Abstract
It has been recently demonstrated that interleukin-1β (IL-1β) plays a central role in monosodium urate (MSU) crystal-induced inflammation and that the NALP3 inflammasome plays a major role in IL-1β production. These discoveries have offered new insights into the pathogenesis of acute gouty arthritis. In this review, we discuss the molecular mechanisms by which MSU crystals induce acute inflammation and examine the mechanisms of action (MOAs) of traditional anti-inflammatory drugs (eg, nonsteroidal anti-inflammatory drugs [NSAIDs], colchicine, and glucocorticoids) and biologic agents (eg, the IL-1β antagonists anakinra, rilonacept, and canakinumab) to understand how their MOAs contribute to their safety profiles. Traditional anti-inflammatory agents may act on the IL-1β pathway at some level; however, their MOAs are broad-ranging, unspecific, and biologically complex. This lack of specificity may explain the range of systemic side effects associated with them. The therapeutic margins of NSAIDs, colchicine, and glucocorticoids are particularly low in elderly patients and in patients with cardiovascular, metabolic, or renal comorbidities that are frequently associated with gouty arthritis. In contrast, the IL-1β antagonists act on very specific targets of inflammation, which may decrease the potential for systemic side effects, although infrequent but serious adverse events (including infection and administration reactions) have been reported. Because these IL-1β antagonists target an early event immediately downstream from NALP3 inflammasome activation, they may provide effective alternatives to traditional agents with minimal systemic side effects. Results of ongoing trials of IL-1β antagonists will likely provide clarification of their potential role in the management of acute gouty arthritis.
Keywords: interleukin-1β, anakinra, canakinumab, rilonacept, inflammation, gouty arthritis, mechanism of action, safety, biologic agents
Introduction
Although gouty arthritis has been recognized as a distinct pathologic entity since ancient times, the molecular pathogenesis of acute gouty attacks and the sequence of pathologic events resulting in chronic tophaceous gout are only now becoming fully understood. Clearly, monosodium urate (MSU) crystals are at the center of the pathophysiologic sequence, and although their role in gout was well described by Garrod in the 19th Century [1], their role in the pathogenesis of acute gouty arthritis had to be rediscovered in 1966 [2]. The broader understanding of the pathogenesis of acute gouty arthritis has led to a new understanding of the mechanism of action (MOA) of agents commonly used to treat gouty arthritis and has resulted in the development of novel therapies for acute gouty arthritis. In this review, we will discuss the molecular mechanisms by which MSU crystals induce acute inflammation, the mechanisms by which current therapies interfere with and suppress the inflammation associated with acute gouty arthritis, the mechanisms associated with typical adverse events (AEs) associated with traditional gouty arthritis anti-inflammatory therapies, and the potential mechanistic advantages of biologic therapies that specifically target the interleukin-1β (IL-1β) inflammation pathway.
Inflammation, an Overview
Inflammation is characterized by rubor, tumor, calor, dolor, and functio laesa (redness, swelling, heat, pain, and loss of function) [3]. Vascular events, including dilatation, leakiness, and expression of molecules involved in the recruitment of leukocytes, play a major role in the first three characteristics and result in the accumulation of neutrophils, macrophages/monocytes, and other inflammatory cells at inflamed sites [3]. The vascular endothelium plays a central role in these events and may be influenced by a variety of intercellular messengers ranging from small molecules (eg, eicosanoids, histamine) to peptide messengers (eg, cytokines and chemokines) [3–6]. In turn, the vascular endothelium will secrete agents including eicosanoids and cytokines, which influence the inflammatory process [3]. Vascular endothelial cells recruit leukocytes through the expression of adhesion molecules at inflamed sites, and different vascular adhesion molecules recruit different cell types. In acute gouty attacks, neutrophils are the predominant cell type, and these cells adhere to the endothelial surface proteins E-selectin, P-selectin, and intercellular adhesion molecule-1 (ICAM-1), which are expressed or upregulated at inflamed sites [7]. Cytokines, such as IL-1β and tumor necrosis factor-α (TNF-α), are the primary stimuli for endothelial expression and upregulation of these adhesive molecules. Older studies have implicated MSU-induced release of IL-1 as central to the initiation of inflammation [4,5], and recent studies indicate that uptake of MSU crystals by cells activates the NALP3 inflammasome, leading to the elaboration of activated IL-1 [8]. In acute gouty attacks, the predominant cellular infiltrate is comprised almost exclusively of neutrophils. IL-8 and its receptor on neutrophils, CXCR2, are required for the development of an acute inflammatory response to MSU crystals [9].
