Abstract
Antimicrobial potential of ethanolic extract of clove (Eugenia caryophyllata) on fresh mutton during storage at 25 ± 2 °C was evaluated. The extract inhibited spoilage and pathogenic microflora of mutton previously treated with acidulants to reduce surface microbial load and the surface pH, up to 4 days without any deleterious change in sensory and physical qualities. Biomarker cadaverine, an indicator of spoiling/spoiled mutton, was present in 1 day stored control samples and absent up to 4 days in treated mutton. The levels of other biomarkers like biogenic amine index (0.31 mg/100 g) and free fatty acids (1.52%) were lower in 4 days stored treated samples than 1 day stored control samples (3.6 mg/100 g and 2.4%, respectively). Thus, ethanolic extract of clove can be effectively used to improve the keeping quality of fresh mutton up to 4 days at 25 ± 2 °C.
Keywords: Fresh mutton, Ethanolic extract, Clove, Biomarker, Preservation
Introduction
Meat industry to minimise microbial spoilage and extend shelf-life of fresh meat is increasingly seeking use of bio-preservatives rather than chemical additives such as antibiotics, salts of sorbic acid, nitrates and nitrites. Spice and herbs are well known to inhibit bacteria, yeasts and moulds. Reports on the use of spices or herbs as preservatives can be traced to 1550 BC, when the ancient Egyptians used certain spices for food preservation. Spices like clove and cinnamon generally used in Indian culinary have been reported to exhibit suppressing action on many food borne pathogens (Bahk et al. 1990; Aureli et al. 1992; Jeyashakila et al. 1996; Lis-Balchin and Deans 1997; Smith-Palmer et al. 1998; Moreira et al. 2005; Mytle et al. 2006). The antimicrobial activity of clove has been extensively reviewed (Subbulakshmi and Naik 2002). The inhibitory activity of spices is known to be due to the action of essential oils, the major component of which is phenolic in nature. For instance, eugenol (2-methoxy-4-2-propenyl phenol), the phenolic component of clove is known to possess antibacterial activity (Burt 2004) and clove is reported to posses highest total phenolic constituents (141 mg/g) compared to other spices like cumin and black mustard. In vitro antimicrobial activity of some plant essential oils on several Gram positive and Gram negative bacteria is reported (Sreenivasan et al. 2006). Inhibitory effect of clove on several spoilage and toxigenic molds is well established (Rajkumar and Berwal 2003). Most of the published literature, while noting the antimicrobial activity of clove against specific cultures, has not exploited this property to preserve fresh mutton which contains heterogeneous organisms (normal flora). There have been too few studies in foods employing clove as a preservative.
In India, the sheep carcasses are brought to the retail shops for sale from the local slaughter houses immediately after slaughter. Consumption of hot meat (unchilled meat) is a common practice throughout the country. The hot meat (35–37 °C) is held at ambient temperature (25 ± 2 °C) and offered for sale for 18–20 h in the retail shops, unlike in western countries where the carcasses are held at chilled temperature (5 °C) for ageing. Ageing helps in tenderisation which is desirable, whereas when carcasses are held at ambient temperature for long duration, it results in microbial spoilage.
In the present investigation, an attempt has been made to use ethanolic extract of clove to improve the shelf-life of fresh mutton during storage at ambient temperature (25 ± 2 °C). The effect of clove extract in inhibiting microbial spoilage and on water holding capacity, texture and metmyoglobin has been studied. The effect on biomarkers like biogenic amines, free fatty acids and phospholipids was also evaluated during storage.
Materials and methods
Mutton (leg portions, 1.5–2 kg) from 18 months old ‘Bannur’ sheep (a local breed) after 6–7 h postmortem was purchased in 6 different batches on different dates from a known source. The mutton was washed in portable water and hung vertically for 1 h to allow the water to drain.
Clove extract
Ethanolic extract of clove (Eugenia caryophyllata) was prepared by soaking clove powder in 95% ethyl alcohol- overnight (1:4 w/v) and filtering through Whattmann No. 41 after agitation for 10–15 min in a rotary flash shaker.
