Abstract
Use of extracts of kinnow and pomegranate by-products as source of natural antioxidant in salted chicken patties during refrigerated storage was evaluated. Five treatments viz., I. Control (meat), II.MS (meat + 2%salt), III. KRP (meat + 2% salt + 2% kinnow-rind-powder extract), IV. PRP (meat + 2% salt + 2% pomegranate-rind-powder extract), and V. PSP (meat + 2% salt + 2% pomegranate seed powder extract). Results showed that salt significantly (P < 0.05) reduced lightness and yellowness but increased chroma and TBARS values. The average increase in TBARS was significantly (P < 0.05) higher in MS (114%) and Control (108%) but lower in KRP (90%), PRP (81%) and PSP (73%). Lipid oxidation (TBARS) in salted meat during the storage was significantly reduced (P < 0.05) by KRP (39%), PRP (43%) and PSP (68%). Thus it was observed that addition of 2% salt accelerated the TBARS formation but inclusion of extracts of pomegranate and kinnow fruit by-products effectively counteracted this effect. The overall antioxidant effect was in the order of PSP>PRP>KRP. Further a significant (P < 0.05) negative correlation between total phenolics contents and TBARS values was also observed. Therefore, it was concluded that extracts of these fruit by-products have potential to be used as natural antioxidants to minimize the oxidative problems in poultry meat products.
Keywords: Chicken patties, Meat colour, Natural antioxidants, Pomegranate, Kinnow, TBARS
Introduction
Chicken breast meat is an important component of healthy diet. Breast meat is further rich source of essential fatty acids that are polyunsaturated and not synthesized by human body (Ashgar et al. 1990). The relatively large proportion of polyunsaturated fatty acids and phospholipids fraction in the composition of poultry breast makes it highly susceptible to lipid oxidation which further affects colour, flavour, texture and nutritional value (Tang et al.2001; Jahan et al.2004). Grinding and addition of salt are common processes used in meat industry. Grinding exposes lipid membranes to metal oxidation catalysts and salt has been demonstrated to accelerate lipid oxidation in chicken meat (O’Neill et al. 1999; Rhee and Ziprin 2001). Synthetic antioxidants like BHT, BHA have been successfully used to prevent the oxidation problems in meat products. However, natural sources of antioxidants are considered to be safer than synthetic antioxidants. Hence there is a growing interest in natural antioxidants in meat products. In this direction, antioxidant effects of grape seed extracts in poultry meat (Brannan. 2008), tea catechins in chicken patties (Mitsumoto et al. 2005), rosemary and sage in chicken nuggets (O’Sullivan et al. 2004) have been investigated.
Pomegranate (Punica granatum) rind is an inedible part/by-product obtained during processing of pomegranate juice. Recently use of pomegranate juice and rind powder as a source of natural antioxidant in chicken patties had been investigated (Naveena et al. 2008). Further, Devatkal et al. (2010) have demonstrated significant antioxidant effect of extracts of pomegranate rind and seed powders in cooked goat meat patties. Kinnow or Tangerine (Citrus reticulata) is a citrus fruit variety grown in north Indian states. Kinnow peel is obtained as a major processing by-product during juice extraction. Further kinnow peel is a rich source of Vitamin C, carotenoids, limonene and polyphenolics antioxidants, (Anwar et al. 2008). Antioxidant effect of extract of kinnow rind powder in cooked goat meat patties had been recently investigated by Devatkal et al. (2010). Moreover there is no published literature available on utilization of kinnow fruit by-product in poultry meat.
Hence, an investigation was carried out to evaluate the effect of extracts from kinnow rind powder (KRP), pomegranate rind powder (PRP) and pomegranate seed powder (PSP) on salt induced lipid oxidation and colour changes in raw chicken patties stored aerobically at 4 ± 1 °C.
Materials and methods
Meat and fruit by-products
Fresh chicken breast meat (broilers of 7–8 weeks) was obtained from a local retail meat processing plant. Meat samples were chilled at 4 °C for 24 h before use. Fresh chicken meat was obtained separately for each of the replication (n = 3). Fresh kinnows (Citrus reticulata) and pomegranate (Punica granatum) fruits were also obtained from retail fruit market. Standard tannic acid (SD Fine Chemicals, Mumbai, India), Thiobarbituric acid (MP Biomedicals Pvt. Ltd. Mumbai, India), and 1, 1, 3, 3- tetraethoxypropane (Sigma Aldrich, New Delhi, India) used in the study were of analytical grade.