Monosodium Urate Crystals and Inflammation
In individuals who suffer from both acute gouty attacks and chronic tophaceous gout, MSU crystals are present in both symptomatic and asymptomatic joint tissue and joint fluid. Many events can set off acute gouty attacks, including overindulgence in alcohol, metabolic stresses such as those that accompany acute myocardial infarctions or surgery, or, most predictably, major shifts in serum uric acid levels leading to resorption of MSU crystals, such as occurs after starting urate-lowering therapy (ULT) [10,11]. It is now clear that in response to MSU crystals, the cells in the joints that initiate the inflammatory cascade are macrophages; these cells phagocytose MSU crystals and release chemo-attractants, such as leukotrienes, IL-8, and others, that recruit neutrophils to the site and start the inflammatory cascade [12,13]. Once recruited to the joint, neutrophils phagocytose MSU crystals and further contribute to the inflammation that characterizes acute gouty attacks.
The mechanisms by which cells take up MSU crystals and activate the inflammatory cascade have been under study for many years, and a number of mechanisms have been proposed and investigated to explain uptake of MSU crystals by leukocytes. MSU crystals are hygroscopic and bind many different proteins to their surface, including immunoglobulin G (IgG) and complement proteins [14–19], which interact with specific receptors on leukocytes to promote leukocyte recruitment and crystal phagocytosis. One experimental problem that has hindered our understanding of the mechanism by which MSU crystals interact with and activate leukocytes is that many MSU preparations used for in vitro studies are contaminated by endotoxin, which directly stimulates Toll-like receptors (TLRs) on leukocytes. Subsequent studies in which endotoxin contamination was eliminated indicated that MSU crystals directly interacted with CD14, a leukocyte cell-surface molecule that interacts with TLR2 and TLR4 to stimulate leukocytes [20], in addition to promoting phagocytosis via complement and immunoglobulin receptors. Regardless of the mechanism by which the MSU crystals are phagocytosed, the crystals interact with TLR2 and TLR4 as well as with the NALP3 inflammasome to stimulate leukocyte activation, leading to the inflammatory cascade [8].
In 2006, Martinon and colleagues [8] first demonstrated that MSU crystals activate a specific inflammatory cascade in leukocytes leading to production of IL-1. Essentially, these authors demonstrated that crystals (MSU and calcium pyrophosphate dihydrate [the crystals that cause calcium pyrophosphate dehydrate disease]) engage the cryopyrin (NALP3) inflammasome, a signaling protein complex in leukocytes that, linked by the adaptor protein apoptosis-associated speck-like protein (ASC), activates caspase-1. Caspase-1, or the neutrophil protease proteinase 3 (PR3) [21], cleaves pro-IL-1β to generate IL-1β, permitting the release of IL-1β into the extracellular space. Once secreted, IL-1β leads to activation of vascular endothelium and production of other chemokines and cytokines, resulting in the recruitment of leukocytes. Based on their homology to caspase-1 and proteinase 3, other neutrophil proteases are likely to play a role in activation of pro-Il-1 to IL-1β as well [22]. In addition to the in vitro evidence of NALP3 activation, increased caspase-1 activation, and increased IL-1 production by cells exposed to crystals, mice lacking NALP3 did not mount much of an inflammatory response to challenge with MSU crystals, with few leukocytes present in the exudate. Thus, the demonstration that the NALP3 inflammasome plays a central role in response to pathogenic crystals leading to IL-1β production and that IL-1β plays the central role in crystal-induced inflammation offered a new insight into the pathogenesis of gouty arthritis [8].
Subsequent work has provided evidence that other factors contribute to MSU-mediated activation of the inflammasome [23,24]. Joosten and colleagues [24] reported that free fatty acids, which activate TLR2, further drive MSU-induced IL-1β production. Moreover, prior studies had demonstrated that MSU crystals induce increased expression of cyclooxygenase-2 (COX-2) with enhanced production of inflammatory prostaglandins that also likely contribute to the enhanced IL-1β production [25–28].
It did not take long for investigators to carry out confirmatory experiments in patients with acute gouty attacks. Administration of an IL-1β/IL-1α blocker (the recombinant IL-1 receptor [IL-1R] antagonist anakinra) [29,30], a chimeric IL-1 TRAP dimeric fusion protein consisting of portions of IL-1R and the IL-1R accessory protein that neutralizes both IL-1β and IL-1α (rilonacept) [31], or a fully human monoclonal antibody that neutralizes the activity of human IL-1β (canakinumab) [32], all have some efficacy in the treatment of acute gouty arthritis [33]. Intracellular IL-1β signals for inflammation via activation of nuclear factor-κB (NFκB) and other studies have shown that signaling molecules downstream of the IL-1 receptor (MyD88) are required for MSU to activate NFκB and for the development of crystal-induced arthritis [34].