Preservative treatment
The washed mutton sample was dipped (1:3 w/v) in hot water (66 °C) containing acidulants (1% each of lactic acid and glacial acetic acid) in a vertical tank (30 cm × 90 cm) and removed immediately. The combination of these acids coupled with higher temperature, helps to reduce surface microbial load of meat (Anderson et al. 1992). The above treatment also aided in reducing the surface pH of mutton from 5.6 to 3.8, thereby increasing the antibacterial activity of phenolic compounds, which are known to be active at low pH (Sara Burt 2004). After the acidulant treatment, the sample was hung vertically for 30 min to drain surface water and sprayed twice with clove extract prepared as above at an interval of 50–60 min (totally, 100 ml extract/kg of mutton) using an electric spray gun (Pilot Power – E88, India). The above treated mutton (T) and control (C) (without any treatment) were stored in a room maintained at 25 ± 2 °C and observed once in 3 h for off- odour development till the samples became unacceptable.
Sensory analysis
Sensory attributes like colour, odour and texture were evaluated using 8 -point Hedonic scale by 7 semi-trained panel.
Microbiological analysis
Microbiological media and media components were obtained from Hi-media Laboratories, Mumbai, India. Ten g sample was homogenised with 90 ml sterile peptone water. Multiple decimal dilutions were made with the sample diluents. The standard plate count (SPC) on plate count agar, yeast and molds (Y and M) on potato dextrose agar acidified to pH 3.5 using 10% tartaric acid and Enterobacteriaceae count on Violet Red Bile Dextrose agar were enumerated. The pathogens Staphylococcus on Baird Parker agar (Harrigan and McCance 1976) and E.coli using 4–methyl umbelliferyl b-D–glucoronide agar (Srihari and Vijaya Rao 1998) were also enumerated.
Water Holding Capacity (WHC)
The WHC was analysed as per the method of Wardlaw et al. (1973) by centrifuging 10 g sample at 10,000 rpm for 10 min in a refrigerated centrifuge (4 °C). The values obtained were expressed in terms of g/100 g mutton.
Texture
The textural changes of the samples were estimated in a texture analyser (Model TA HDi, Stable Microsystems, UK) using a software, ‘Texture expert’ version 1.22. Longitudinal sections of debonned mutton chunks (2.5 cm3) were subjected to a force (kg) to compress to 25% at a speed of 0.5 mm/s and the results were expressed in Newtons.
Chemical parameters
All the reagents and chemicals used were of AR grade obtained from M/s Qualigens Fine Chemicals, India. Standard palmitic acid and putrescine, cadaverine, and spermidine were obtained from Sigma Chemical Co., USA.
Metmyoglobin (MMb)
MMb was estimated as per the method described by Trout (1989) by homogenising 10 g minced mutton in 100 ml of cold phosphate buffer of pH 6.8 and measuring OD at 525, 572 and 700 nm, using the following formula,
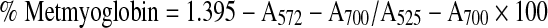 |
and expressed as g/100 g of total pigment.
Free Fatty Acids (FFA)
Total lipids from the samples were extracted using chloroform and methanol mixture according to the method of Folch et al. (1957). After removal of excess solvent, the fat was dried, weighed and analysed for FFA by colorimetric procedure (Lowry and Tinsley 1976). The amount of FFA was calculated as palmitic acid equivalent from the calibration curve using different aliquots of standard palmitic acid and expressed as% on lipid basis.
Phospholipids (PL)
Total lipid P from the lipid extract of samples was analysed colorimetrically using 4–amino-3-naphthol-sulfonic acid (ANSA) reagent (Fiske and Subba Row 1925). The amount of lipid P present was determined with the help of a calibration graph using standard potassium phosphate solution (80–400 μg). Phospholipid (PL) content was determined from lipid P levels 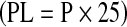 (Leseigneur-Meynier and Grandemer 1991) and expressed in terms of% on lipid basis.
(Leseigneur-Meynier and Grandemer 1991) and expressed in terms of% on lipid basis.
