Abstract
Objectives
Patients with rheumatoid arthritis (RA) have a reduced life expectancy due to increased cardiovascular disease. The lack of a suitable animal model resembling both RA and atherosclerosis has hindered studies demonstrating a direct link between systemic inflammation in RA and the development of atherosclerosis. Our objective was to overcome this barrier by generating an animal model (K/BxAg7) that spontaneously develops both RA-like disease and atherosclerosis.
Methods
Arthritis severity was evaluated using clinical indices and immunohistochemical staining of ankle joint specimens. Aortic atherosclerosis was delineated via Sudan IV staining and immunohistochemical analysis. Serum cholesterol and lipoprotein levels were measured using enzymatic assays. Serum levels of cytokines, chemokines and adipokines were determined by Luminex assays.
Results
K/BxAg7 mice developed a destructive arthropathy followed by prominent aortic atherosclerosis. These animals also displayed dyslipidaemia, characterised by reduced serum levels of total cholesterol and high-density lipoprotein, and increased low-density lipoprotein (LDL)/vLDL compared with control mice. Further, there were higher levels of circulating inflammatory mediators, such as interleukin-6, sRANKL and CCL5 in atherosclerotic K/BxAg7 mice compared with controls. Treatment with etanercept reduced arthritis and atherosclerosis development in K/BxAg7 mice.
Conclusions
K/BxAg7 mice recapitulate the same sequence of events occurring in patients with RA, namely an erosive, inflammatory arthritis followed by atherosclerosis. These data suggest that the K/BxAg7 mouse is a novel system for investigating the interplay between systemic inflammation occurring in RA and the development of atherosclerosis.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory and destructive arthropathy that affects 1% of the population.1 Patients with RA are at increased risk for premature cardiovascular (CV) events, which contributes greatly to their high mortality.2 3 This elevated CV risk is independent of traditional risk factors and may be related to increased systemic inflammation. Further, dyslipidaemia, characterised by elevated serum levels of total cholesterol (TC) and low-density lipoprotein (LDL) and decreased high-density lipoprotein (HDL), also raises CV risk in RA.4 To date, advancement in understanding the relationships among inflammatory arthritis, dyslipidaemia and atherosclerosis in RA has been limited by the lack of a representative animal model.
Mice expressing the KRN T-cell receptor (TCR) in the context of the major histocompatibility complex (MHC) Class II Allele IAk (Ag7) develop a spontaneous, erosive arthritis that resembles RA.5 Ag7 MHC class II alleles present endogenous glucose-6-phosphate isomerase (G6PI) peptides that are recognised by the KRN TCR. Innate and adaptive immune components including B cells, T cells, neutrophils, mast cells, macrophages, complement factors, inflammatory cytokines and Fc receptors have been shown to be instrumentalfor the development of arthritis in these animals.6–8 The arthritis is transferrable to naïve mice through injection of serum containing anti-G6PI antibodies,9 with the resulting inflammatory arthritis in recipient mice resembling the effector phase. K/BxAg7 mice also develop cardiac valvulitis, another feature occurring in RA.10 Thus, the K/BxAg7 mouse represents an ideal animal model to examine the influence of inflammatory arthritis on the development of atherosclerosis.
Methods
Animals
KRN mice (C57BL/6) were a gift from Drs Diane Mathis and Christophe Benoist. Ag7 (C57BL/6) mice were provided by Dr Paul Allen. C57BL/6 (Jackson Laboratory, Bar Harbor, Maine, USA), and the NOD mice (Taconic, Germantown, New York, USA) were purchased. Genotyping was confirmed by Transnetyx (Memphis, Tennessee, USA) and flow cytometry. Equal numbers of male and female mice were fed chow or Harlan Teklad atherogenic diet TD.94059 (Harlan, Houston, Texas, USA) containing 15.8% fat and 1.2% cholesterol. A subset of K/BxAg7 mice was injected subcutaneously with phosphate buffered saline (PBS) or 0.8 mg/kg etanercept twice weekly for 13 weeks. All studies were approved by the IACUC at Northwestern University, and mice were maintained within the Center for Comparative Medicine.
Scoring and induction of arthritis
Ankle width was measured using calipers. Clinical score (total=12) for four paws measured disease severity, scored as 0=normal, 1=swollen wrist/ankle, 2=swelling extending to forepaw/hindpaw, 3=swelling extending to digits. Clinical damage index (total=40) for four paws was determined by summing the number of irreversibly hyper-extended or flexed joints. Ankle joints were fixed in 10% formalin and decalcified. For the serum transfer-induced arthritis (STIA) experiments, C57BL/6 mice were injected intraperitoneally with 100 µl of K/BxN serum in 200 µl PBS.
