Abstract
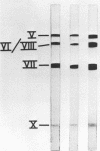
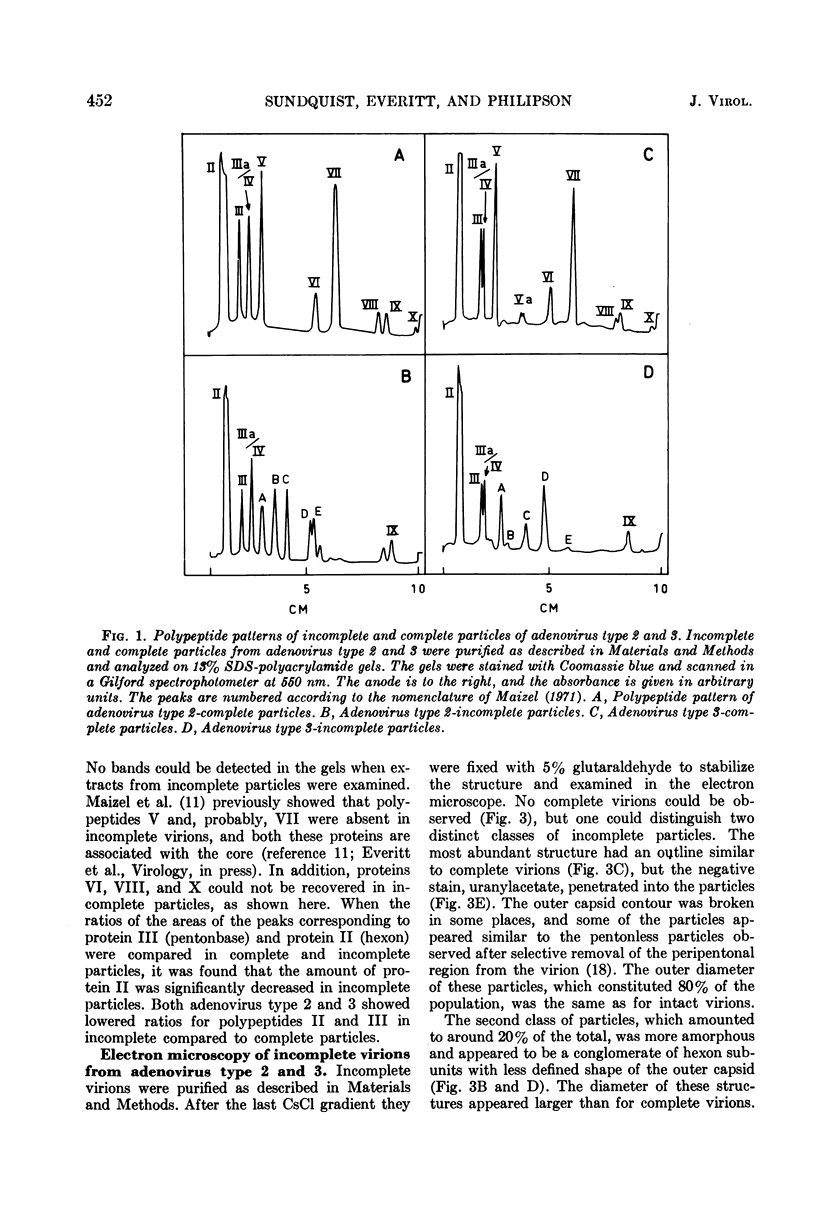
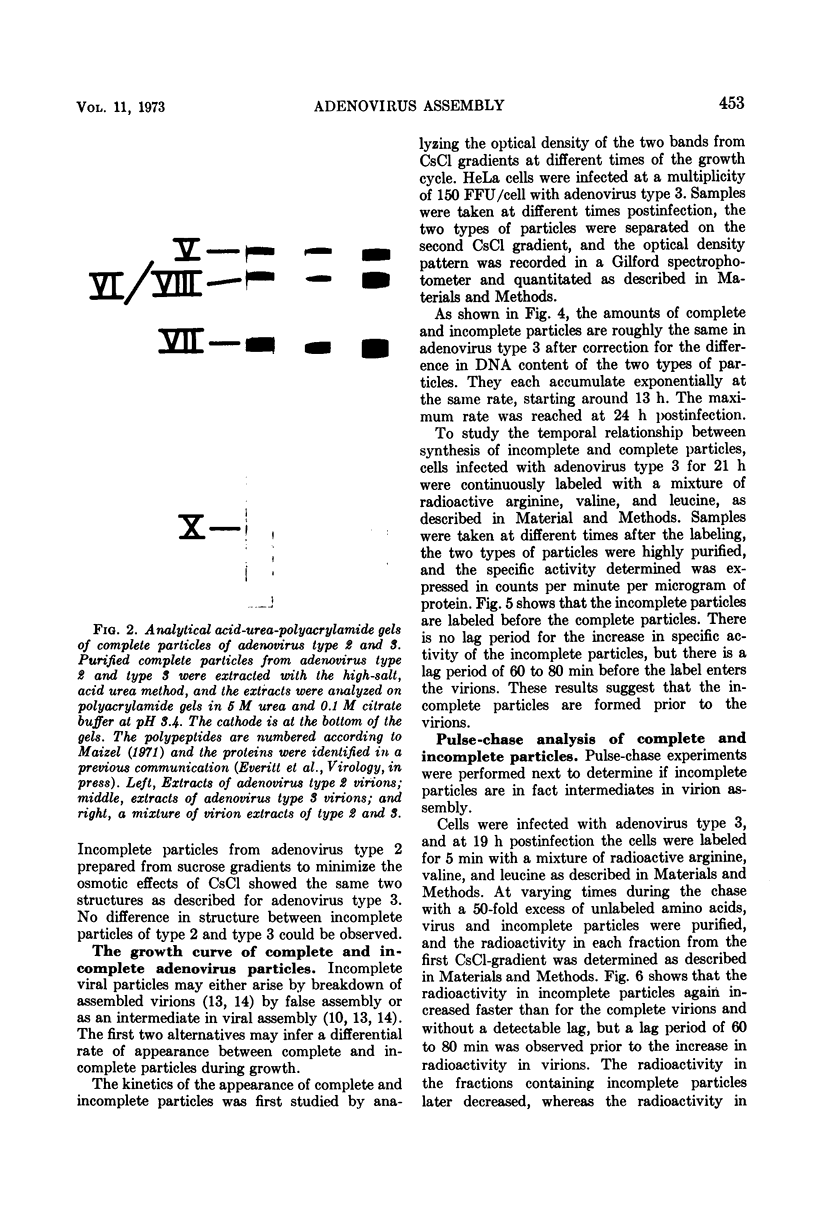
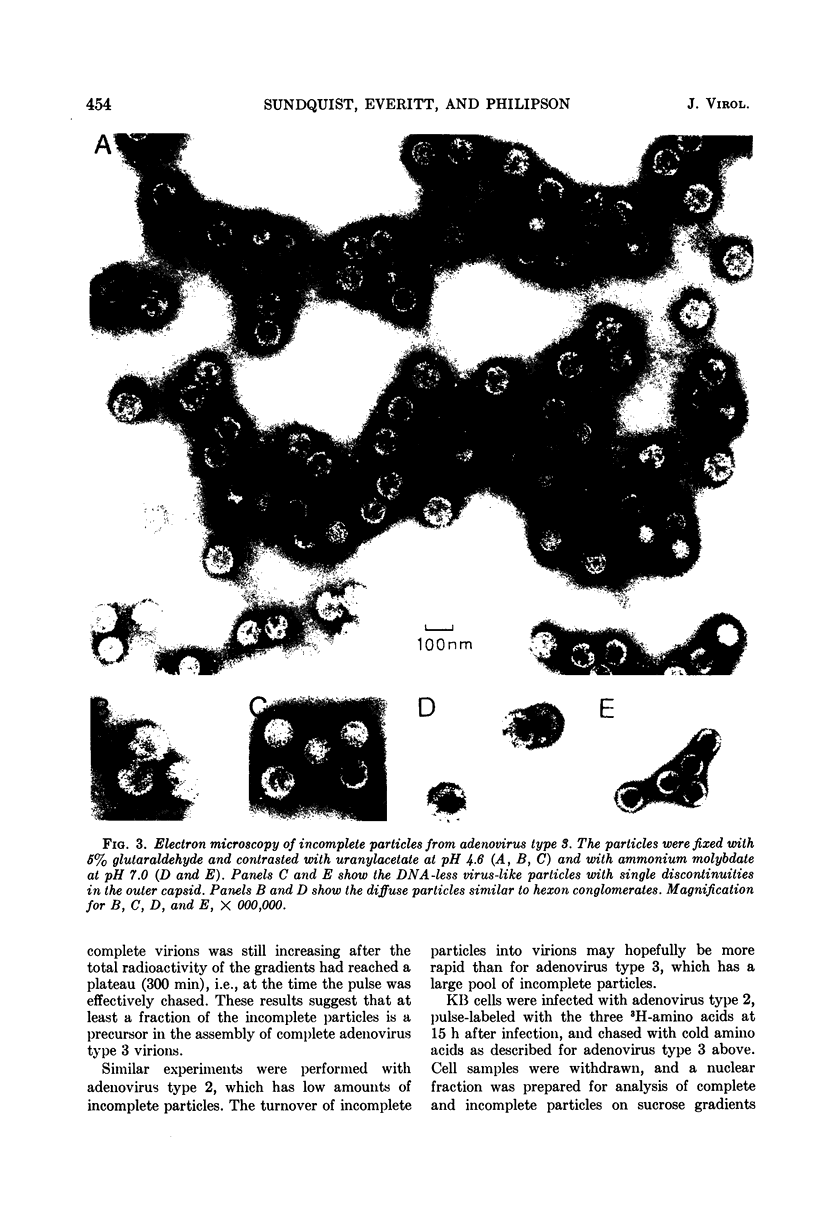
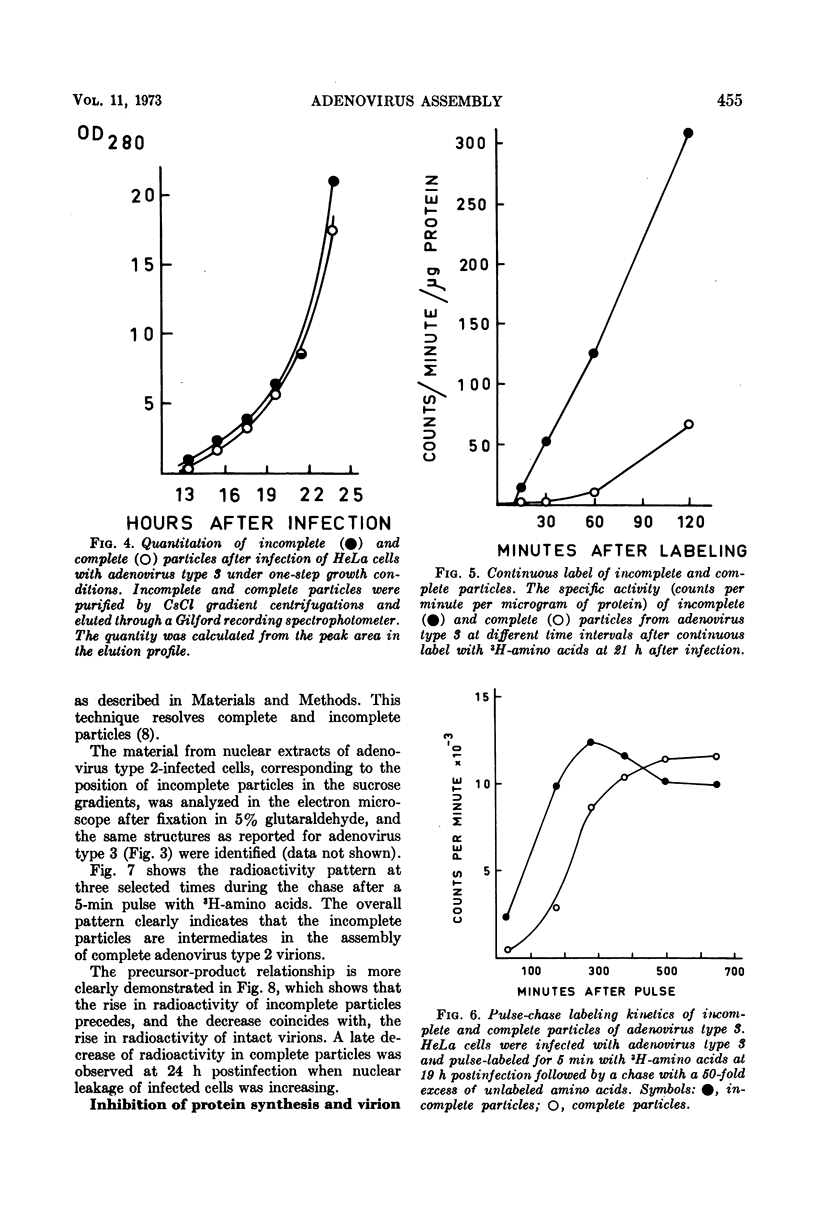
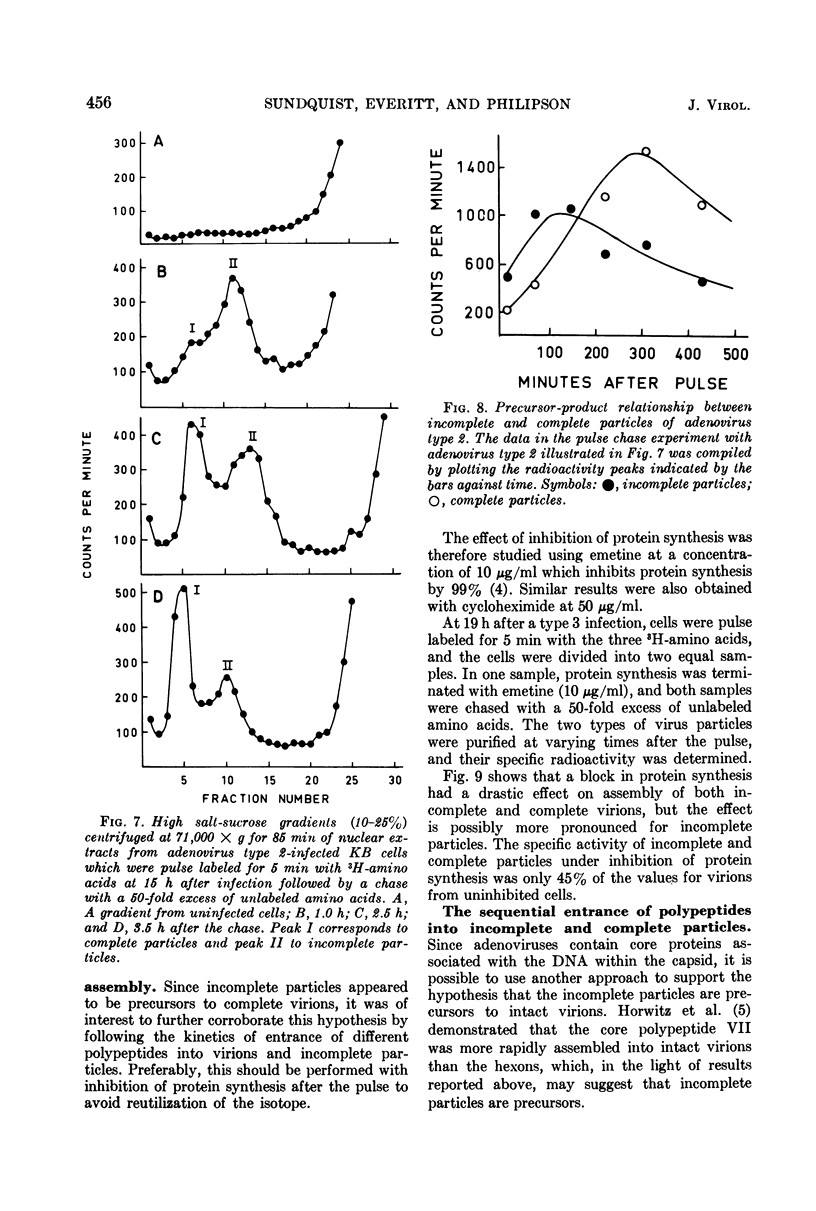
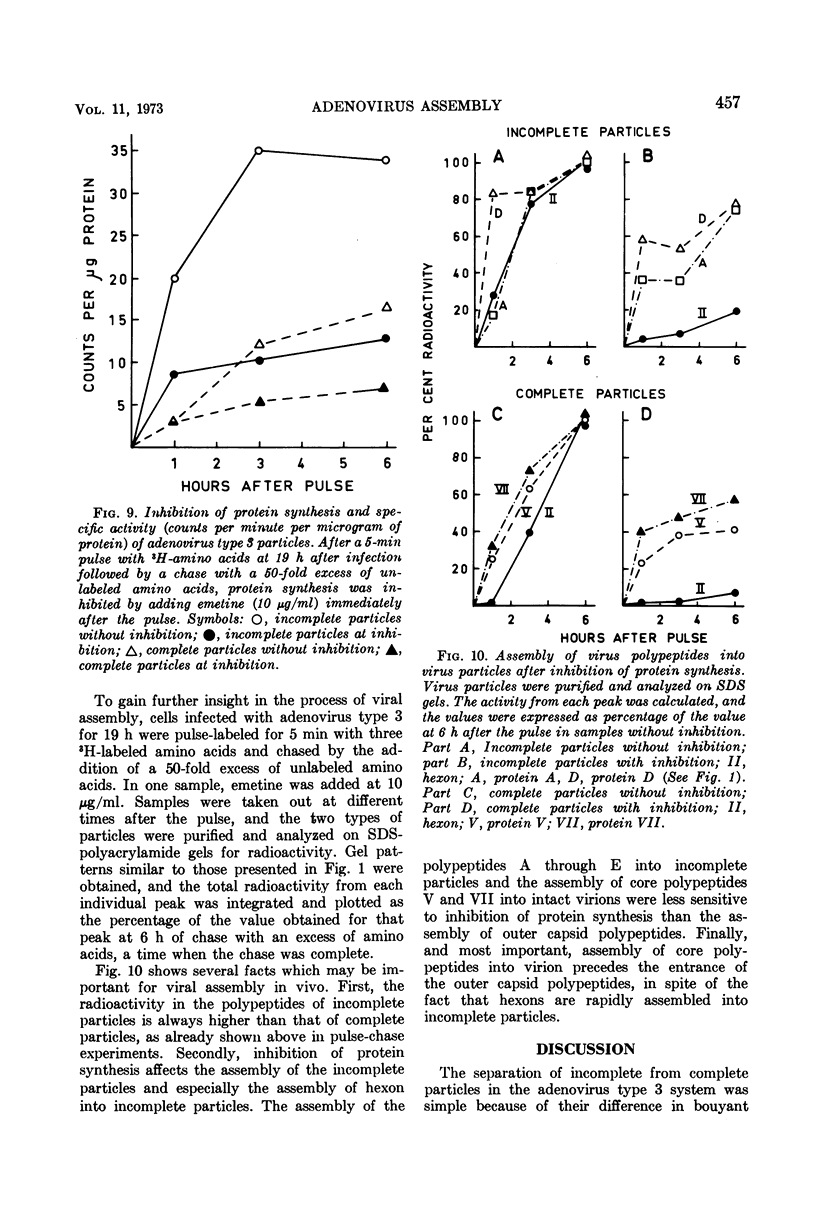
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purified incomplete particles of adenoviruses type 2 and 3 revealed that core proteins V and VII and capsid proteins VI, VIII, and X were absent in these particles, but they contained polypeptides not present in complete particles. Two types of incomplete particles were observed in the electron microscope, appearing as deoxyribonucleic acid-less particles with single discontinuities in the capsid structure (about 80%), or more amorphous particles resembling hexon aggregates (about 20%). The amount of incomplete and complete particles increased in parallel during the infectious cycle. Detectable amounts were found at 13 h with a maximum rate of synthesis for both particles at 24 h after infection. 