Biogenic amines
Extraction of amines from sample and their derivatization was done as reported by Rosier and Petegham (1988) with a slight modification. Twenty five g of sample was homogenised with 50 ml of 5% hot (80–90 °C) trichloroacetic acid (TCA) solution for 2 min in a homogeniser. The homogenate was centrifuged at 5000 rpm for 10 min. The supernatant was filtered and 5 ml filtrate was dansylated using 2 ml dansyl chloride (10 mg/2 ml acetone), 1 ml buffer (pH 9) and 5 drops of 4 N NaOH solution. After 1 h at 55 °C for the completion of dansylation, the sample was centrifuged at 20,000 rpm for 3 min and supernatant used for HPLC analysis. Similarly, a stock standard dansyl amine of putrescine, cadaverine and spermidine was prepared using 17–20 mg of standard amine in 10 ml of 5% TCA. From this, working standard was prepared by diluting the stock 10 folds and 1 ml of working standard was subjected to dansylation as above. The dansylated solution (25 ml) was analysed on HPLC (Waters Model 1525, India) using C18 micro bondapack column using a gradient elution of methanol-water, starting with 70 ml methanol and 30 ml water and ending with 100 ml methanol over 15 min. UV detector at 254 nm was used for detection. Peak identity was established with the help of standards and peak area was measured using a microprocessor-based integrator. Various amine levels in mutton were quantified as mg/100 g mutton on fresh weight basis.
Biogenic Amine Index (BAI)
An index based on the sum of putrescine, cadaverine and spermidine, the major biogenic amines found in mutton was drawn for the acceptance of mutton as per Hernandez-Jover et al. (1996).
Statistical analysis
All the results presented are the means of 6 batches of experiments in which duplicate samples were taken for each parameter from each batch. Data obtained were subjected to 1 way analysis of variance and the levels were differentiated using Duncan’s multiple range test (Steel and Torrie 1980). Significance was ascertained at p < 0.01.
Results and discussion
Samples with acidulant and clove extract treatment (T) had the highest shelf life of 4 days compared to 1 day for control (C) samples (Table 1). C samples were analysed only up to 2 days, as they spoiled completely by that time. For all the analyses, the data obtained for 1 day stored control samples were taken as cut off value for acceptance (since sensorially they were not acceptable) and compared with T samples. Higher scores for colour, odour and texture were obtained for T samples till the end of 4 days, as compared with 1 day stored C samples. But at the end of 5th day, slight off-odour was perceptible and the texture was slightly ‘dried like’, because of surface dryness, though the colour score was still acceptable (Table 1).
Table 1.
Changes in sensory and microbiological quality of mutton treated with clove extract and stored at 25 ± 2 °C
| Storage period, days | |||||||
|---|---|---|---|---|---|---|---|
| Sensory scores (n = 7 panelists) | 0 | 1 | 2 | 3 | 4 | 5 | |
| Colour | C | 8.0 ± 0.09x,a | 3.3 ± 0.19x,b | 1.2 ± 0.05x,c | ND | ND | ND |
| T | 7.9 ± 0.07x,a | 6.7 ± 0.13y,a | 6.2 ± 0.11y,ab | 6.2 ± 0.09ab | 6.1 ± 0.07ab | 6.0 ± 0.06ab | |
| Odour | C | 8.0 ± 0.11x,a | 2.1 ± 0.12x,b | 1.1 ± 0.07x,c | ND | ND | ND |
| T | 8.0 ± 0.14x,a | 7.2 ± 0.09y,a | 6.1 ± 0.14y,ab | 6.1 ± 0.18ab | 6.0 ± 0.04ab | 4.0 ± 0.05b | |
| Texture | C | 7.8 ± 0.11x,a | 4.3 ± 0.19x,b | 1.3 ± 0.08x,c | ND | ND | ND |
| T | 7.9 ± 0.17x,a | 7.8 ± 0.12y,a | 6.6 ± 0.13y,a | 5.9 ± 0.09ab | 5.4 ± 0.11ab | 3.1 ± 0.08b | |
| Microbiological, counts (log cfu/g) | |||||||
| SPC | C | 5.3 ± 0.13x,a | 7.9 ± 0.61x,b | 9.1 ± 0.16x,c | ND | ND | ND |