Immunohistochemistry
Paraffin-embedded ankle sections were stained with H&E. Paraffin-embedded aortic sinus sections were stained with Masson's Trichrome, rat antimouse F4/80 (clone BM8), or rat antimouse CD45 (Caltag) antibodies. Imaging was performed using an Olympus DP40 microscope (Tokyo, Japan) equipped with a DP71 camera.
Evaluation of atherosclerosis
Peripheral blood was harvested by cardiac puncture, followed by ventricular perfusion with 20 ml of PBS. Aortas were excised, fixed with 10% formalin, and stained with Sudan IV (Sigma-Aldrich, St Louis, Missouri, USA). Atherosclerosis was quantitated by computer-assisted morphometric analysis (ImagePro 6.3 software) of whole aortic (aortic root to iliac bifurcation) specimens. Results were expressed as the percentage of Sudan IV stained lesion area (calculated by Sudan IV stained area/total aortic area ×100).
Serum analysis
Serum cytokine and chemokine levels were assessed using Luminex-based (Austin, Texas, USA) 27-plex assays (Affymetrix, Santa Clara, California, USA) according to the manufacturer's instructions. Serum TC, low-density lipoprotein/very low-density lipoprotein (LDL/vLDL) and HDL levels were measured using enzymatic assays according to the manufacturer's instructions (AbCam, Cambridge, Massachusetts, USA).
Statistical analysis
Repeated-measure, two-way analysis of variance with Bonferroni post-tests were used for arthritis analyses. Student's t tests were used to compare Sudan IV staining, lipid and cytokine levels between groups. Linear regression analysis was used with TC, LDL/vLDL or HDL as X variable predictors, and atherosclerotic burden as the Y variable. Statistical significance was established at p<0.05.
Results
K/BxAg7 mice develop a severe, destructive arthropathy
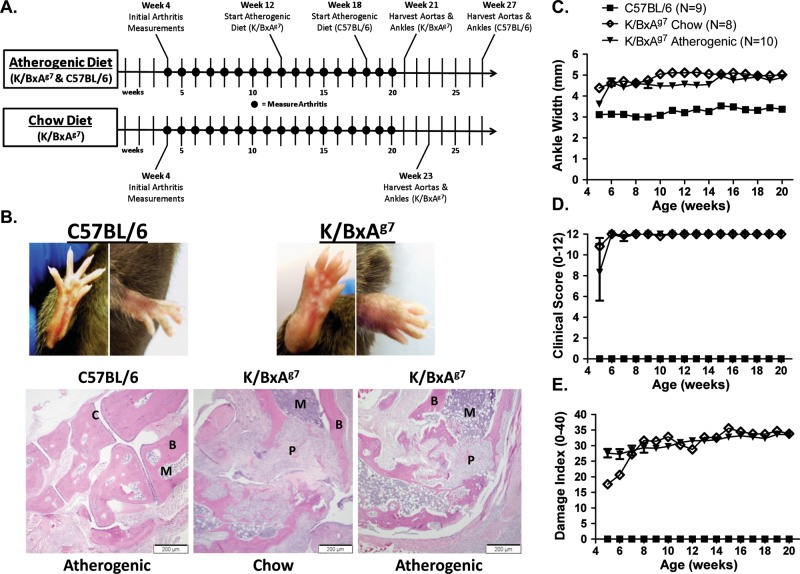
In order to understand the interplay between systemic inflammation that occurs in RA-like disease and atherosclerosis, we generated mice (K/BxAg7) that express the KRN TCR transgene (K), and the MHC class II Allele H-2k (Ag7), which were backcrossed for several generations onto a C57BL/6 (H-2b) background.11 While K/BxN mice12 have been used extensively to model RA, the non-obese diabetic (NOD (N)) background, which predisposes K/BxN animals toward diabetes, may confound the atherosclerosis studies. At 4–5 weeks of age (figure 1A), K/BxAg7 mice developed a severe arthropathy, as measured by histologic ankle joint analysis (figure 1B), ankle width (figure 1C), clinical score (figure 1D) and damage index (figure 1E). In contrast, no arthritis was observed in control animals (C57BL/6) on a mixed H-2b and H-2k (Ag7), or a pure H-2b background (unpublished data). There were also no gender differences identified between mice. Our data suggest that K/BxAg7 mice develop a spontaneous, destructive arthritis that mimics RA, which is in agreement with previous findings.13
Figure 1.
