Abstract
During embryonic development, surface ectoderm differentiates to form corneal, conjunctival, and eyelid epidermal epithelia, and glandular epithelium (lacrimal and meibomian glands). Periocular mesenchymal cells of neural crest origin migrate and differentiate, leading to the formation of corneal endothelium and the stromas of the cornea, conjunctiva, eyelids, and trabecular meshwork. The formation of functional ocular surface tissues requires coordinated spatial and temporal expression of transcription factors and signaling molecules of various cytokines and signaling pathways, and the synthesis and remodeling of unique extracellular matrix. Although bidirectional interactions and signaling between mesenchyme and epithelium are considered necessary for embryonic formation of ocular surface tissues and homeostasis in adults, the molecular and cellular mechanisms that regulate such processes remain largely unknown. To investigate possible mechanisms, we have developed mouse models in which the gene functions of ocular surface epithelia and stromas can be altered by Doxycycline induction in spatial and temporal specific manners.
Keywords: Cre-LoxP system, gene targeting, genetically modified mouse lines, ocular surface tissues morphogenesis, Tet-On system, transgenesis
I. Ontogeny of Ocular Surface Tissues
Morphogenesis of ocular surface tissue during embryonic development involves two major cellular events. 1) The differentiation of surface ectoderm gives rise to corneal, conjunctival, and eyelid epidermal epithelia, as well as glandular epithelium (lacrimal and meibomian glands).1 2) The migration and differentiation of periocular mesenchymal cells of neural crest origin lead to the formation of corneal endothelium and the stroma of the cornea, conjunctiva, eyelids, and trabecular meshwork.2,3 It should be noted that the periocular mesenchymal cells also contribute to stromal cells of the iris and scleral fibrocytes (our unpublished observation).
A. Formation of Corneal Endothelium and Stroma of Ocular Surface Tissues
During chicken embryonic development, the corneal epithelium synthesizes components of the extracellular matrix (ECM) for the formation of primary stroma when the lens detaches from the surface ectoderm. This is followed by a two-wave periocular mesenchymal cell migration. Mesenchymal cells of the first wave migrate underneath the primary stroma, undergo mesenchymal/endothelial transformation, and become corneal endothelial cells. The mesenchymal cells of the second wave invade the primary stroma and become keratocytes that are responsible for the formation of secondary stroma of cornea in adult vertebrates.2
Similarly, during murine embryonic development, corneal morphogenesis is also characterized by the invasion of neural crest-derived mesenchymal cells into the primary stroma at embryonic day (E)12.5. However, unlike in avian development, the mesenchymal cells invading the stroma do not immediately form a distinct endothelium at this stage; instead, fibroblastic cells are randomly distributed throughout the stroma. Between E12.5 and E13.5, the endothelium forms via a yet undefined () mechanism(s).4,5
Migrating neural crest cells express Wnt1 between E8.5 and E10.5 during embryonic development. To determine the cell lineages of ocular surface stroma (cornea, conjunctiva, eyelids, and trabecular meshwork), we used bitransgenic Wnt1-Cre/ROSA26mTmG (Wnt1-Cre/mTmG) mice to determine the contribution of Wnt1+ cells in ocular surface tissues of adult mice. The mTmG mouse is a dual reporter mouse line in which mTmG transgene cassette is under the control of a ubiquitous chicken actin promoter that is targeted into the ROSA26 allele of mouse chromosome 6. The reporter gene normally and ubiquitously expresses membrane-bound Tomato red fluorescent protein in most, if not all, somatic cells. In cells expressing Cre recombinase of a transgenic mouse driven by a cell type-specific promoter, the Tomato red transgene is excised and EGFP transgene is activated to label the cells with membrane-bound EGFP.6 For example, in bitransgenic Wnt1-Cre/mTmG mice, the red fluorescent gene is deleted in neural crest cells expressing Wnt1, and this leads to the expression of EGFP in neural crest cells and their progeny. Interestingly, almost all stromal cells found in cornea, conjunctiva, eyelids, and trabecular meshwork of adult Wnt1-Cre/mTmG mice are EGFP+ (our unpublished observations), suggesting that the Wnt1+ neural crest cells are the progenitors of the stromal cells of ocular surface tissues in adults. In contrast, only a portion of corneal endothelial cells show green fluorescence in Wnt1-Cre/ROSAmTmG mice, indicating that two or more populations of periocular mesenchymal cells of neural crest origin contribute to the formation of corneal endothelium in adults (Figure 1).