| T | 4.6 ± 0.34x,a | 5.1 ± 0.16y,a | 5.4 ± 0.26y,a | 5.6 ± 0.19a | 6.0 ± 0.16ab | 7.7 ± 0.19b | |
| Yeast and molds | C | 2.1 ± 0.43x,a | 4.6 ± 0.21x,b | 7.9 ± 0.72x,c | ND | ND | ND |
| T | 1.9 ± 0.19x | Absent | Absent | Absent | Absent | Absent | |
| Entero-bacteriaceae | C | 2.9 ± 0.43x,a | 7.1 ± 0.09x,b | 8.6 ± 0.07x,c | ND | ND | ND |
| T | 2.8 ± 0.03x,a | 3.8 ± 0.06y,a | 3.9 ± 0.09y,a | 4.3 ± 0.09a | 5.2 ± 0.17ab | 6.8 ± 0.13b | |
| E.coli | C | 2.7 ± 0.27x,a | 4.6 ± 0.44x,b | 6.7 ± 0.42x,c | ND | ND | ND |
| T | 2.4 ± 0.05x | Absent | Absent | Absent | Absent | Absent | |
| S.aureus | C | 2.1 ± 0.15x,a | 5.8 ± 0.54x,b | 6.5 ± 0.14x,c | ND | ND | ND |
| T | 1.9 ± 0.41x | Absent | Absent | Absent | Absent | Absent | |
C Control; T Treated
The means having different superscripts (a,b,c,) in a row are significantly different (p < 0.01). The means having different superscripts (x, y) in a column pertaining to specific group/parameter differs significantly (p < 0.01) n = 12 ND= Not done
Lower (p < 0.01) counts in SPC and Enterobacteriaceae were observed in T samples till 4 days as compared 1 day for C samples (Table 1). In T samples, Y and M, E.coli and S. aureus were absent till the end of 5th day. The SPC levels were well within the acceptable limits till 4th day (Sahoo and Kumar 2005). By 5th day, the SPC levels reached to 7 log cfu/g, which correlated with slight off-odour development. In case of C samples, there was a steady increase in all the counts and the sample was completely spoiled (putrid) by 2nd day. The inhibitory effect of clove extract E. coli, S. aureus, B. cereus and spoilage organisms has been well established (Karur Sofia et al. 2007, Baohua Kong et al. 2007) and on certain fungi (Omidbeygi et al. 2007; Tullio et al. 2007; Guo and Cheng 2006; Viuda Martos et al. 2007). Present investigation revealed the inhibitory effect of clove extract on meat spoilage organisms, the effect being more profound because of the low surface pH of mutton caused by acidulant treatment (Sara Burt 2004). Essential oils are capable of inducing bacterial cell lysis, damaging both cell wall and membrane (Rhayour et al. 2003). The hydrophobicity of essential oils enables them to partition in the lipids of the cell membrane and mitochondria, rendering them permeable and leading to leakage of cell contents causing cell death (Sara Burt 2004).
Lower (p < 0.01) losses in the WHC was noticed in treated samples during storage up to 4 days, whereas in control samples around 16 and 28% loss was noticed during first and second day of storage, respectively (Table 2). The minimal loss in WHC observed in the treated mutton is due to lower pH and lesser extent of microbial spoilage (Verma and Sahoo 2000). Similar correlation was reported during storage of fresh mutton treated with green tea extract (Kumudavally et al. 2008).
Table 2.
Changes in physico-chemical quality of mutton treated with clove extract and stored at 25 ± 2 °C
| Storage period, days | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| Water holding capacity,% | C | 69.9 ± 1.12x,a | 62.3 ± 1.84x,b | 52.4 ± 1.01x,c | ND | ND | ND |
| T | 70.1 ± 1.43x,a | 68.1 ± 2.14y,a | 67.0 ± 2.10y,a | 66.4 ± 1.81a | 65.0 ± 2.92ab | 63.2 ± 2.10b | |
| Hardness, N | C | 810 ± 3.14x,a | 642 ± 2.14x,b | 412 ± 3.21x,c | ND | ND | ND |
| T | 805 ± 3.48x,a | 800 ± 2.24y,a | 792 ± 3.21y,a | 785 ± 2.45a | 772 ± 3.14ab | 765 ± 3.21b | |
| Metmyo-globin,% | C | 42.3 ± 1.12x,a | 59.2 ± 2.14x,b | 63.5 ± 3.51x,c | ND | ND | ND |
| T | 42.2 ± 1.23x,a | 42.3 ± 2.02y,a | 43.0 ± 1.24y,a | 44.2 ± 1.43a | 45.2 ± 1.21ab | 50.2 ± 1.25b | |
| Free fatty acid, g/100 g lipid | C | 1.2 ± 0.18x,a | 2.4 ± 0.34x,b | 4.1 ± 0.11x,c | ND | ND | ND |