K/BxAg7 mice develop a severe, destructive arthropathy. (A) Experimental timeline. (B) Forepaw and hindpaw images (upper panel) from 6-week-old C57BL/6 and K/BxAg7 mice. H&E staining of representative ankle joint specimens (lower panel) from C57BL/6 and K/BxAg7 mice fed an atherogenic diet, or K/BxAg7 mice fed chow. (P)=pannus (B)=bone (C)=cartilage (M)=bone marrow. Mean±SEM (C) ankle width, (D) clinical score and (E) damage index in C57BL/6 (filled squares), and K/BxAg7 (filled triangles) mice fed an atherogenic diet or K/BxAg7 (open diamonds) mice fed chow. Statistically significant differences (p<0.0001) were observed between K/BxAg7 (atherogenic or chow diet) and C57BL/6 mice at all time points.
K/BxAg7 mice are susceptible to atherosclerosis
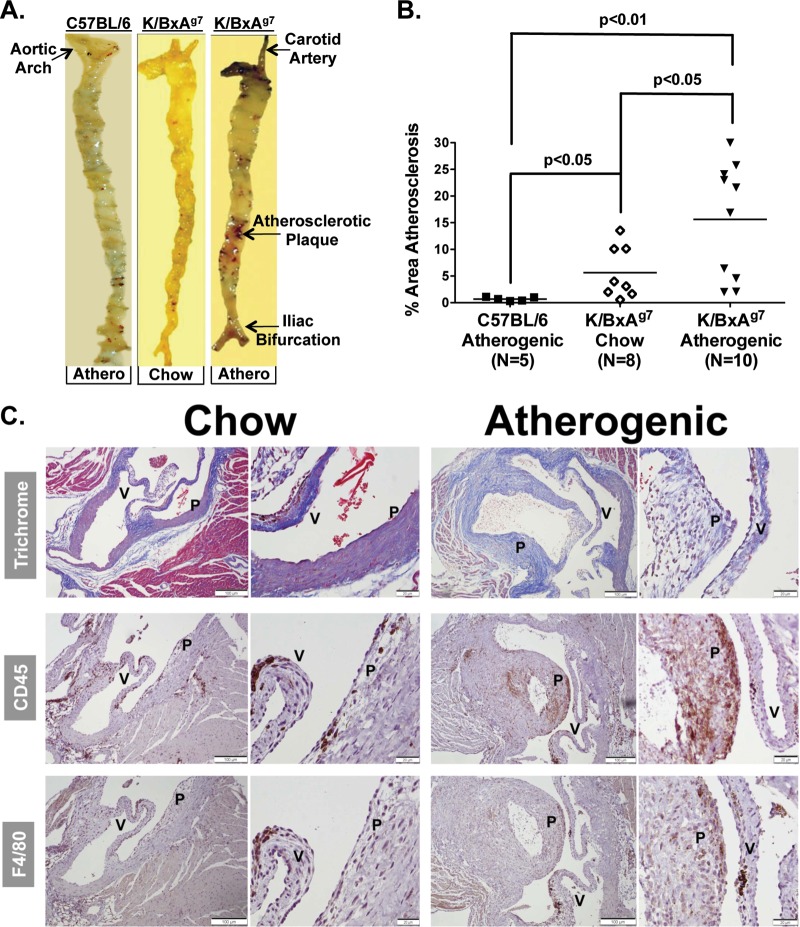
Patients with RA are predisposed toward atherosclerotic disease.2 3 To determine whether K/BxAg7 animals are susceptible to atherosclerosis, subgroups of mice were fed chow for 12 weeks followed by an atherogenic diet, or continued on the chow diet (figure 1A). K/BxAg7 mice displayed a 22-fold increase in aortic atherosclerosis compared with control mice, which exhibited negligible lesion formation even on an atherogenic diet (figures 2A,B). Further, plaque area was 2.3-fold greater in K/BxAg7 mice fed an atherogenic diet compared with chow (figures 2A,B). Gender-specific differences between mice were not observed. Leucocytes and macrophages were highly present in atherosclerotic plaques from K/BxAg7 mice (figure 2C), similar to RA patients.14 These data suggest that K/BxAg7 arthritic/atherosclerotic mice develop a histological and cellular plaque composition that is reminiscent of patients with RA.