Preparation of powders and extracts of kinnow rind (KRP), pomegranate rind (PRP) and pomegranate seed (PSP)
Mature and healthy kinnow and pomegranate fruits were washed, cut manually with knife and peeled off. The rind (peel) thus obtained was cut into small pieces using a sharp knife and dried in an air circulatory tray drier (Narang Scientific Works, New Delhi, India) at 60 °C for 48 h. Dried pieces were powdered in a heavy duty kitchen grinder and sieved using a sieve, ASTM No.10 (1.651 mm) and packed into 100 g units and stored in high density polyethylene bags at room temperature. Similarly powder from pomegranate seeds was prepared by drying the pomegranate fruits seeds in a tray drier and grinding in a heavy duty kitchen grinder and sieving (using a sieve ASTM No.10).
About 10 g of dried powder was mixed with 25 ml boiled distilled water and left for 1 h. The extract obtained by filtration was used in further experiments. Freshly prepared extract was used for each replication.
Preparation patties
About 3 kg of chicken breast meat was minced twice (10 mm plate followed by 8 mm plates using a meat mincer (Sirman, Italy). After mincing, the meat samples (500 g each) were assigned to one of the following five treatments : I) Control (meat without salt and natural antioxidant); II). MS (meat with 2% w/w salt); III) KRP (meat with 2% w/w salt and 5% w/v kinnow extract); IV) PRP (meat with 2% w/w salt and 5% w/v pomegranate rind extract and V) PSP (meat with 2% w/w salt and 5% w/v pomegranate seed extract). Immediately after adding all ingredients, meat samples were thoroughly mixed and made into patties manually (75 g each). These patties were packed individually in low density polyethylene bags and stored at 4 ± 1 °C for 6 days. During the storage period instrumental colour, TBARS values and total phenolics content were measured at an interval of 2 days.
Estimation of total phenolics
Total phenolics from meat patties were estimated by Folin–Ciocalteus (F–C) assay. (Escarpa and Gonzalez 2001). Five gram meat was blended with 25 ml boiled distilled water and extracted for 1 h. Suitable aliquots of extracts were taken in different test tubes and the volume was made to 0.5 ml with distilled water followed by the addition of 0.25 ml F–C (1 N) reagent and 1.25 ml sodium carbonate solution (20%). The tubes were vortexed and the absorbance was recorded at 725 nm after 40 min. The amount of total phenolics was calculated as tannic acid equivalent from the calibration curve using standard tannic acid solution (0.1 mg/ml). During storage period total phenolics were measured at an interval of 2 days. The average decrease in total phenolics from 0th day to 6th day was also calculated arithmetically and expressed in percentage.
Instrumental colour evaluation
The colour changes during storage was monitored by evaluating Hunter ‘L’, ‘a’, ‘b’, Hue and Chroma values at an interval of 2 days. Colourimetric analysis on surface of chicken patties was performed using a Hunter Lab Miniscan XE Plus colourimeter (Hunter Associates Laboratory Inc., Reston, VA, USA) with 25 mm aperture set for illumination D65, 10° standard observer angle. Hunter L (lightness), a (redness) and b (yellowness) values were measured on the outer surface of chicken patties from four randomly chosen spots. Hue angle (Tan -b/a) and chroma values (a2 + b2)1/2 were calculated according to Hunter and Harold (1987).
Thiobarbituric acid reacting substances (TBARS) value
Lipid oxidization was monitored by measuring thiobarbituric acid reactive substances at an interval of 2 days during storage. TBARS were determined using extraction method described by Witte et al. (1970). TBARS were extracted in chilled 20% trichloroacetic acid. Thiobarbituric acid extracts of each sample were used for measuring the absorbance at 520 nm. 1, 1, 3, 3, tetraethoxypropane (Sigma Aldrich, New Delhi, India) was used as standard for TBARS assay. TBARS numbers were calculated as mg of malonaldehyde per kg of meat sample. The average increase in TBARS from 0 day to 6th day was also calculated and expressed in percentage.