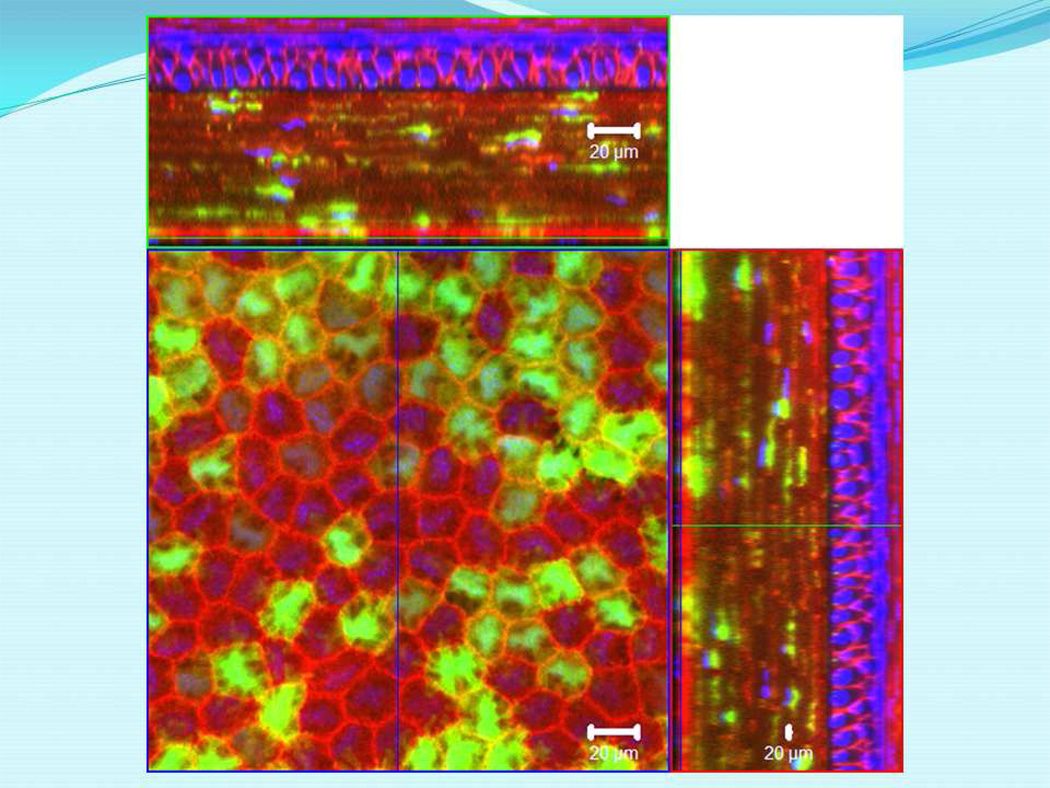
Figure 1.
Expression of EGFP by corneal endothelial cells of a bitransgenic adult Wnt1-Cre/mTmG mouse. Bitransgenic Wnt1-Cre/mTmG mice were obtained by cross breeding Wnt1-Cre transgenic and ROSA26mTmG knock-in mice. The excised cornea was examined by a ZEISS LSM 510 confocal microscope. Endothelial cells derived from Wnt1-Cre-positive cells express EGFP by converting the ROSA26mTmG allele to ROSA26ΔTmG allele. Those red fluorescence-labeled cells are derived from periocular mesenchymal cells that did not express Wnt1-Cre transgene. This observation is consistent with the notion that two or more neural crest cell populations contribute to corneal endothelial cells.
We have used a similar strategy with Kera-Cre/mTmG bitransgenic mice that begin keratocan expression by periocular mesenchymal cells of neural crest origin at E13.5, a day after the formation of corneal endothelium. We have confirmed that possibly three waves of periocular mesenchymal cell migration contribute to the formation of corneal endothelium and ocular surface tissue stromas (keratocytes, eyelid stromal cells, and trabecular fibrocytes) during embryonic development (Figure 2).