Figure 2.
K/BxAg7 mice are susceptible to atherosclerosis. (A) Sudan IV staining of representative aortic specimens from C57BL/6 or K/BxAg7 mice fed an atherogenic diet (‘athero’) or a K/BxAg7 mouse fed chow. (B) Mean±SEM percentage atherosclerotic area of Sudan IV stained aortic specimens from K/BxAg7 (filled triangles) and C57BL/6 (filled squares) mice fed an atherogenic diet, or K/BxAg7 (open diamonds) mice fed chow. (C) Masson's trichrome, anti-CD45 antibody or anti-F4/80 antibody staining of aortic sinus specimens from representative K/BxAg7 mice fed chow, or an atherogenic diet. 10× (left) and 40× (right) magnification, are depicted for aortic specimens. P=atherosclerotic plaque, V=aortic valve.
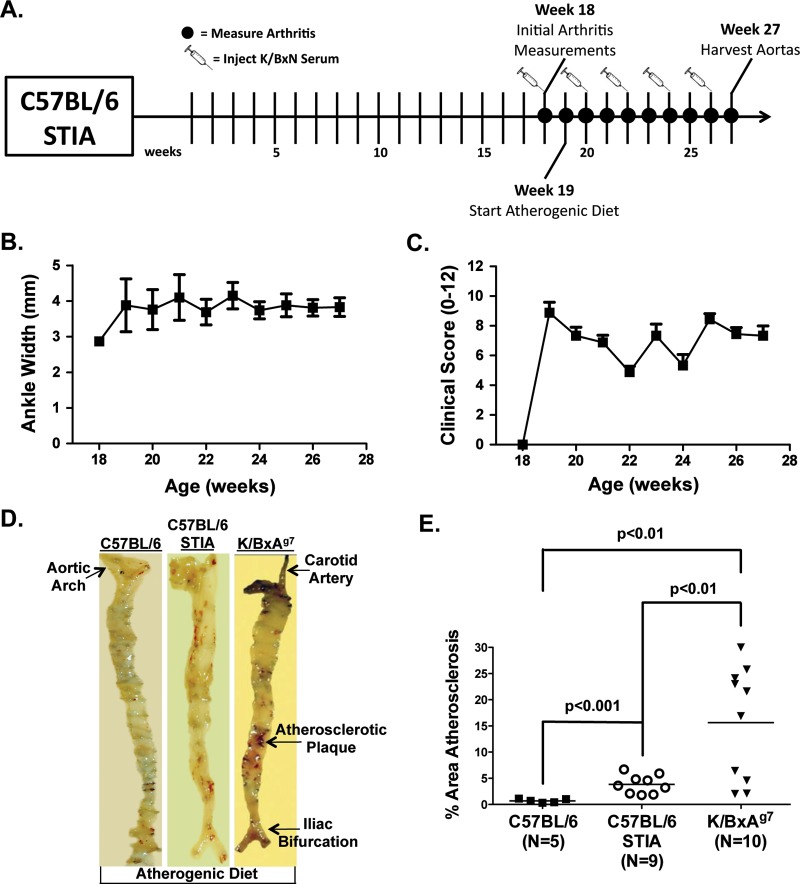
The K/BxN STIA model9 resembles the effector phase of RA. Eighteen-week-old C57BL/6 mice were injected bimonthly with K/BxN serum, and were fed an atherogenic diet for 9 weeks (figures 3A–C). Atherosclerosis levels were 5.4-fold higher (p<0.001) in STIA mice than in controls fed an atherogenic diet (figures 3D,E). However, plaque area was still 4.1-fold greater in K/BxAg7 compared with STIA mice (figures 3D,E). Together, these data indicate that induction of inflammatory arthritis via endogenous (K/BxAg7) or transferred (K/BxN) arthritogenic serum provokes the development of atherosclerosis. Furthermore, arthritis and atherosclerosis in the STIA model does not require specific T-cell receptors or MHC class II alleles.
Figure 3.
Induction of arthritis triggers the development of atherosclerosis in C57BL/6 mice. (A) Experimental timeline. C57BL/6 serum transfer-induced arthritis (STIA) mice were intraperitoneally injected with K/BxN serum bimonthly, and (B) joint width and (C) clinical score were assessed weekly, thereafter (mean±SEM). (D) Sudan IV staining of a representative aortic specimen from a C57BL/6 STIA mouse. C57BL/6 and K/BxAg7 mice treated as in (A) are included for comparison. (E) Percentage (mean±SEM) atherosclerotic area of Sudan IV stained aortic specimens from C57BL/6 (filled squares), C57BL/6 STIA (open circles) and K/BxAg7 (filled triangles) mice.