3H-labeled amino acids were incorporated into incomplete particles without a detectable lag period, but the label appeared in complete particles with a 60- to 80-min lag. Early after the pulse in pulse-chase experiments, the radioactivity was higher for incomplete particles than for complete particles and leveled off before the activity of complete particles reached a maximum. In the adenovirus type 2 system, pulse-chase experiments suggested a precursor-product relationship between incomplete and complete particles. After a short pulse, 19 h postinfection, entrance of 3H-labeled amino acids into the hexon polypeptide of complete particles was delayed for 80 min, but no delay was observed for the labeling of the hexon polypeptide of incomplete particles. The core polypeptides appear in complete particles without a delay, also suggesting that incomplete particles were precursors to complete particles. Incorporation of 3H-labeled amino acids into the hexon polypeptide of complete and incomplete particles was drastically decreased by inhibition of protein synthesis with emetine. However, the uptake of label into core proteins of complete particles was only decreased to 50% on inhibition of protein synthesis. The results suggest that incomplete particles are intermediates in virus assembly in vivo and that the assembly of capsid polypeptides into incomplete and complete particles is dependent on continuing protein synthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S. Isopycnic separation of subcellular components from poliovirus-infected and normal HeLa cells. Science. 1968 Nov 1;162(3853):572–574. doi: 10.1126/science.162.3853.572. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Philipson L. Mechanism of the arginine requirement for adenovirus synthesis. I. Synthesis of structural proteins. J Virol. 1971 Nov;8(5):742–753. doi: 10.1128/jvi.8.5.742-753.