Statistical analysis
The experiment was replicated thrice and all parameters were measured in duplicate. The data presented in the paper are all mean values of six replications. All data were analyzed using statistical software, Agristat (Indian Agriculture Statistical Research institute, New Delhi, India). Storage data of total phenolics, Hunter colour and TBARS values were analyzed using two-way ANOVA with treatment and storage time as main effects. Statistical significance was identified at the 95% confidence level (P < 0.05). Correlation between total phenolics content and TBARS values of different treatments during storage period was also calculated using Microsoft Excel 2003 (Microsoft Corporation, Sacramento, USA).
Results and discussion
Total phenolics content
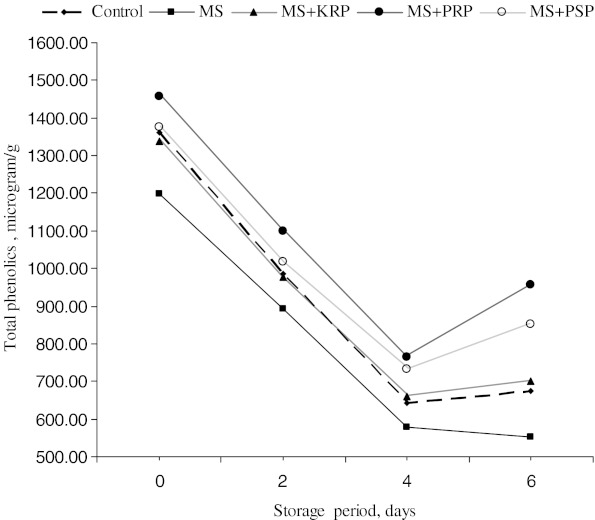
The average phenolics content was significantly (P < 0.05) higher in KRP (893 μg/g), PRP (1069 μg/g) and PSP (993 μg/g) treatments than control (792 μg/g) and MS (726%). Among fruit by-product extracted treatments, PRP significantly (P < 0.05) increased the phenolics content followed by PSP and KRP. The overall changes in phenolics content (Fig. 1) indicated a significant (P < 0.05) decrease in phenolics up to 4th day of storage However, total phenolics increased on 6th day of storage. Total phenolics are synthesized by phenylpropanoid pathway involving an enzyme phenylalanine ammonia-lyase.It has been reported that chilling temperature increases the activity of this enzyme and accelerates build up of phenolics (Padda and Picha 2008).
Fig. 1.
Overall changes in phenolics content during refrigerated storage of ground chicken patties. MS, KRP, PRP, PSP-see Table 1
Percentage decrease of total phenolics during storage was significantly (P < 0.05) higher in control (103%) followed by MS (78%) than KRP (55%), PRP (34%) and PSP (38%). These results indicated that treatments with extracts of fruit by-products showed significantly (P < 0.05) higher phenolics during storage period. Similarly Leheska et al. (2006) observed an increase in phenolics content of precooked pork breakfast sausages prepared with fruit purees. Naveena et al. (2008) have reported a significant increase in phenolics content of cooked chicken patties treated with pomegranate juice and rind extract. Recently Devatkal et al. (2010) have also reported the higher phenolics content of goat meat patties treated with KRP, PRP and PSP extracts. Pomegranate fruit parts are known to contain phenolics compounds and antioxidant characteristics of pomegranate seeds and rind powder have also been reported (Negi and Jayaprakasha 2003).