Termination of Acute Gouty Attacks
Acute gouty arthritis attacks are generally self-limited and last less than 3 to 4 weeks. Very little is understood at present about how gouty arthritis attacks spontaneously terminate despite the ongoing presence of the inciting agent. One suggestion has been that monocytes/macrophages that take up the apoptotic neutrophils from the joint secrete transforming growth factor-β1 (TGF-β1) and other anti-inflammatory mediators that terminate the attack [35]. Similar uptake of apoptotic neutrophils occurs in rheumatoid arthritis without termination or amelioration of inflammation, so there must be other factors at work as well [36]. Recent work has indicated that interaction of free fatty acids with TLR2 is required for urate crystal-mediated activation of the inflammasome, production of active IL-1, and acute inflammation [24]. This finding has suggested that the elevation of free fatty acids following a heavy meal plays a critical role in the induction of acute gouty attacks. Interestingly, it has long been known that low density lipoprotein (LDL; specifically apolipoprotein B in the LDL), commonly elevated in many patients with gout, bind to MSU crystals and prevent neutrophil activation by crystals, another potential mechanism for suppression of inflammation during intercurrent gout.
Clinical Management of Inflammation in Acute Gouty Arthritis: How Do Therapies Work and How Do Their Mechanisms of Action Affect Safety?
Overview of Acute Gouty Arthritis Management and Treatment Limitations
The goals of gouty arthritis treatment are 2-fold. First, rapid anti-inflammatory therapy is necessary to manage the significant pain, swelling, and disability associated with acute attacks [10,11]. Once an acute attack has terminated, ULT should be initiated to prevent future acute attacks and long-term complications associated with chronic tophaceous gout (eg, joint destruction) [11].
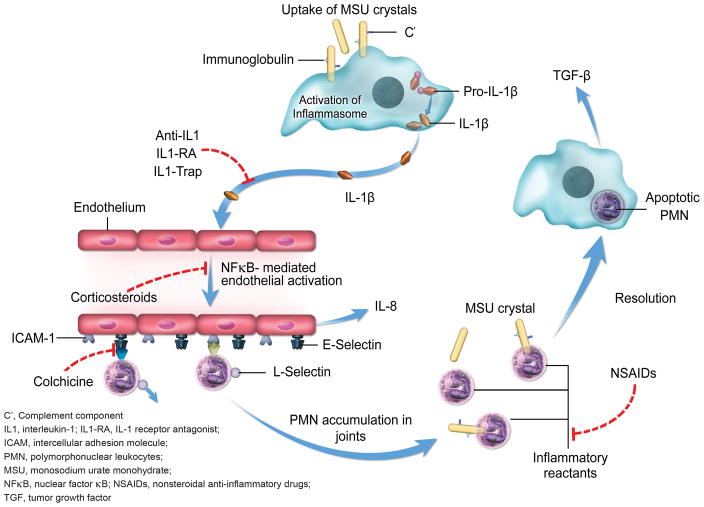
Long before the development of biologic agents that target IL-1β, other therapies had been used successfully and are still currently used for the prevention and treatment of acute gouty attacks, including nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and glucocorticoids. Figure 1 provides an overview of the sites of action of traditional therapies and new biologic agents involved in mediating crystal-induced inflammation. These traditional therapies are effective in reducing inflammation, with symptomatic relief occurring within 24 hours [37,38]. Research on traditional agents continues, with the AGREE study demonstrating that low doses of colchicines are as effective as high doses over 24 hours, with safety similar to placebo [38]. Glucocorticoids are a good option in patients with contraindications to NSAIDs and colchicine or in refractory cases [39], although a meta-analysis showed inconclusive evidence for their effectiveness compared with other anti-inflammatory agents [40].
Figure 1. The most common mechanisms of therapeutic anti-inflammatory action of gouty arthritis drugs.
Colchicine, NSAIDs, and glucocorticoids act on many different molecular targets; the mechanisms displayed herein are the most likely targets for reduction of monosodium urate crystal-induced inflammation when these drugs are administered at the recommended therapeutic doses. Anti-IL-1, IL1-RA, and IL1-Trap therapies act specifically to block IL-1β.
Current gouty arthritis treatment guidelines recommend oral NSAIDs or colchicine a first-line systemic treatment for acute attacks [41] However, the MOAs through which NSAIDs and colchicine reduce inflammation are not specific, and the systemic actions of these drugs are known to cause severe AEs in some patients [42,43]. For example, NSAIDs are associated with adverse gastrointestinal, renal, and cardiovascular effects [43,44], and at therapeutic doses, colchicine is associated with safety concerns such as blood dyscrasias, drug-drug interactions, neuromuscular toxicity, and gastrointestinal AEs [42].
