Abstract
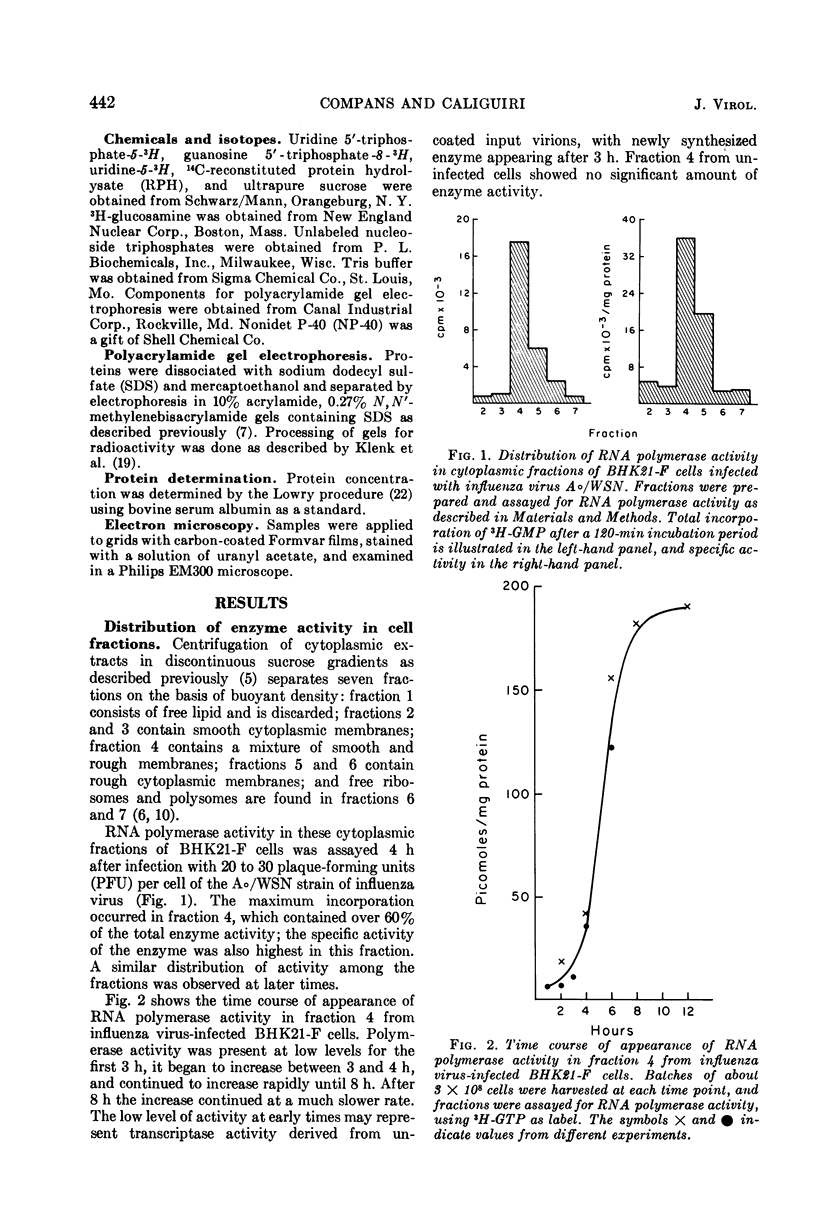
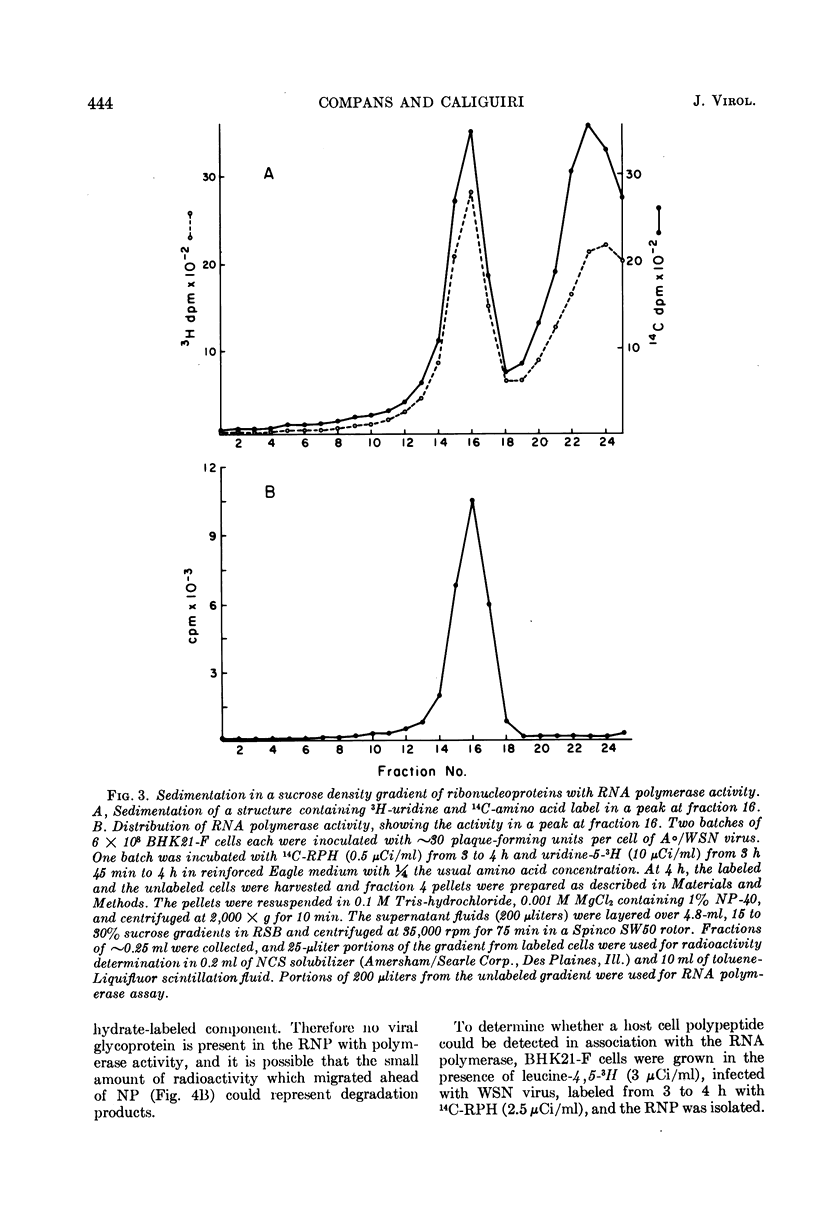
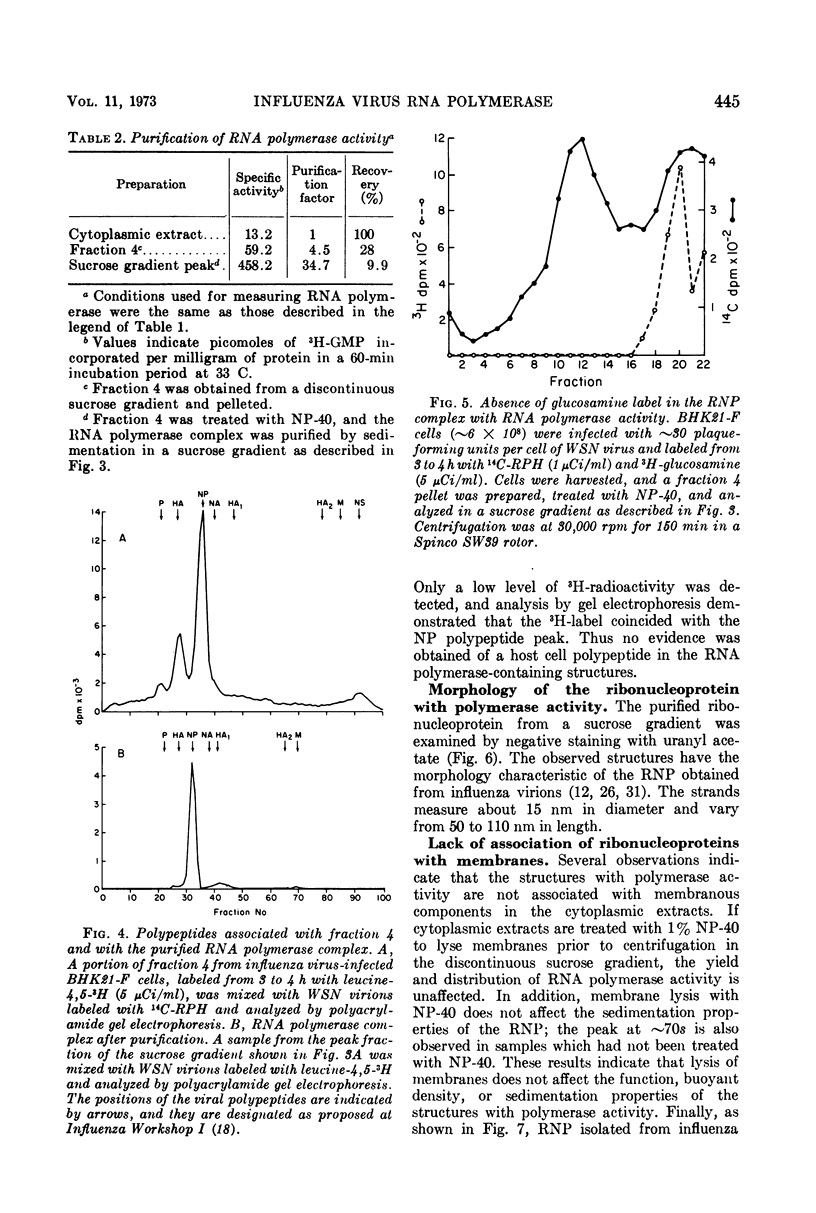
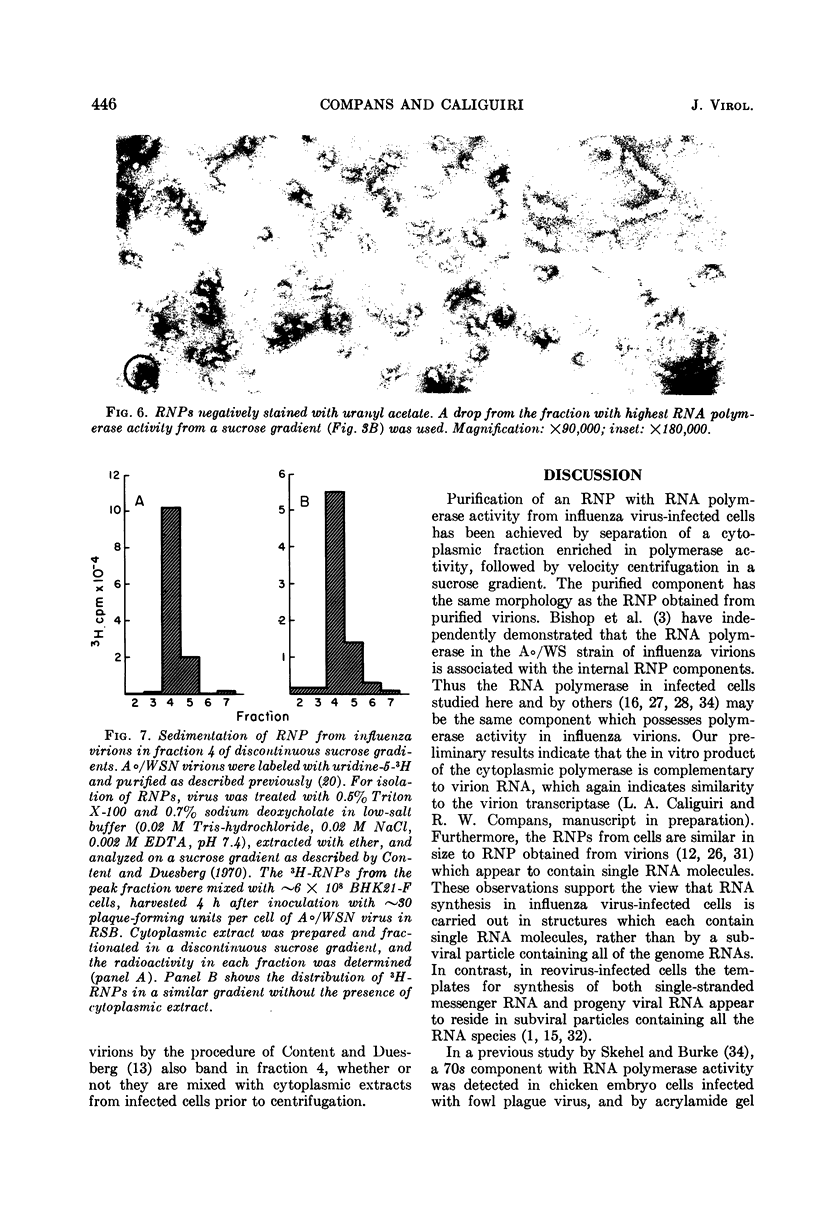
Structures with RNA polymerase activity were isolated from influenza virus-infected cells, and consisted of ribonucleoprotein (RNP) complexes, similar in morphology to the viral internal component or nucleocapsid. The isolation procedure involved fractionation of infected cells in a discontinuous sucrose gradient, in which enzyme activity was concentrated in a fraction of intermediate density which contains both smooth and rough cytoplasmic membranes. The RNPs with polymerase activity were further purified in a velocity gradient, after which the peak fractions showed a 35-fold purification of the polymerase activity when compared with cytoplasmic extracts. The NP polypeptide, which is the subunit of the virion RNP, was the only virus-specific polypeptide detected in these RNP structures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acs G., Klett H., Schonberg M., Christman J., Levin D. H., Silverstein S. C. Mechanism of reovirus double-stranded ribonucleic acid synthesis in vivo and in vitro. J Virol. 1971 Nov;8(5):684–689. doi: 10.1128/jvi.8.5.684-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. I. Optimal conditions for in vitro activity of the ribonucleic acid-dependent ribonucleic acid polymerase. J Virol. 