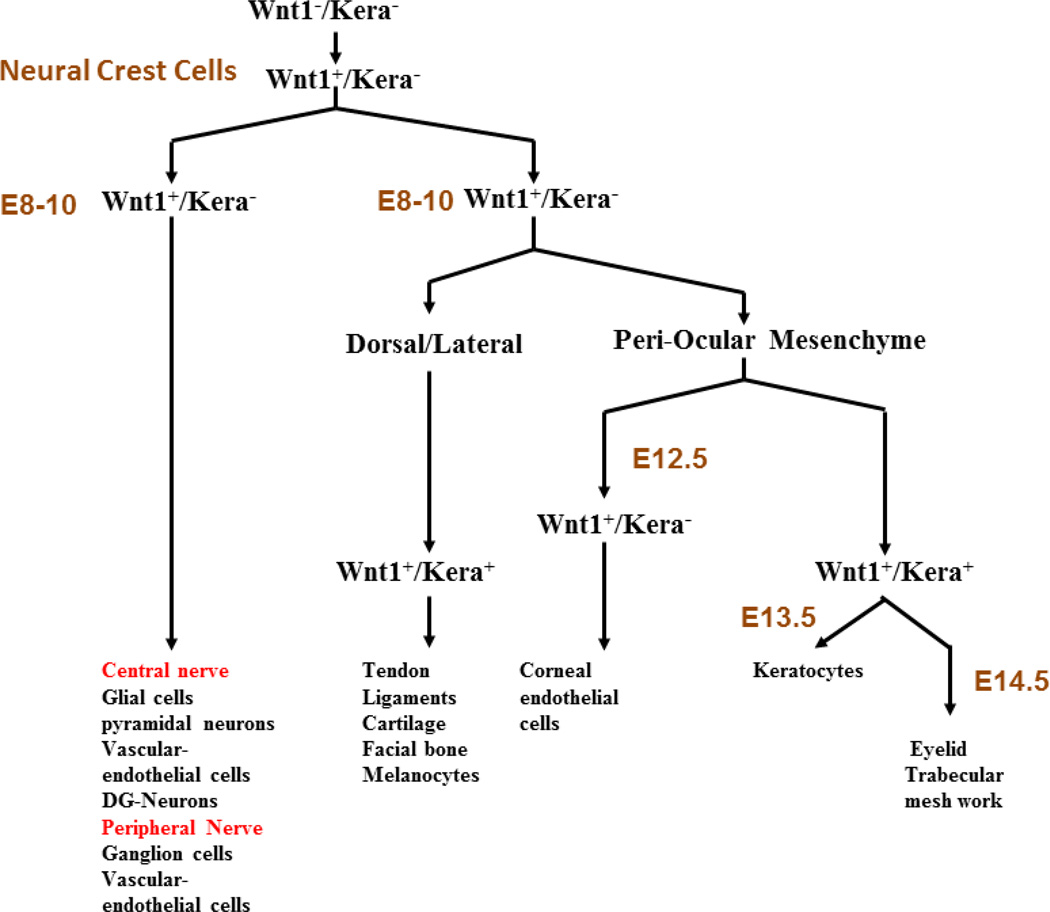
Figure 2.
Schematic diagram of migration and cell fates. Determination of periocular mesenchymal cells of neural crest origin during embryonic ocular surface morphogenesis. (See text for details.)
The first wave involves the migration of Wnt1-positive but Kera-negative (keratocan) periocular mesenchymal cells of neural crest origin to form the endothelium between E12.0 and E13.0 and form ZO-1 tight junction complexes by E18.5.