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman A. P. Inhibitors of protein biosynthesis. V. Effects of emetine on protein and nucleic acid biosynthesis in HeLa cells. J Biol Chem. 1968 Aug 10;243(15):4089–4094. [PubMed] [Google Scholar]
- Horwitz M. S., Scharff M. D., Maizel J. V., Jr Synthesis and assembly of adenovirus 2. I. Polypeptide synthesis, assembly of capsomeres, and morphogenesis of the virion. Virology. 1969 Dec;39(4):682–694. doi: 10.1016/0042-6822(69)90006-3. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Wood W. B., Okinaka R. Bacteriophage T4 head morphogenesis. On the nature of gene 49-defective heads and their role as intermediates. J Mol Biol. 1971 May 14;57(3):555–573. doi: 10.1016/0022-2836(71)90109-4. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. II. Soluble proteins, cores, top components and the structure of the virion. Virology. 1968 Sep;36(1):126–136. doi: 10.1016/0042-6822(68)90122-0. [DOI] [PubMed] [Google Scholar]
- Okubo C. K., Raskas H. J. Thermosensitive events in the replication of adenovirus type 2 at 42 degrees. Virology. 1971 Nov;46(2):175–182. doi: 10.1016/0042-6822(71)90020-1. [DOI] [PubMed] [Google Scholar]