1971 Jul;8(1):66–73. doi: 10.1128/jvi.8.1.66-73.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Roy P., Bean W. J., Jr, Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. 3. Completeness of the transcription process. J Virol. 1972 Oct;10(4):689–697. doi: 10.1128/jvi.10.4.689-697.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliguiri L. A., Klenk H. D., Choppin P. W. The proteins of the parainfluenza virus SV5. 1. Separation of virion polypeptides by polyacrylamide gel electrophoresis. Virology. 1969 Nov;39(3):460–466. doi: 10.1016/0042-6822(69)90094-4. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Mosser A. G. Proteins associated with the poliovirus RNA replication complex. Virology. 1971 Nov;46(2):375–386. doi: 10.1016/0042-6822(71)90039-0. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Membranous structures associated with translation and transcription of poliovirus RNA. Science. 1969 Nov 14;166(3907):885–886. doi: 10.1126/science.166.3907.885. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. The role of cytoplasmic membranes in poliovirus biosynthesis. Virology. 1970 Sep;42(1):100–111. doi: 10.1016/0042-6822(70)90242-4. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Chow N. L., Simpson R. W. RNA-dependent RNA polymerase activity associated with virions and subviral particles of myxoviruses. Proc Natl Acad Sci U S A. 1971 Apr;68(4):752–756. doi: 10.1073/pnas.68.