Instrumental colour
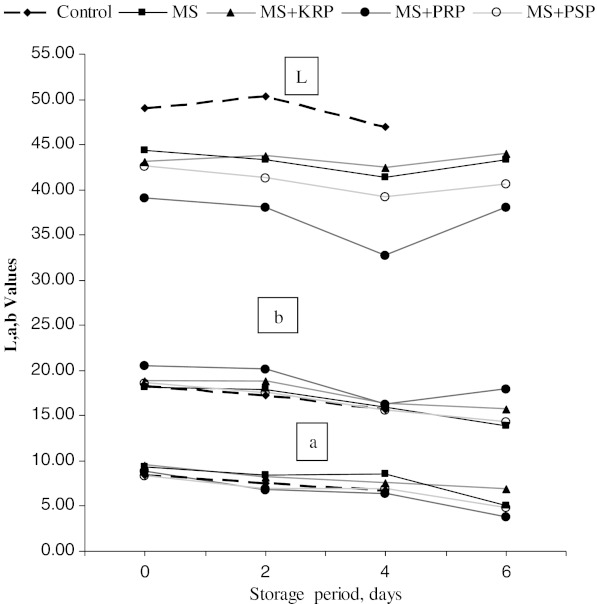
Mean hunter colour values, hue and chroma values are presented in Table 1. Overall mean values of ‘L’ (lightness) was significantly (P < 0.05) higher in control than other treatments. Use of salt and extracts of fruit by-products powder resulted in the dark colour (lower L Value).There was no significant difference between different storage intervals for L values. Overall means of redness (Hunter a value) value was significantly (P < 0.05) higher in control, MS and KRP as compared to PRP and PSP. Redness did not vary significantly between PRP and PSP treatments but significantly (P < .0.05) decreased during storage period in all treatments (Fig. 2) Yellowness (Hunter b value) was significantly (P < 0.05) higher in PRP followed by control and KRP. No significant difference was noticed for yellowness between MS and PSP .Yellowness did not vary significantly during initial days of storage. However it decreased after 4th day of storage (Fig. 2). This trend was similar in all the treatments except PSP which showed a decreasing trend during all storage intervals.
Table 1.
Overall means of Hunter colour values of ground chicken patties treated with different natural antioxidants and stored aerobically at 4 ± 1 °C
| Treatments | L value | a value | b value | Hue | Chroma |
|---|---|---|---|---|---|
| Meat (Control) | 48.80 ± 1.39d | 7.53 ± 0.55 b | 17.13 ± 0.49 b | 57.69 ± 1.04a | 18.76 ± 0.49 b |
| Meat + Salt (MS) | 42.05 ± 0.49c | 7.86 ± 0.32b | 16.47 ± 0.79a | 64.71 ± 1.03b | 18.32 ± 0.56ab |
| KRP1 + Meat + Salt | 42.48 ± 0.39c | 7.98 ± 0.29b | 17.44 ± 0.38b | 65.44 ± 0.91b | 19.21 ± 0.37c |
| PRP2 + Meat + Salt | 36.95 ± 0.39a | 6.39 ± 0.36a | 18.68 ± 0.64c | 71.10 ± 1.28d | 19.86 ± 0.62c |
| PSP3 + Meat + Salt | 40.87 ± 0.64b | 6.71 ± 0.27a | 16.46 ± 0.43a | 67.96 ± 0.91c | 17.83 ± 0.42a |
Means with different superscript within a column are significantly different (P < 0.05). (n = 6)
1Kinnow Rind Powder extract, 2Pomegranate Rind Powder extract, 3Pomegranate seed Powder extract
Fig. 2.
Overall changes in Hunter colour values during refrigerated storage of ground chicken patties. MS, KRP, PRP, PSP-see Table 1
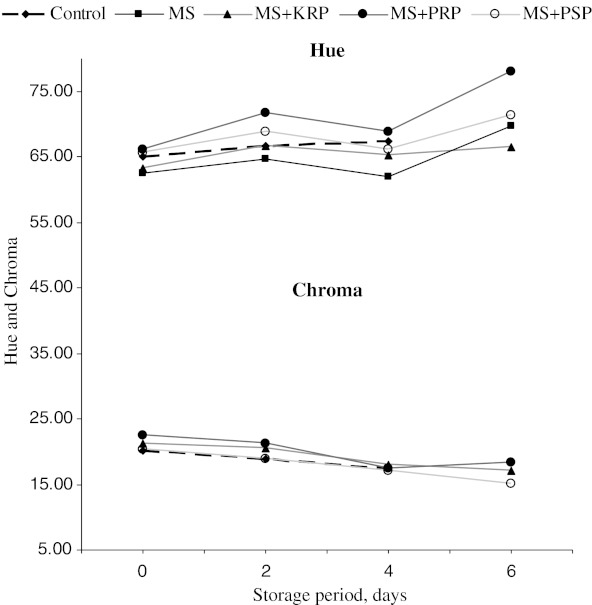
Mean Hue values were significantly (P < 0.05) higher in PRP followed by PSP and lowest in control as compared to MS and KRP treatments which showed no significant difference . It showed an increasing trend with storage interval in all the treatments (Fig. 3) Overall means of chroma value (colour intensity) was significantly higher in KRP and PRP than other treatments. Chroma value did not differ significantly between MS, PSP and control treatments. Chroma values further decreased with storage intervals in all the treatments (Fig. 3).