The second wave of periocular mesenchymal cell migration commences at E12.5/E13.0. These keratocan-expressing periocular mesenchymal cells are progeny of Wnt1-positive cells at E8.5/E10.5, but they are now Wnt1- negative. They invade the primary stroma and become keratocytes of the adult cornea. The number of keratocytes increases steadily due to the continued invasion of mesenchymal cells and the proliferation of resident keratocytes, and reaches a plateau between E16.5-E18.5, concomitant with the cessation of neural crest cell invasion into corneal stroma and decreased proliferation of keratocytes.4 It has been demonstrated that keratocytes are still engaged in proliferation until postnatal day 4 in neonatal rabbits, at a time when expression of Ki67 decreases. The keratocytes of adults are quiescent, as evidenced by the lack of Ki67 expression.
A less characterized third wave of periocular mesenchymal cell migration is noted at E13.5-E14.0 for the formation of eyelid stroma and trabecular meshwork (data not shown). These mesenchymal cells contribute to the formation of tarsal plate and levator muscle and supporting stromal cells of meibomian glands, as well as mesenchymal cells of eyelash dermal papilae.7 A population of mesoderm-derived fibroblastic cells forms the orbicularis oculi. However, the molecular and cellular mechanisms of eyelid stroma morphogenesis remain elusive.
B. Formation of Ocular Surface Epithelium
From E12.5 through E14.5 of mouse embryonic development, the corneal surface maintains a two-cell-layered peridermal epithelium, which expresses keratin 14 (Krt14). The corneal epithelium begins to express Krt12, a marker of corneal type epithelium differentiation, at E13.5/E14.0. Corneal epithelium stratifies into multicell layers after birth, and the expression of Krt14 becomes limited to the basal cell layer like many other stratified epithelia, eg, skin, tongue, etc., while Krt12 is found in all cell layers of corneal epithelium of adult mice (older than 12 weeks).1,8 The conjunctival epithelium undergoes similar changes of Krt14 and Krt13 expression patterns during mouse development.
Folds in the surface ectoderm appear at E13.0 as eyelid formation begins. Between E14.0 and E15.0, the eyelid grows and covers about half of the cornea. The eyelid folds fuse around E15.5 and remain closed until postnatal days 13–14. The outer epithelium of eyelid differentiates to assume the epidermal epithelium and express the epidermal epithelium-specific Krt1/Krt10 keratin pair, whereas the cells of the inner surface of the eyelid become conjunctival epithelial cells expressing the Krt4/Krt13 keratin pair and goblet cells expressing MUC5AC.1 Krt4 is first observed between E14.0 and E15.0 as a subpopulation of epithelial cells derived from surface ectoderm to form the lid buds. From E16.0 on, Krt4 is detected in all areas of developing conjunctival epithelium. In postnatal and adult eyes, the Krt4/Krt13 keratin pair is localized exclusively to the conjunctiva of the ocular surface. Krt14 is first noted in epithelium comprising the eyelid bud at E13.0. Concomitantly, some epithelial cells in the putative fornix of the conjunctiva do not yet express Krt14. These findings suggest that the invagination of surface ectoderm to form the presumptive eyelid may associate with the commencement of ocular surface epithelium differentiation to form corneal and conjunctival epithelium.
Lacrimal gland formation begins with the thickening of peridermal epithelium at the fornix of the conjunctiva at E12.5. Early budding is observed at E13.5, which continues to elongate and branch. The glands are well developed by E18.5. Mesenchymal/epithelial interactions via FGF10 play a pivotal role in lacrimal gland morphogenesis.9 In contrast, the morphogenesis of meibomian glands takes place much later.10 At E18.5, thickening of the epithelium at the lid margins is noted. The budding, elongation, and branching of meibomian glands takes place primarily postnatally.10 Nevertheless, the molecular and cellular mechanism of meibomian gland morphogenesis remains elusive.