| T | 1.26 ± 0.12x,a | 1.3 ± 0.15y,a | 1.36 ± 0.11y,a | 1.44 ± 0.10a | 1.52 ± 0.20ab | 2.31 ± 0.26b | |
| Phospholipids,% lipid | C | 7.8 ± 0.11x,a | 5.5 ± 0.13x,b | 3.8 ± 0.09x,c | ND | ND | ND |
| T | 7.8 ± 0.09x,a | 7.2 ± 0.13y,a | 6.8 ± 0.21y,ab | 6.7 ± 0.19ab | 6.6 ± 0.17ab | 5.9 ± 0.11b | |
| Cadaverine, mg/100 g | C | 0.00 | 0.51 ± 0.09 | 0.76 ± 0.16 | ND | ND | ND |
| T | Absent | Absent | Absent | Absent | Absent | 0.19 ± 0.06 | |
| Biogenic amine Index, mg/100 g | C | 0.21 ± 0.04x,a | 3.6 ± 0.41x,b | 5.6 ± 0.21x,c | ND | ND | ND |
| T | 0.2 ± 0.06x,a | 0.22 ± 0.03y,a | 0.24 ± 0.04y,a | 0.27 ± 0.06a | 0.31 ± 0.06a | 0.98 ± 0.09b | |
C Control; T Treated
The means having different superscripts (a, b, c, ..) in a row are significantly different (p < 0.01). The means having different superscripts (x, y) in a column differ significantly (p < 0.01). ND-Not done
The T samples exhibited least changes in hardness at the end of 4 days of storage whereas in C, more than 50% textural loss was noticed at the end of 2 days (Table 2). Pseudomonas species responsible for spoilage of mutton stored aerobically at 25 °C are known to be proteolytic and excessive proteolysis could produce abnormal softness and unacceptable mashiness (Stanbridge and Davies 1998; Tabilo et al. 1999) as observed in C samples. The firm texture noticed in the treated samples during storage up to 4 days correlated (r > 0.97) with the sensory and microbiological (SPC) characteristics, indicating that the clove extract inhibited growth and proliferation of proteolytic flora to a great extent. The metmyoglobin levels recorded a significant (p < 0.01) increase during storage up to 2 days in C samples (Table 2) whereas in T, the levels were almost the same as that of fresh mutton up to 4 days. Armstrong (1993) reported that signs of brownish discolouration were observed when at least 60% of unstable myoglobin pigment in a particular area becomes oxidised, as observed in the present study in C at the end of 2 days. A positive correlation (r > 0.95) was noticed between metmyoglobin levels and SPC in both C and T samples as also observed by Verma and Sahoo (2000) during storage of minced chevon at 4 °C. Aerobic bacteria such as pseudomonas associated with spoilage of meat stored aerobically at 25 °C, cause discoloration due to metmyoglobin formation by reducing oxygen tension (Stanbridge and Davies 1998). The lower extent of discolouration in treated samples during storage is due to the inhibitory effect of clove extract on this aerobic bacteria.
FFA increased steadily as PL levels decreased and a negative linear correlation (r > 0.98) was noticed between FFA and PL in C (Table 2). Alasnier et al. (2000) studied lipolysis in muscle during refrigerated storage and reported that FFA formation is due to the breakdown of triglycerides and PL. Patil et al. (2007) observed decrease in the levels of PL during frozen storage of chevon. In the present study, PL content negatively correlated (r > 0.94) with SPC levels indicating that it decreased steadily as bacterial load increased. Both PL and FFA levels have been reported as good indicators of bacterial quality of fresh mutton (Vasundhara et al. 1983; Vasundhara and Kumudavally 1989). Psychrotropic bacteria, mainly Pseudomonas species are reported to produce lipase and phospholipase causing an increase in FFA (Koka and Weimer 2001; Chung Wang et al. 1997). In treated samples, both FFA and PL levels did not register significant (p > 0.05) change during storage up to 4 days as compared to 0 day treated samples, indicating the inhibitory action of clove oil on the growth of Pseudomonas.