- Ozer H. L. Synthesis and assembly of simian virus 40. I. Differential synthesis of intact virions and empty shells. J Virol. 1972 Jan;9(1):41–51. doi: 10.1128/jvi.9.1.41-51.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L., Tegtmeyer P. Synthesis and assembly of simian virus 40. II. Synthesis of the major capsid protein and its incorporation into viral particles. J Virol. 1972 Jan;9(1):52–60. doi: 10.1128/jvi.9.1.52-60.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Hirsch M., Penman S. Utilization of messenger in adenovirus-2-infected cells at normal and elevated temperatures. Nat New Biol. 1972 Aug 2;238(83):143–144. doi: 10.1038/newbio238143a0. [DOI] [PubMed] [Google Scholar]
- Pettersson U. Structural proteins of adenoviruses. VI. On the antigenic determinants of the hexon. Virology. 1971 Jan;43(1):123–136. doi: 10.1016/0042-6822(71)90230-3. [DOI] [PubMed] [Google Scholar]
- Prage L., Höglund S., Philipson L. Structural proteins of adenoviruses. 8. Characterization of incomplete particles of adenovirus type 3. Virology. 1972 Sep;49(3):745–757. doi: 10.1016/0042-6822(72)90531-4. [DOI] [PubMed] [Google Scholar]
- Prage L., Pettersson U., Höglund S., Lonberg-Holm K., Philipson L. Structural proteins of adenoviruses. IV. Sequential degradation of the adenovirus type 2 virion. Virology. 1970 Oct;42(2):341–358. doi: 10.1016/0042-6822(70)90278-3. [DOI] [PubMed] [Google Scholar]
- Rekosh D. Gene order of the poliovirus capsid proteins. J Virol. 1972 Mar;9(3):479–487. doi: 10.1128/jvi.9.3.479-487.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Skehel J. J. The polypeptides of adenovirus-infected cells. J Gen Virol. 1972 Apr;15(1):45–57. doi: 10.1099/0022-1317-15-1-45. [DOI] [PubMed] [Google Scholar]
- Winters W. D., Russell W. C. Studies on the assembly of adenovirus in vitro. J Gen Virol. 1971 Feb;10(2):181–194. doi: 10.1099/0022-1317-10-2-181. [DOI] [PubMed] [Google Scholar]