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Content J., Duesberg P. H. Structure of the ribonucleoprotein of influenza virus. J Virol. 1972 Oct;10(4):795–800. doi: 10.1128/jvi.10.4.795-800.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W. Influenza virus proteins. II. Association with components of the cytoplasm. Virology. 1973 Jan;51(1):56–70. doi: 10.1016/0042-6822(73)90365-6. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Content J., Duesberg P. H. Electrophoretic distribution of the proteins and glycoproteins of influenza virus and Sendai virus. J Virol. 1970 Dec;6(6):707–716. doi: 10.1128/jvi.6.6.707-716.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E. A., Pons M. W. The effect of polyvinylsulfate on the ribonucleoprotein of influenza virus. Virology. 1970 Jun;41(2):382–384. doi: 10.1016/0042-6822(70)90093-0. [DOI] [PubMed] [Google Scholar]
- Gomatos P. J. Comparison of the virion polymerase of reovirus with the enzyme purified from reovirus-infected cells. J Virol. 1970 Nov;6(5):610–620. doi: 10.1128/jvi.6.5.610-620.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P. P., Walters C. P. Influenza virus-induced ribonucleic acid nucleotidyltransferase and the effect of actinomycin D on its formation. Biochemistry. 1966 Jan;5(1):231–235. doi: 10.1021/bi00865a030. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med. 1966 Sep 1;124(3):501–520. doi: 10.1084/jem.124.