Biogenic amine levels are positively related with storage time (Min et al. 2007) and are monitored as a measure of proteolytic activity. Cadaverine has been reported as a reliable biomarker for threshold level of spoilage (Kumudavally et al. 2001). In the present investigation, cadaverine was absent in fresh mutton and in T up to 4 days of storage (Table 2). It appeared in 1 day stored C and 5 days stored T, indicating that the treated samples are not spoiled up to 4 days. Cadaverine concentrations increase only when high numbers of presumptive Enterobacteriaceae are present (Edwards et al. 1983) and clove exhibited a strong inhibitory action on Enterobacteriaceae and thus on cadaverine formation (Kumudavally et al. 2005). The BAI also registered a steep increase in control samples up to 2 days of storage. In T, the BAI was significantly lower (p < 0.001) than 1 day stored C till 4 days and a significant increase (p < 0.01) was noticed only on 5th day of storage (Table 2). The lower level of BAI noticed in T is due to the inhibitory effect of ethanolic extract of clove on decarboxylases which are responsible for biogenic amine formation (Chitra and Morihiko 1995). Hernandez-Jover et al. (1996) reported a BAI value of <5 mg/kg for fresh meat. In the present study also, BAI levels were of <5 mg/kg in fresh C and in 4 days stored T mutton indicating that T samples are acceptable up to 4 days. Cadaverine and BAI levels in C and T correlated positively (r > 0.97) with SPC and odour scores.
Conclusion
Ethanolic extract of clove exerted a strong inhibitory effect on meat spoilage organisms and pathogenic organisms, which in turn is reflected in the levels of WHC, hardness, metmyoglobin, cadaverine, FFA and PL. The clove extract was effective in preserving fresh mutton at 25 ± 2 °C up to 4 days, without any adverse effect on the sensory qualities.
References
- Alasnier C, David Briand E, Gandemer G. Lipolysis in muscle during refrigerated storage as related to the metabolic type of the fibres in the rabbit. Meat Sci. 2000;54:127–134. doi: 10.1016/S0309-1740(99)00075-3. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Marshall RT, Dickson JS. Efficacies of acetic, lactic and two mixed acids in reducing numbers of bacteria on surfaces of lean meat. J Food Saf. 1992;12:139–147. doi: 10.1111/j.1745-4565.1991.tb00072.x. [DOI] [Google Scholar]
- Armstrong H. Extending shelf-life with vitamin E. Pigsmisset. 1993;9:18–19. [Google Scholar]
- Aureli P, Costintini A, Zolea S. Antimicrobial activity of some plant essential oils against Listeria monocytogenes. J Food Prot. 1992;55:344–348. doi: 10.4315/0362-028X-55.5.344. [DOI] [PubMed] [Google Scholar]
- Bahk J, Yousef AE, Marth EH. Behaviour of Listeria monocytogenes in the presence of selected spices. Lebensm Wiss Technol. 1990;22:66–69. [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods – A review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Chitra N, Morihiko S. Inhibition of amino acid decarboxylase activity of Enterobacter aerogenes by active components in spices. J Food Prot. 1995;58:280–283. doi: 10.4315/0362-028X-58.3.280. [DOI] [PubMed] [Google Scholar]
- Chung Wang YJ, Bailey ME, Marshall RT (1997) Reduced oxidation of fresh pork in the presence of exogenous hydrolases and bacteria at 20 °C. J Appl Microbiol 82:317-324 [DOI] [PubMed]
- Edwards RA, Dainty RH, Hibbard CM. The relationship of bacterial numbers and types to diamine concentration in fresh and aerobically stored beef, pork and lamb. J Food Technol. 1983;18:777–788. doi: 10.1111/j.1365-2621.1983.tb00316.x. [DOI] [Google Scholar]
- Fiske N, Subba Row Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–379. [Google Scholar]
- Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Guo JZ, Cheng SR. The inhibition of ten kinds of Chinese herbal medicines against fungus causing deterioration of fruits and vegetables and analysis of available compositions. Food Sci Technol. 2006;4:68–71. [Google Scholar]