K/BxAg7 mice are dyslipidaemic
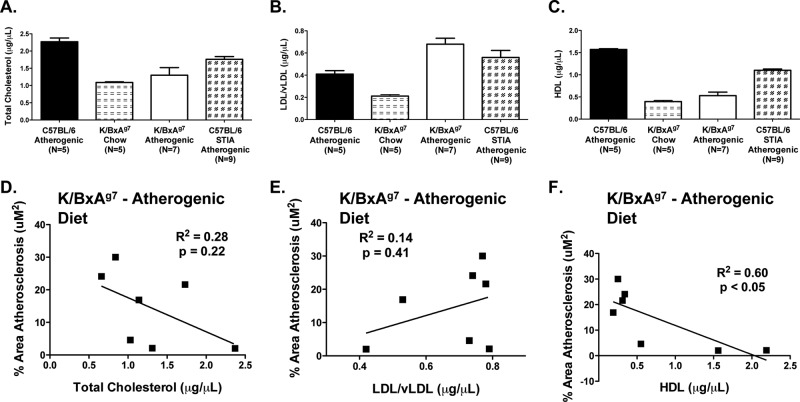
Dyslipidaemia may be an important contributor to increased CV mortality in RA.4 K/BxAg7 mice exhibited marked dyslipidaemia, characterised by reduced serum levels of TC (figure 4A, p<0.01), increased LDL/vLDL (figure 4B, p<0.01), and diminished HDL (figure 4C, p<0.01) compared with non-arthritic controls on an atherogenic diet. However, LDL/vLDL levels were increased by 3.2-fold (p<0.0001) in K/BxAg7 mice fed an atherogenic diet versus chow, while TC and HDL levels were unaffected by diet (figure 4). Lower HDL levels in K/BxAg7 mice were associated with greater amounts of atherosclerosis (R2=0.60, p<0.05, figure 3F) whereas, TC (figure 3D) and LDL/vLDL (figure 3E) were not significantly related to atherosclerotic burden. Similar to K/BxAg7 mice, STIA mice demonstrated reduced levels of TC (figure 3A, p<0.01) and HDL (figure 3C, p<0.01), and elevated LDL/vLDL (figure 3B, p=0.12) compared with non-arthritic controls. Altogether, these findings show that dyslipidaemia may contribute to the elevated atherosclerosis observed in K/BxAg7 mice, and that induction of arthritis elicits dyslipidaemia in normal animals.
Figure 4.
K/BxAg7 mice develop dyslipidaemia. Serum was harvested from 27-week-old C57BL/6 (filled bars), 27-week-old C57BL/6 serum transfer-induced arthritis (STIA) (chequered bars), and 21-week-old K/BxAg7 (open bars) mice fed an atherogenic diet for 9 weeks, or 23-week-old K/BxAg7 mice maintained on chow (horizontal bars). Mean±SEM (A) total cholesterol (TC), (B) low-density lipoprotein (LDL)/vLDL and (C) high-density lipoprotein (HDL) are depicted. All t test comparisons between groups were statistically significant (p<0.05) with the following exceptions: TC – K/BxAg7 atherogenic versus chow, LDL/vLDL–K/BxAg7 and C57BL/6 atherogenic versus C57BL/6 STIA atherogenic, and HDL–K/BxAg7 atherogenic versus chow. Linear regression analysis of (D) TC, (E) LDL/vLDL and (F) HDL versus atherosclerosis.