II. Roles of Mesenchymal-Epithelial Interactions in Ocular Surface Tissue Morphogenesis
The formation of functional ocular surface tissues (transparent cornea, conjunctiva, eyelids, and lacrimal and meibomian glands) requires coordinated spatial and temporal expression of transcription factors and signaling molecules of various cytokines and signaling pathways. This leads to the unique organization of ECM in stroma and the proper differentiated epithelia derived from surface ectoderm (cornea, conjunctiva, eyelid epidermal epithelium, and lacrimal and meibomian glands). The maintenance of such functional ocular surface tissues also depends on coordinated gene expression in adults. Bidirectional interactions and signaling between mesenchyme and epithelium are considered to be essential for morphogenesis, organogenesis, cell differentiation and growth during embryogenesis and maintenance of homeostasis in adults. It is suggested that tissue morphogenesis during embryonic development involves orderly cellular migration and differentiation, which is controlled by cues from various growth factors and the components of ECM, which are under constant remodeling during embryogenesis. Many growth factors, eg, HGF, FGF-7 (KGF), EGF and TGF-βs, have been characterized as autocrine and paracrine mediators of stromal-epithelial interactions. The expression of such growth factors and their respective specific receptors implies that they are important for the maintenance of ocular surface tissue functions.
Use of transgenic and knockout mice has been highly informative for the identification of growth factors that are important for ocular surface morphogenesis. Many transgenic and knockout mice for growth factors and their respective receptors exhibit abnormal ocular surface morphogenesis. For example, ablation of TGF-β2 results in maldevelopment of the anterior segment, a phenotype resembling Axenfield-Rieger and Peters anomaly in humans.4 Similarly, the EGF receptor (EGFR)-mediated pathways involve epithelial growth, differentiation, and migration, as shown in mice in which ablation of EGFR itself, or TGF-α, an EGFR ligand, results in eye-open at birth phenotype similar to that seen in Mekk1-mutant mice. A temporal upregulation of TGF-α expression has been observed at the leading edge of the eyelid epithelium, suggesting that the EGFR pathway may be activated during lid closure.1 However, the precise growth factors and the mechanisms by which they modulate corneal morphogenesis remain largely unknown.
Due to the lack of appropriate experimental animal models, we do not know the precise molecular mechanisms by which transcription factors modulate ocular surface tissue morphogenesis. For example, it has been shown that deletion of specific transcription factors, eg, Pax6, Klfs via gene targeting techniques, disrupt the formation of normal ocular surface tissues, but the molecular mechanisms by which the transcription factors modulate cellular functions and determine cell fates are unknown.
III. Challenges of Future Studies
Many questions remain unanswered regarding the morphogenesis of ocular surface tissues during embryonic development. For instance, how many different cell lineages of periocular mesenchymal cells contribute to corneal endothelium? What is the cell lineage of meibomian glands-- epidermal epithelium, conjunctival epithelium, or a yet-to-be defined epithelium cell lineage? What are the positional cues for the differentiation of peridermal epithelial cells of surface ectoderm to various ocular surface epithelia (corneal, conjunctival, eyelid epidermal epithelium, and glandular epithelium of lacrimal and meibomian glands)? What are the molecular cellular mechanisms regulating epithelial/mesenchymal interaction for tissue morphogenesis during development and homeostasis in adults. To address these and many other questions, it is imperative to develop experimental animal models, such as genetically modified mouse lines of inducible cell type-specific overexpression and gene ablations.
Several mouse driver lines that are capable of perturbing genetic functions in ocular surface tissues have been created in our laboratories and others. Tables 1 and 2 summarize the driver mouse lines that are designed to target cells derived from periocular mesenchymal cells of neural crest origin and ocular surface epithelia derived from peridermal epithelium of surface ectoderm, respectively. Both Wnt1-Cre and P0-Cre mouse lines can have deleted floxed genes in neural crest cells and overexpression of transgene that is driven by a ubiquitous promoter, eg, chicken actin promoter, and its expression is prevented until the excision of a floxed stop element preceding the reporter gene by Cre recombinase (Figure 3), Such genetic modifications result in perturbation of gene functions in migrating neural crest cells during embryonic development and their progeny, such as corneal endothelium, keratocytes and stromal cells of conjunctiva and eyelids, and dermal papillae of hair and eyelash (Table 1).
Table 1.
