao T, Kitatani F, Watanabe N, Yagi A, Sakata KA. Simple Screening Method for Antioxidants and Isolation of Several antioxidants Produced by Marine Bacteria from Fish and shellfish. Biosci Biotechnol Biochem. 1994;58:1780–1783. doi: 10.1271/bbb.58.1780. [DOI] [Google Scholar]
- Teijo Y, Li P, Jari S, Anu H, Heikki V. Free radical scavenging activity of phenolics by reversed phase TLC. JAOCS. 2003;80(1):9–14. doi: 10.1007/s11746-003-0642-z. [DOI] [Google Scholar]
- Ukeda H. Flow-injection analytical system for the evaluation of antioxidative activity. Bunseki Kagaku. 2004;53:221. doi: 10.2116/bunsekikagaku.53.221. [DOI] [Google Scholar]
- Ukeda H, Adachi Y, Sawamura M. Flow injection analysis of DPPH radical based on electron spin resonance. Talanta. 2002;58:1279. doi: 10.1016/S0039-9140(02)00411-3. [DOI] [PubMed] [Google Scholar]
- Vít K, Daniel J, Lubos O, Ludek J, Kamil K. Assay of radical scavenging activity of antidotes against chemical warfare agents by DPPH test using sequential injection technique. J Appl Biomed. 2007;5:81–84. [Google Scholar]
- Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical scavenging activity of foods by using 1,1,-Diphenyl-2-Picrylhydrazyl. Biosci Biotech Biochem. 1998;62:1201–1204. doi: 10.1271/bbb.62.1201. [DOI] [PubMed] [Google Scholar]
- Yepez B, Espinosa M, Lopez S, Bolanos G. Producing antioxidant fractions from herbaceous matrices by supercritical fluid extraction. Fluid Phase Equil. 2002;194:879–884. doi: 10.1016/S0378-3812(01)00707-5. [DOI] [Google Scholar]
- Yoichiro I. Efficient preparative counter-current chromatography with a coil planet centrifuge. J Chromato. 1981;214(1):122–125. doi: 10.1016/S0021-9673(00)80907-3. [DOI] [Google Scholar]
- Yu LL. Free radical scavenging properties of conjugated linoleic acids. J Agric Food Chem. 2001;49:3452–3456. doi: 10.1021/jf010172v. [DOI] [PubMed] [Google Scholar]
- Zhihong C, Moore J, Liangli Y. High-Throughput Relative DPPH Radical Scavenging Capacity Assay. J Agric Food Chem. 2006;54:7429–7436. doi: 10.1021/jf0611668. [DOI] [PubMed] [Google Scholar]
- Zhu QY, Hackman RM, Ensunsa JL, Holt RR, Keen CL. Antioxidative activities of oolong tea. J Agric Food Chem. 2002;50:6929–6934. doi: 10.1021/jf0206163. [DOI] [PubMed] [Google Scholar]