Serum cytokines/chemokines are altered in K/BxAg7 mice
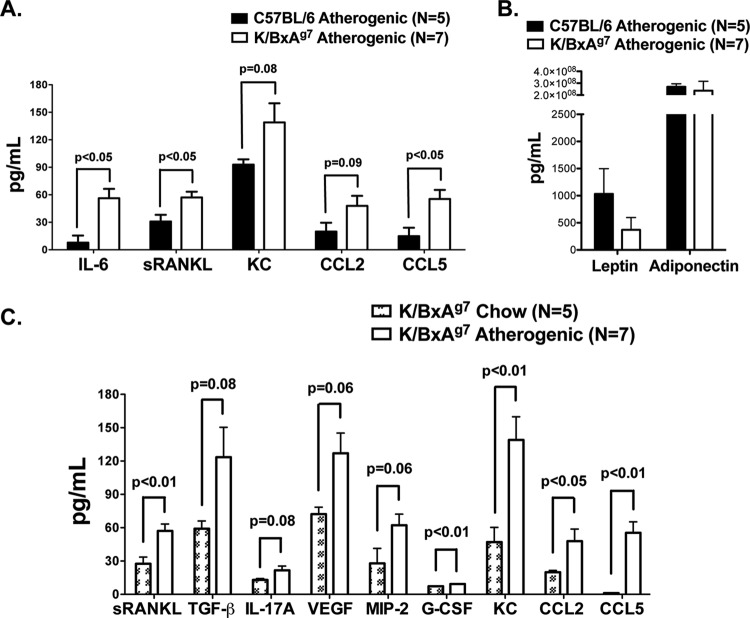
Cytokines, chemokines and adipokines are central to the development of atherosclerosis.15 16 Serum levels of interleukin-6, sRANKL and CCL5 were significantly greater (p<0.05), while KC and CCL-2 trended toward significance in K/BxAg7 mice compared with C57BL/6 controls fed an atherogenic diet (figure 5A). Elevations in IL-21, IL-12/23 p40, tumour necrosis factor (TNFα), MIP-2, CXCL10 and GM-CSF were also detected, but were not significant in K/BxAg7 mice compared with controls (online figure 1). In contrast, VEGF, TGF-β, IL-4, IL-13, IL-1α, IL-1β, IL-17A, G-CSF, CCL3, leptin and adiponectin were expressed at similar levels (figure 5B and online figure 1) and IL-10, interferon γ, IL-2, IL-12 p70 and IL-23 p19 were undetectable (unpublished data). Further, sRANKL, G-CSF, KC, CCL2 and CCL5 were increased in K/BxAg7 mice fed an atherogenic diet compared with chow (figure 5C), while levels of the remaining cytokines and chemokines were unaffected by diet (online figure 1). Together, these data suggest that higher levels of circulating cytokines and chemokines, but not adipokines, may contribute to increased atherosclerosis in K/BxAg7 mice.
Figure 5.
Altered serum cytokine/chemokine profiles in K/BxAg7 mice. (A) Serum cytokines and chemokines and (B) adipokines (mean±SEM) in C57BL/6 (filled bars) and K/BxAg7 (open bars) mice fed an atherogenic diet were measured via Luminex. (C) Serum cytokines and chemokines were measured via Luminex in 23-week-old K/BxAg7 mice fed chow (chequered bars), or 21-week-old K/BxAg7 mice fed an atherogenic diet for 9 weeks (open bars).
TNF blockade improves arthritis and atherosclerosis in K/BxAg7 mice
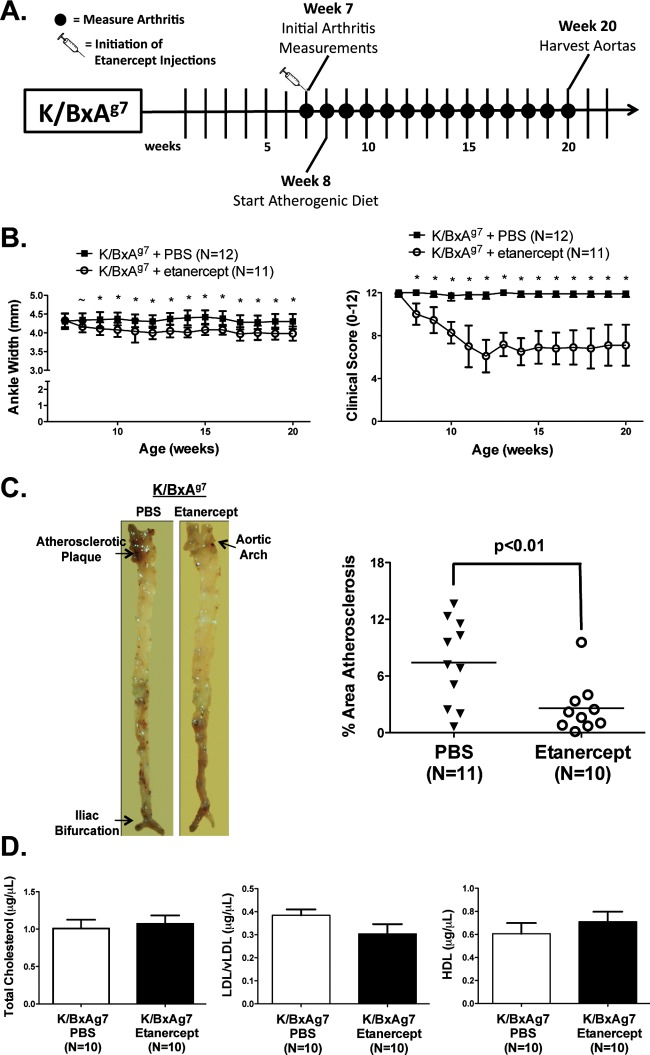
Etanercept is a TNF antagonist commonly employed to treat RA.17 K/BxAg7 mice on an atherogenic diet were treated biweekly with PBS or etanercept for 13 weeks. Administration of etanercept reduced arthritis severity (figure 6B), as indicated by a 0.2–0.4 mm decrease in ankle width, and by a 2.0-fold reduction in joint clinical score, as compared with PBS-treated controls. Atherosclerotic burden was reduced by 3.0-fold in etanercept-treated mice compared with controls (figure 6C). Further, etanercept therapy in K/BxAg7 mice reversed aortic valvulitis thickening (figure 3),10 and impaired valvular function (online Videos 1–4). TC, LDL/vLDL and HDL levels were unaffected by etanercept treatment (figure 6D). Together, these data demonstrate a link between arthritis severity and the development of atherosclerosis in K/BxAg7 mice.
Figure 6.
Improved arthritis, atherosclerosis and dyslipidaemia in K/BxAg7 mice treated with etanercept. (A) Experimental timeline. (B) Arthritis severity, (C) mean±SEM% area aortic atherosclerosis and (D) TC, LDL/vLDL and HDL levels in K/BxAg7 animals treated with phosphate buffered saline (open) or etanercept (filled). ∼ denotes statistical significance at p<0.05, *at p<0.01.