Driver Mouse Lines for Genetic Modification in Stromas of Ocular Surface Tissues
| Mouse Lines | Embryonic Stages |
Targeted Cell Types | Induction | Function | ||||
|---|---|---|---|---|---|---|---|---|
| Cornea | Conjunctiva | Eyelid | Eyelash | |||||
| Endo | Keratocyte | Stromal cells | Stromal cells | Dermal papillae | ||||
| Wnt1-Cre/Xf/f | E8–E10 | + | + | + | + | + | N.I. | Gene ablation |
| P0-Cre/Xf/f | E9 | + | + | + | ? | ? | N.I. | Gene ablation |
| Kera-Cre/Xf/f | E13 | − | + | + | + | + | N.I. | Gene ablation |
| Kera-rtTA/tet-O-Cre/Xf/f | E13 Adult |
− − |
+ + |
+ − |
+ − |
+ ? |
Dox | Gene ablation |
| Kera-rtTA/tet-O-X | E13 Adult |
− − |
+ + |
+ − |
+ − |
+ ? |
Dox | Over expression |
Table 2.
Driver Mouse Lines for Genetic Modification in Ocular Surface Epithelia, and Lacrimal and Meibomian Glands
| Mouse Lines | Embryonic Stages |
Targeted Epithelium & Glands | Induction | Function | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cor. | Conj. | Eyelid | Eyelash | Lacrimal | Meibomian | ||||
| Le-Cre/Xf/f | E8 | + | + | + | + | + | + | N.I. | Gene ablation |
| Krt14-Cre/Xf/f | E13 | + | + | + | + | + | + | N.I. | Gene ablation |
| Krt5-Cre/Xf/f | E13 | + | + | + | + | + | + | N.I. | Gene ablation |
| Krt12-Cre/Xf/f | E14 | + | − | − | − | − | − | N.I. | Gene ablation |
| Krt14-rtTA/TC/Xf/f | E13 Adult |
+ + |
+ + |
+ + |
+ + |
+ + |
+ + |
Dox | Gene ablation |
| Krt5-rtTA/TC/Xf/f | E13 Adult |
+ + |
+ + |
+ + |
+ + |
+ + |
+ + |
Dox | Gene ablation |
| Krt12-rtTA/TC/Xf/f | E14 Adult |
+ + |
− − |
− − |
− − |
− − |
− − |
Dox | Gene ablation |
| Krt14-rtTA/tet-O-X | E13 Adult |
+ + |
+ + |
+ + |
+ + |
+ + |
+ + |
Dox | Overexpression |
| Krt5-rtTA/ tet-O-X | E13 Adult |
+ + |
+ + |
+ + |
+ + |
+ + |
+ + |
Dox | Overexpression |
| Krt12-rtTA/ tet-O-X | E14 Adult |
+ + |
− − |
− − |
− − |
− − |
− − |
Dox | Overexpression |
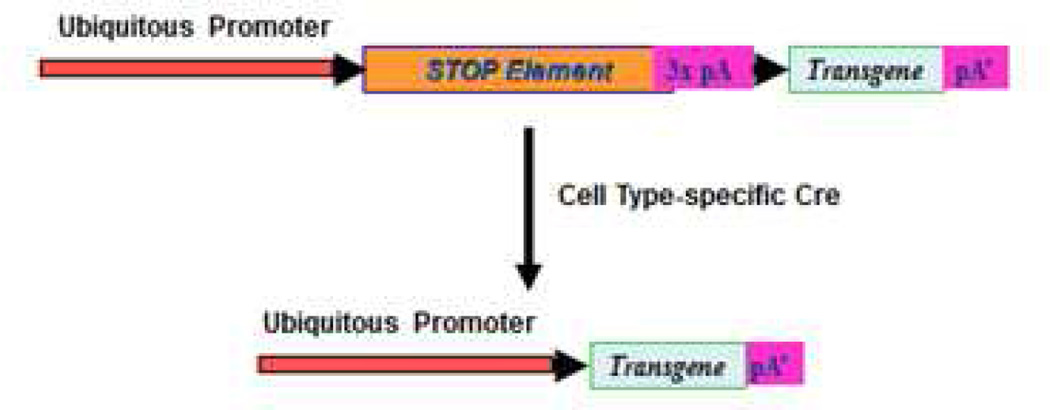
Figure 3.