- Harrigan WF, McCance ME. Laboratory methods in food and dairy microbiology. London: Academic; 1976. [Google Scholar]
- Hernandez-Jover T, Izquierdo-Pulido M, Vecina-Nogues MT, Vidal-Carou MC. Biogenic amine sources in cooked cured shoulder pork. J Agric Food Chem. 1996;44:3097–3101. doi: 10.1021/jf960250s. [DOI] [Google Scholar]
- Jeyashakila R, Vasundhara TS, Vijaya Rao D. Inhibitory effect of spices on in vitro histamine production and histidine decraboxylase activity of Morganella morganii & on the biogenic amine formation in mackerel stored at 30 °C. Z Lebensm Unter Forchung. 1996;203:71–76. doi: 10.1007/BF01267773. [DOI] [PubMed] [Google Scholar]
- Karur Sofia P, Prasad R, Kumar Vijay V, Kumar Srivastava A. Evaluation of antimicrobial activity of Indian spices against common food borne pathogens. J Food Sci Technol. 2007;42:910–915. doi: 10.1111/j.1365-2621.2006.01308.x. [DOI] [Google Scholar]
- Koka R, Weimer BC. Influence of growth conditions on heat stable phospholipase activity in Pseudomonas. J Dairy Res. 2001;68:109–116. doi: 10.1017/S0022029900004647. [DOI] [PubMed] [Google Scholar]
- Kong B, Wang J, Xiong YL. Antimicrobial activity of several herb and spice extracts in culture medium and in vacuum-packaged pork. J Food Prot. 2007;70:641–647. doi: 10.4315/0362-028x-70.3.641. [DOI] [PubMed] [Google Scholar]
- Kumudavally KV, Shobha A, Vasundhara TS, Radhakrishna K. Chromatographic analysis of cadaverine to detect incipient spoilage in mutton. Meat Sci. 2001;59:411–415. doi: 10.1016/S0309-1740(01)00094-8. [DOI] [PubMed] [Google Scholar]
- Kumudavally KV, Padmini S, Radhakrishna K, Bawa AS. Effect of surface treatment of fresh mutton with spices on lipolytic bacteria, Enterobacteriaceae and their degradation products during storage. J Food Sci Technol. 2005;42:249–253. [Google Scholar]
- Kumudavally KV, Phanindra Kumar HS, Tabassum A, Radhakrishna K, Bawa AS. Green tea – A potential preservative for extending the shelf-life of fresh mutton at ambient temperature (25 ± 2 °C) Food Chem. 2008;107:426–433. doi: 10.1016/j.foodchem.2007.08.045. [DOI] [Google Scholar]
- Leseigneur-Meynier A, Grandemer G. Lipid composition of pork muscle in relation to metabolic type of the fibers. Meat Sci. 1991;29:229–241. doi: 10.1016/0309-1740(91)90052-R. [DOI] [PubMed] [Google Scholar]
- Lis-Balchin M, Deans SG. Bioactivity of selected plant essential oils against Listeria monocytogenes. J Appl Microbiol. 1997;82:759–762. doi: 10.1046/j.1365-2672.1997.00153.x. [DOI] [PubMed] [Google Scholar]
- Lowry RR, Tinsley JJ. Rapid colorimetric determination of free fatty acids. J Am Oil Chem Soc. 1976;53:470–472. doi: 10.1007/BF02636814. [DOI] [PubMed] [Google Scholar]
- Min JS, Lee SO, Jang A, Jo C, Park CS, Lee M. Relationship between the concentration of biogenic amines and volatile basic nitrogen in fresh beef, pork, and chicken meat. Asian Australian J Anim Sci. 2007;20:1278–1284. [Google Scholar]
- Moreira MR, Pouce AG, Valle CE, Roura SI. Inhibitory parameters of essential oils to reduce a food borne pathogen. Lebensm Wiss Technol. 2005;38:565–570. [Google Scholar]
- Mytle N, Anderson GL, Doyle MP, Smith MA. Antimicrobial activity of clove (Syzgium aromaticum) oil in inhibiting Listeria monocytogenes on chicken frankfurters. Food Control. 2006;17:102–107. doi: 10.1016/j.foodcont.2004.09.008. [DOI] [Google Scholar]
- Omidbeygi M, Barzegar M, Hamidi Z, Naghdibadi H. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control. 2007;18:1518–1523. doi: 10.1016/j.foodcont.2006.12.003. [DOI] [Google Scholar]
- Patil DA, Gunjal BB, Pawar VD, Machewad GM, Surve VD. Effects of chilling, freezing and frozen storage on quality of chevon. J Food Sci Technol. 2007;44:174–177. [Google Scholar]