Discussion
Here, we characterised K/BxAg7 mice as a unique model of arthritis/atherosclerosis that recapitulates the same sequence of events occurring in RA. Even passive transfer of arthritis triggers the development of atherosclerosis, suggesting that both disease states occur independently of the unique TCR (KRN) and MHC class II allele (Ag7) present in K/BxAg7 mice. While previous studies have shown that mice lacking Fas18 or FasL,19 or that are on a genetically predisposed background (BWF1),20 develop SLE-like disease and enhanced atherosclerosis when fed a high fat diet, we are the first to generate a murine model of RA-like disease that is also susceptible to atherosclerosis. K/BxAg7 mice also develop dyslipidaemia, and display increased serum levels of pro-inflammatory cytokines and chemokines. TNF blockade diminishes arthritis and atherosclerosis in K/BxAg7 mice. Collectively, these data link arthritis and atherosclerosis as interdependent disease processes, and establish the K/BxAg7 mouse as a unique model for preclinical testing of therapeutics for these disorders.
K/BxAg7 mice develop a destructive inflammatory arthritis mirroring RA patients with severe or untreated disease. Unlike RA, there was no evidence of calcified aortic lesions or thrombotic events despite extensive atherosclerosis in K/BxAg7 animals (unpublished data). Yet, K/BxAg7 mice demonstrated a lipid paradox (diminished TC and HDL) that resembles the dyslipidaemia seen in RA patients with the greatest inflammatory burden.21 There was a significant inverse association between HDL levels and plaque area in K/BxAg7 mice, consistent with previous studies showing an atheroprotective role of HDL in RA.4 However, treatment with etanercept improves arthritis and atherosclerosis without affecting lipid levels in K/BxAg7 mice, suggesting a non-causative relationship between dyslipidaemia and atherosclerosis in these animals. Future studies will determine whether altering dyslipidaemia impacts the development of atherosclerosis in K/BxAg7 mice.
Since cytokines and chemokines are potent orchestrators of inflammation, altered levels of these factors may contribute to the development of arthritis and vascular disease in K/BxAg7 mice. Indeed, elevated levels of several inflammatory mediators were evident in K/BxAg7 mice and TNF blockade led to improved arthritis and atherosclerosis. However, epidemiologic studies have yielded conflicting data on the effects of disease-modifying treatments on RA disease activity and CV disease. Thus, dissecting the disease-specific effects of individual mediators will be critical to understanding the relationships among inflammatory arthritis, dyslipidaemia and atherosclerosis in K/BxAg7 mice as a model for RA-like disease.