Fig. 3.
Overall changes in Hue and Chroma values during refrigerated storage of ground chicken patties. MS, KRP, PRP, PSP-see Table 1
A significant decease in ‘L’ value due to addition of salt to comminuted chicken patties had been reported by Swatland and Barbut (1999). In a recent study, Naveena et al. (2008) reported a reduction in ‘L’ value and an increase in ‘a’ values due to addition of PRP in chicken patties. Decrease in ‘a’ value indicates the change in colour from red to brown which could be due to the formation of metmyoglobin in salt containing treatments. It has been further reported that salt greatly accelerate the process of meat discolouration due to pro-oxidative activity which can be attributed to its ability to release iron from heme pigments and other heme binding molecules (Rhee and Ziprin 2001)
TBARS values
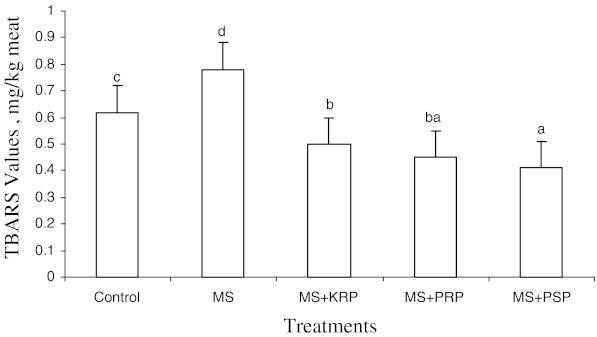
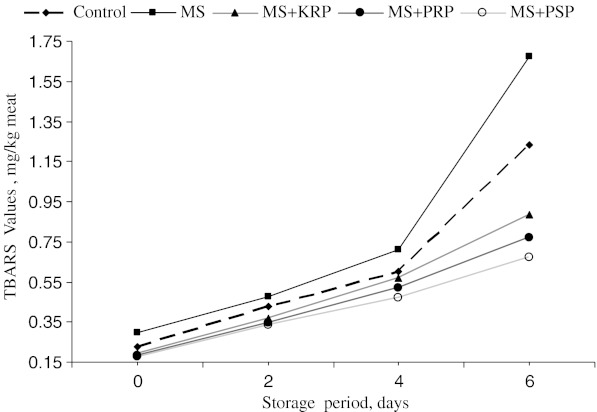
Mean TBARS values (Fig. 4) were significantly (P < 0.5) higher in MS followed by control. Treatments with extracts showed a significantly (P < 0.5) lower TBARS values than control and MS. Lowest TBARS was found in PSP sample followed by PRP and KRP. During all storage intervals TBARS was significantly higher in MS followed by control samples. PRP and PSP samples showed lowest TBARS values during all storage intervals. Thus treatments with fruit by-products extract (KRP, PRP, and PSP) showed a significant (P < 0.05) lower TBARS values than control and MS samples. Irrespective of treatment, TBARS gradually increased with increase in storage period (Fig. 5).
Fig. 4.
Overall means of TBARS values of ground chicken patties. MS, KRP, PRP, PSP-see Table 1. Bars with different letters differ significantly (P < 0.05)
Fig. 5.
Overall changes in TBARS values during refrigerated storage of ground chicken patties. MS, KRP, PRP, PSP-see Table 1
Average percent increase in TBARS (Table 2) was maximum in MS followed by control. Fruit by-products treated samples showed a significant (P < 0.05) lower percent increase in TBARS as compared to control and MS. Among three by-products, PSP extract was having higher antioxidant activity followed by PRP and KRP. Similar observations on% reduction of TBARS revealed that KRP, PRP and PSP reduced TBARS by 23%, 27% and 40% respectively in comparison to control sample. Similarly as compared to salted meat sample TBARS was reduced by 39%, 43% and 68% in KRP, PRP and PSP respectively. Thus these extracts effectively delayed the formation of TBARS during refrigerated storage of chicken patties. Further pro-oxidant effect of salt was also minimized by the use of these natural extracts in chicken patties. These results clearly demonstrated that addition of extracts of KRP, PRP and PSP significantly reduced the auto oxidation in control sample and salt induced oxidation in MS.
Table 2.