Schematic illustration of Cre-transgenic mouse lines for cell-type-specific overexpression of transgenes. Cell type-specific overexpression of bitransgene mice can be achieved by cross-breeding a cell type-specific promoter. Cre transgenic mouse and a ubiquitous promoter dual reporter mouse in which the target second transgene is preceded by a loxP flanked STOP elements. The second target transgene is silenced except in cells expressing Cre recombinase.
Kera-Cre transgenic mice express Cre recombinase driven by keratocan promoter in migrating periocular mesenchymal cells of neural crest origin, which leads to perturbation of gene functions of corneal keratocytes and stromal cells of conjunctiva and eyelids and dermal papillae of hair and eyelash. Kera-rtTA transgenic mice are used to generate bitransgenic Kera-rtTA/tet-O-X (cDNA of X gene of interest) and tri-transgenic Kera-rtTA/tet-O-Cre/Xf/f mice for overexpression of transgenes and deletion of floxed genes in corneal keratocytes, and stromal cells of conjunctiva and eyelids, respectively.
With use of a strategy similar to that shown in Table 2, Le-Cre transgenic mice with modified Pax6 promoter express Cre recombinase by ocular peridermal epithelial cells of surface ectoderm that give rise to epithelial cells of lens, cornea, conjunctiva, lacrimal and meibomian glands, and hair. Thus, genetic modification with bi-transgenic Le-Cre/Xf/f mice can potentially perturb morphogenesis of lens and ocular surface epithelia (cornea, conjunctiva, and epithelium-derived lacrimal and meibomian glands). Transgenic mouse lines using Krt14 and Krt5 promoters will perturb gene functions in epithelial cells of all stratified epithelia (skin, cornea, conjunctiva, mouth mucosa, esophagus, vagina, etc). In contrast, Krt12 promoter is active only in corneal epithelial cells and provides unique models for modifying gene functions in corneal epithelium. The greatest remaining challenge is to prepare inducible driver mouse lines that will allow genetic modifications in stem cells of various ocular surface epithelia at postnatal stages.
Acknowledgments
Supported by Grants NIH/NEI EY011845, EY013755, EY021768, Research To Prevent Blindness and Ohio Lions Eye Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no commercial or proprietary interest in any concept or product discussed in this article.
References
- 1.Kao WW, Xia Y, Liu CY, Saika S. Signaling pathways in morphogenesis of cornea and eyelid. Ocul Surf. 2008;6:9–23. [PubMed] [Google Scholar]
- 2.Hay ED. Development of the vertebrate cornea. Int Rev Cytol. 1980;63:263–322. doi: 10.1016/s0074-7696(08)61760-x. [DOI] [PubMed] [Google Scholar]
- 3.Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26:374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saika S, Saika S, Liu CY, et al. TGFbeta2 in corneal morphogenesis during mouse embryonic development. Dev Biol. 2001;240:419–432. doi: 10.1006/dbio.2001.0480. [DOI] [PubMed] [Google Scholar]
- 5.Kao WW. Ocular surface tissue morphogenesis in normal and disease states revealed by genetically modified mice. Cornea. 2006;25(Suppl 1):S7–S19. doi: 10.1097/01.ico.0000247207.55520.a4. [DOI] [PubMed] [Google Scholar]
- 6.Muzumdar MD, Tasic B, Miyamichi K, et al. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi Y, Liu CY, Jester JJ, et al. Excess biglycan causes eyelid malformation by perturbing muscle development and TGF-alpha signaling. Dev Biol. 2005;277:222–234. doi: 10.1016/j.ydbio.2004.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanifuji-Terai N, Terai K, Hayashi Y, et al. Expression of keratin 12 and maturation of corneal epithelium during development and postnatal growth. Invest Ophthalmol Vis Sci. 2006;47:545–551. doi: 10.1167/iovs.05-1182. [DOI] [PubMed] [Google Scholar]
- 9.Makarenkova HP, Ito M, Govindarajan V, et al. FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development. 2000;127:2563–2572. doi: 10.1242/dev.127.12.2563. [DOI] [PubMed] [Google Scholar]
- 10.Nien CJ, Massei S, Lin G, et al. The development of meibomian glands in mice. Mol Vis. 2010;16:1132–1140. [PMC free article] [PubMed] [Google Scholar]