- Rajkumar V, Berwal JS. Inhibitory effect of clove (Eugenia caryophyllus) on toxigenic molds. J Food Sci Technol. 2003;40:416–418. [Google Scholar]
- Rhayour K, Buchikhi T, Tantaoui EA, Sendide K, Rammal A. The mechanism of bactericidal action of oregago and clove essential oils and their phenolic major components on Escherichia coli and Bacillus subtilis. J Essential Oil Res. 2003;15:356–362. [Google Scholar]
- Rosier J, Petegham CV. A screening method for the simultaneous determination of putrescine, cadaverine, histamine, spermidine and spermine in fish by means of high pressure liquid chromatography of their 5-diamine naphthalene 1-sulphonyl derivatives. Z Lebens Unters Forchung. 1988;86:25–28. doi: 10.1007/BF01027175. [DOI] [PubMed] [Google Scholar]
- Sahoo J, Kumar N. Quality of vacuum packaged muscle foods stored under frozen conditions: A review. J Food Sci Technol. 2005;42:209–213. [Google Scholar]
- Smith-Palmer A, Stewart J, Fyfe L. Antimicrobial properties of plant essential oils and essences against five important foodborne pathogens. Lett Appl Microbiol. 1998;26:118–122. doi: 10.1046/j.1472-765X.1998.00303.x. [DOI] [PubMed] [Google Scholar]
- Sreenivasan P, Manickkam J, Savarimuthu I. In vitro antibacterial activity of some plant essential oils. BMC Comple Alternat Med. 2006;6:39–47. doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srihari KA, Vijaya Rao D. Relative efficacy of 4-methyl umbelliferyl D-glucuronide (MUG) growth media for the detection of Escherichia coli in processed foods. J Food Sci Technol. 1998;35:314–319. [Google Scholar]
- Stanbridge LH, Davies AR. The microbiology of chill stored meat. In: Board RG, Davies AR, editors. The microbiology of meat and poultry. London: Blackie Academic and Professional; 1998. pp. 174–219. [Google Scholar]
- Steel RGD, Torrie JH. Principle and procedure of statistics: A biometrical approach. 2. New York: Mc Graw-Hill; 1980. [Google Scholar]
- Subbulakshmi G, Naik M. Nutritive value and technology of spices: current status and future perspectives. J Food Sci Technol. 2002;39:319–344. [Google Scholar]
- Tabilo G, Flores M, Fiszman SM, Toldra F. Post mortem meat quality and sex affect textural properties and protein breakdown of dry cured ham. Meat Sci. 1999;51:255–260. doi: 10.1016/S0309-1740(98)00125-9. [DOI] [PubMed] [Google Scholar]
- Trout GR. Variations in myoglobin denaturation and colour of cooked beef, pork and turkey meat as influenced by pH, sodium chloride, sodium tripolyphosphate and cooking temperature. J Food Sci. 1989;54:536–540. doi: 10.1111/j.1365-2621.1989.tb04644.x. [DOI] [Google Scholar]
- Tullio V, Nostro A, Mandras N, Dugo P, Banche G, Cannatelli MA, Cuffini AM, Alonzo V, Carll NA. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact meyhods. J Appl Microbiol. 2007;102:1544–1550. doi: 10.1111/j.1365-2672.2006.03191.x. [DOI] [PubMed] [Google Scholar]
- Vasundhara TS, Kumudavally KV. Proteolytic and lipolytic degradation products as indicators for quality assessment of canned mutton curry. J Food Sci Technol. 1989;26:314–317. [Google Scholar]
- Vasundhara TS, Kumudavally KV, Sharma TR. Altered free fatty acid levels in fresh or canned mutton as indicators of spoilage. J Food Prot. 1983;46:1050–1054. doi: 10.4315/0362-028X-46.12.1050. [DOI] [PubMed] [Google Scholar]
- Verma SP, Sahoo J. Extension of shelf-life of ground chevon during refrigerated storage by using ascorbic acid. J Food Sci Technol. 2000;37:565–570. [Google Scholar]
- Viuda Martos M, Ruiz Navajas Y, Fernandez Lopez J, Perez Alvarez JA. Antifungal activities of thyme, clove and oregano essential oils. J Food Safety. 2007;27:91–101. doi: 10.1111/j.1745-4565.2007.00063.x. [DOI] [Google Scholar]
- Wardlaw FR, Mc Caskill LH, Acton JC. Effects of post-mortem changes on poultry meat loaf properties. J Food Sci. 1973;38:421–423. doi: 10.1111/j.1365-2621.1973.tb01444.x. [DOI] [Google Scholar]