Acknowledgments
This work was supported by grants Driskill Fellow Scholarship award, NIH grant (AR07611) and NIH LRP to Shawn Rose, HHMI (57006753) to C Shad Thaxton, NIH grants (HL051387 and HL108795) to Douglas Vaughan, NIH grants (EB005866 and CA151880) to Thomas Meade, and NIH grants (AR050250, AR054796, AI092490 and HL108795) and Funds provided by Northwestern University, Feinberg School of Medicine to Harris Perlman. The authors thank Jungwha Lee for advice on statistical analyses.
Footnotes
Contributors: All authors contributed to the manuscript.
Funding: NIH.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: We will share all data and resources that relate to this manuscript.
References
- 1.Pope RM, Perlman H. Rheumatoid Arthritis. In: Tsokos GC, eds. Current Molecular Medicine: Principles of Molecular Rheumatology. Totowa: Humana Press; 2000:325–61 [Google Scholar]
- 2.Aviña-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008;59:1690–7 [DOI] [PubMed] [Google Scholar]
- 3.del Rincón ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 2001;44:2737–45 [DOI] [PubMed] [Google Scholar]
- 4.Toms TE, Symmons DP, Kitas GD. Dyslipidaemia in rheumatoid arthritis: the role of inflammation, drugs, lifestyle and genetic factors. Curr Vasc Pharmacol 2010;8:301–26 [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto I, Staub A, Benoist C, et al. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science 1999;286:1732–5 [DOI] [PubMed] [Google Scholar]
- 6.Ji H, Ohmura K, Mahmood U, et al. Arthritis critically dependent on innate immune system players. Immunity 2002;16:157–68 [DOI] [PubMed] [Google Scholar]
- 7.Kyburz D, Corr M. The KRN mouse model of inflammatory arthritis. Springer Semin Immunopathol 2003;25:79–90 [DOI] [PubMed] [Google Scholar]
- 8.Monach PA, Nigrovic PA, Chen M, et al. Neutrophils in a mouse model of autoantibody-mediated arthritis: critical producers of Fc receptor gamma, the receptor for C5a, and lymphocyte function-associated antigen 1. Arthritis Rheum 2010;62:753–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korganow AS, Ji H, Mangialaio S, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity 1999;10:451–61 [DOI] [PubMed] [Google Scholar]
- 10.Binstadt BA, Hebert JL, Ortiz-Lopez A, et al. The same systemic autoimmune disease provokes arthritis and endocarditis via distinct mechanisms. Proc Natl Acad Sci USA 2009;106:16758–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu D, Horvath S, Matsumoto I, et al. Molecular basis for recognition of an arthritic peptide and a foreign epitope on distinct MHC molecules by a single TCR. J Immunol 2000;164:5788–96 [DOI] [PubMed] [Google Scholar]
- 12.Korganow AS, Duchatelle V, et al. Organ-specific disease provoked by systemic autoimmunity. Cell 1996;87:811–22 [DOI] [PubMed] [Google Scholar]
- 13.Mandik-Nayak L, Wipke BT, Shih FF, et al. Despite ubiquitous autoantigen expression, arthritogenic autoantibody response initiates in the local lymph node. Proc Natl Acad Sci USA 2002;99:14368–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aubry MC, Maradit-Kremers H, Reinalda MS, et al. Differences in atherosclerotic coronary heart disease between subjects with and without rheumatoid arthritis. J Rheumatol 2007;34:937–42 [PubMed] [Google Scholar]
- 15.Ait-Oufella H, Taleb S, Mallat Z, et al. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol 2011;31:969–79 [DOI] [PubMed] [Google Scholar]
- 16.Surmi BK, Hasty AH. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vascul Pharmacol 2010;52:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray KM, Dahl SL. Recombinant human tumor necrosis factor receptor (p75) Fc fusion protein (TNFR:Fc) in rheumatoid arthritis. Ann Pharmacother 1997;31:1335–8 [DOI] [PubMed] [Google Scholar]
- 18.Ma Z, Choudhury A, Kang SA, et al. Accelerated atherosclerosis in ApoE deficient lupus mouse models. Clin Immunol 2008;127:168–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aprahamian T, Rifkin I, Bonegio R, et al. Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease. J Exp Med 2004; 199:1121–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahn BH, Lourencço EV, McMahon M, et al. Pro-inflammatory high-density lipoproteins and atherosclerosis are induced in lupus-prone mice by a high-fat diet and leptin. Lupus 2010;19:913–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis 2009;68:460–9 [DOI] [PubMed] [Google Scholar]