Relationship between total phenolics and TBARS values
| Treatments | Average decrease in phenolics (%) | Average increase in TBARS (%) | Correlation coefficient |
|---|---|---|---|
| Meat (Control) | 103d | 108.70c | −0.96 |
| Meat + Salt (MS) | 78c | 114.16c | −0.93 |
| KRP1 + Meat + Salt | 55b | 90.78b | −0.91 |
| PRP2 + Meat + Salt | 34a | 81.94ab | −.096 |
| PSP3 + Meat + Salt | 38a | 73.50a | −0.95 |
Means with different superscript within a column are significantly different (P < 0.05). (n = 6)
1Kinnow Rind Powder extract, 2Pomegranate Rind Powder extract, 3Pomegranate seed Powder extract
Results of TBARS formation indicated a strong pro-oxidant effect of salt. Further, it was also observed that pro-oxidant effect of salt was minimized by the fruits extracts. Use of extracts of KRP, PRP and PSP in chicken patties meat significantly (P < 0.05) decreased TBARS values. Among three extracts, PSP was having highest antioxidant effect followed by PRP and KRP. A similar antioxidant effect of PRP in chicken patties had been reported by Naveena et al. (2008).
Earlier studies have shown that the addition of salt to meat and meat products increases the TBARS values. O’Neill et al. (1999) reported the pro-oxidative effect of salt in chicken meat. Hernandez et al. (2002) reported that salt at the higher ionic strength decreased the glutathione peroxide activity and increased TBARS content of ground meat during refrigeration temperature. O’Sullivan et al. (2004) observed that malonaldehyde contents in salted chicken nuggets were significantly higher than those in the control nuggets. The possible reasons for salt induced lipid oxidation are reduction in the activity of antioxidant enzyme like catalase, glutathione peroxidase and superoxide dismutase, stimulation of lipid oxidation via iron activation by chloride ions, displacement of iron molecule from myoglobin structure by sodium ions thereby providing free iron for the catalysis of lipid oxidation (O’Neill et al. 1999; Hernandez et al. 2002).
Correlation between total phenolics and TBARS values
Evaluation of relationship between TBARS values and total phenolics through analysis of correlation coefficients (Table 2) suggested a significant ((P < 0.05) negative correlation between these parameters. Thus TBARS increased gradually and total phenolics decreased with increase in storage period. A significant relation between phenolics content and antioxidant effect of pomegranate peel extract has been reported by Negi and Jayaprakasha (2003).
Conclusion
This study clearly demonstrated the pro-oxidant effect of salt and antioxidant effect of kinnow and pomegranate by-products. These extracts significantly reduced auto-oxidation and salt promoted oxidation in chicken patties during refrigerated storage. Among three extracts, PSP was more effective in reducing TBARS formation. Therefore it was concluded that extracts of these fruits by-products could be successfully added to meat to function as antioxidant. Further it is important to note that while processing kinnow and pomegranate into juice, the rind and pulp are discarded and unutilized. The food industry can make use of the by-products as a source of natural anti-oxidants in processed food products. As promising as these results are, additional research will be required to determine how these powders could be used as health promoting functional ingredients in poultry meat processing.