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Caliguiri L. A., Choppin P. W. The proteins of the parainfluenza virus SV5. II. The carbohydrate content and glycoproteins of the virion. Virology. 1970 Oct;42(2):473–481. doi: 10.1016/0042-6822(70)90290-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landsberger F. R., Lenard J., Paxton J., Compans R. W. Spin-labeled electron spin resonance study of the lipid-containing membrane of influenza virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2579–2583. doi: 10.1073/pnas.68.10.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Penhoet E., Miller H., Doyle M., Blatti S. RNA-dependent RNA polymerase activity in influenza virions. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1369–1371. doi: 10.1073/pnas.68.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W., Schulze I. T., Hirst G. K., Hauser R. Isolation and characterization of the ribonucleoprotein of influenza virus. Virology. 1969 Oct;39(2):250–259. doi: 10.1016/0042-6822(69)90045-2. [DOI] [PubMed] [Google Scholar]
- Pons M. W. Studies on the replication of influenza virus RNA. Virology. 1972 Mar;47(3):823–832. doi: 10.1016/0042-6822(72)90574-0. [DOI] [PubMed] [Google Scholar]
- Ruck B. J., Brammer K. W., Page M. G., Coombes J. D. The detection and characterization of an induced RNA polymerase in the chorioallantoic membranes of embryonated eggs infected with influenza A2 virus. Virology. 1969 Sep;39(1):31–41. doi: 10.1016/0042-6822(69)90345-6. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Becht H., Rott R. Inhibition of influenza RNA polymerase by specific antiserum. Virology. 1971 Jan;43(1):137–143. doi: 10.1016/0042-6822(71)90231-5. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rott R. Ribonucleic acid nucleotidyl transferase induced in chick fibroblasts after infection with Newcastle disease virus. J Gen Virol. 1969 Jun;4(4):565–570. doi: 10.1099/0022-1317-4-4-565. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rott R. Ribonucleic acid nucleotidyl transferase induced in chick fibroblasts after infection with an influenza virus. J Gen Virol. 1969 Jan;4(1):125–137. doi: 10.1099/0022-1317-4-1-125. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Astell C., Levin D. H., Schonberg M., Acs G. The mechanisms of reovirus uncoating and gene activation in vivo. Virology. 1972 Mar;47(3):797–806. doi: 10.1016/0042-6822(72)90571-5. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Burke D. C. Ribonucleic acid synthesis in chick embryo cells infected with fowl-plague virus. J Virol. 1969 Apr;3(4):429–438. doi: 10.1128/jvi.3.4.429-438.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J. RNA-dependent RNA polymerase activity of the influenza virus. Virology. 1971 Sep;45(3):793–796. doi: 10.1016/0042-6822(71)90197-8. [DOI] [PubMed] [Google Scholar]