References
- Anwar F, Naseer R, Bhanger MI, Ashraf S, Talpur FN, Aladededune FA. Physicochemical characteristics of citrus seeds and oils from Pakistan. J Am Oil Chem Soc. 2008;85:321–330. doi: 10.1007/s11746-008-1204-3. [DOI] [Google Scholar]
- Ashgar A, Lin CF, Buckely DJ, Booren AM, Flegal CJ. Effect of dietary oils and α- tocopherol supplementation on membrane lipid oxidation in broiler meat. J Food Sci. 1990;55:46–50. doi: 10.1111/j.1365-2621.1990.tb06013.x. [DOI] [Google Scholar]
- Brannan RG. Effect of grape seed extract on physicochemical properties of ground, salted, chicken thigh meat during refrigerated storage at different relative humidity levels. J Food Sci. 2008;73:36–39. doi: 10.1111/j.1750-3841.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- Devatkal SK, Narsaiah K, Borah A. Anti-oxidant effect of extracts of kinnow rind, pomegranate rind and seed powders in cooked goat meat patties. Meat Sci. 2009;85:155–159. doi: 10.1016/j.meatsci.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Escarpa A, Gonzalez MC. Approach to the content of total extractable phenol compounds from different food samples by comparison of Chromatographic and spectrophotometer methods. Anal Chim Acta. 2001;427:119–127. doi: 10.1016/S0003-2670(00)01188-0. [DOI] [Google Scholar]
- Hernandez P, Park D, Rhee KS. Chloride salt type/ionic strength, muscle site and refrigeration effects on antioxidant enzymes and lipid oxidation in pork. Meat Sci. 2002;61:405–410. doi: 10.1016/S0309-1740(01)00212-1. [DOI] [PubMed] [Google Scholar]
- Hunter RS, Harold RW. The measurement of appearance. New York: Wiley; 1987. [Google Scholar]
- Jahan K, Paterson A, Spickett CM. Fatty acid composition, antioxidants and lipid oxidation in chicken pattiess from different production regimes. Int J Food Sci Technol. 2004;39:443–453. doi: 10.1111/j.1365-2621.2004.00799.x. [DOI] [Google Scholar]
- Leheska JM, Boyce J, Brooks JC, Hoover LC, Thompson LD, Miller F. Sensory attributes and phenolics content of precooked pork breakfast sausage with fruit purees. J Food Sci. 2006;71:S249–S252. doi: 10.1111/j.1365-2621.2006.tb15649.x. [DOI] [Google Scholar]
- Mitsumoto M, O’grady MN, Kerry JP, Buckely DJ. Addition of tea catechins and vitamin C on sensory evaluation, colour and lipid stability during chilled storage in cooked or raw beef and chicken patties. Meat Sci. 2005;69:773–779. doi: 10.1016/j.meatsci.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Naveena BM, Sen AR, Vaithiyanathan S, Babji Y, Kondaiah N. Comparative efficacy of pomegranate juice, pomegranate rind powder and BHT in cooked chicken patties. Meat Sci. 2008;80:1304–1308. doi: 10.1016/j.meatsci.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Negi PS, Jayaprakasha GK. Antioxidant and antibacterial activities of Punica granatum peel extracts. J Food Sci. 2003;68:1473–1477. doi: 10.1111/j.1365-2621.2003.tb09669.x. [DOI] [Google Scholar]
- O’Neill LM, Galvin K, Morrissey PA, Buckley JJ. Effect of carnosine, salt & dietary vitamin E on the oxidative stability of chicken meat. Meat Sci. 1999;52:89–94. doi: 10.1016/S0309-1740(98)00152-1. [DOI] [PubMed] [Google Scholar]
- O’Sullivan CM, Lynch AM, Lynch PB, Buckley DJ, Kerry JP. Use of antioxidants in chicken nuggets manufactured with and without the use of salt and/or sodium tripolyphosphate: Effects on product quality and shelf- life stability. Int J Poultry Sci. 2004;3:345–353. doi: 10.3923/ijps.2004.345.353. [DOI] [Google Scholar]
- Padda MS, Picha DH. Effect of low temperature storage on phenolics composition and antioxidant activity of sweet potatoes. Postharvest Biol Technol. 2008;47:176–180. doi: 10.1016/j.postharvbio.2007.06.014. [DOI] [Google Scholar]
- Rhee KS, Ziprin YA. Pro-oxidative effects of NaCl in microbial growth controlled and uncontrolled beef and chicken. Meat Sci. 2001;57:105–112. doi: 10.1016/S0309-1740(00)00083-8. [DOI] [PubMed] [Google Scholar]
- Swatland HJ, Barbut S. Sodium chloride levels in commented chicken muscle in relation to processing characteristics and Fresnel reflectance detected with a polarimetric probe. Meat Sci. 1999;51:377–381. doi: 10.1016/S0309-1740(98)00138-7. [DOI] [PubMed] [Google Scholar]
- Tang S, Kerry JP, Sheehan D, Buckely DJ, Barbut S. A comparative study of tea catechins and α-tocopherol as antioxidants in cooked beef and chicken meat. Supplementation on membrane lipid oxidation in broiler meat. Eu Food Research Technol. 2001;213:286–289. doi: 10.1007/s002170100311. [DOI] [Google Scholar]
- Witte VC, Krauze GF, Bailey ME. A new extraction method for determining 2- thiobarbituric acid values of pork and beef during storage. J Food Sci. 1970;35:582–585. doi: 10.1111/j.1365-2621.1970.tb04815.x. [DOI] [Google Scholar]