Because gouty arthritis is associated with several comorbidities (including cardiovascular disease, hypertension, type 2 diabetes mellitus, obesity, hyperlipidemia, metabolic syndrome, chronic kidney disease [CKD], and nephrolithiasis) [45,46], contraindications to NSAIDs and/or colchicine are common [47]. In a study that reviewed medical records from the Department of Veterans Affairs, of 575 patients diagnosed with gouty arthritis, more than 88% of patients had at least 1 comorbid condition [47]. In the same study, more than 90% of patients had at least 1 contraindication to NSAIDs and approximately 50% of patients had at least 1 contraindication to colchicines [47].
In patients who cannot tolerate NSAIDs or colchicine, and in patients with polyarticular gouty arthritis or CKD, glucocorticoids are recommended for the management of acute gouty arthritis attacks [48]. However, even with short-term use, glucocorticoids have been associated with AEs, such as hypertension and diabetes mellitus [49]. In patients with pre-existing impaired glucose tolerance, the diabetogenic effects associated with glucocorticoids can be particularly substantial [49].
Below, we present a detailed examination of the MOAs of traditional anti-inflammatory drugs and how their varied actions may contribute to the safety profiles of these agents. We then discuss newer agents that act specifically on the IL-1β pathway.
Nonsteroidal Anti-inflammatory Drugs
Prostaglandins generated by the inducible COX-2 enzyme play a major role in the stimulation of inflammatory responses and contribute to the development of the cardinal signs of acute inflammation in gouty arthritis attacks [3,50]. Unlike COX-1, which is expressed constitutively in most cells and is responsible for homeostatic functions (including epithelial cytoprotection, platelet aggregation, and regulation of renal blood flow), COX-2 is the product of an immediate-early gene that is rapidly inducible and tightly regulated [51]. COX-2 is the dominant source of prostaglandins in inflammation. However, recent evidence suggests that both COX-1 and COX-2 may contribute to prostanoid production during both acute inflammatory responses and the resolution phase of inflammation [3]. COX-1 accounts for approximately 10% to 15% of prostanoid formation induced by lipopolysaccharide, and both COX-1 and COX-2 are expressed in circulating inflammatory cells ex vivo [52]. Human data indicate that COX-1–derived prostanoids drive the initial phase of acute inflammation, while COX-2 upregulation may not occur until several hours later [3,52]. The roles of different prostanoids formed by COX-1 and/or COX-2 are extremely complex; depending on whether a given prostanoid is formed by COX-1 or COX-2, the same molecule may either stimulate or resolve inflammation [3].
The biological consequences of COX inhibition with NSAIDs are potentially broad-ranging and not well understood. It is hypothesized that NSAID-based COX inhibition can be tolerated in most patients because prostanoid formation is a homeostatic response system. Under most physiologic conditions, only small amounts of prostanoids are formed, and their biological importance may be minimal. However, under conditions of physiologic stress (ie, in elderly patients and patients with comorbid renal, cardiovascular, or gastrointestinal conditions), alterations in prostanoid expression are associated with increased safety concerns [3]. These safety concerns can be particularly problematic when relatively high doses of NSAIDs are prescribed, as is common practice in the management of acute gouty arthritis attacks [53].
Safety of Nonsteroidal Anti-inflammatory Drugs
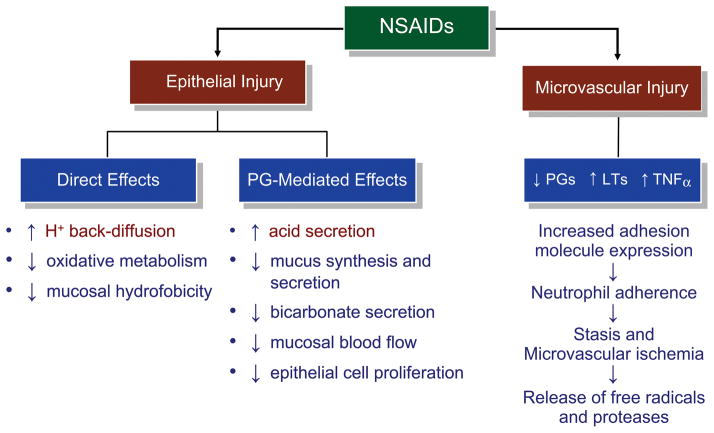
It is well established that NSAIDs are associated with an increased risk of gastrointestinal side effects. The mechanisms associated with NSAID-induced gastric damage are outlined in Figure 2 [43]. Most nonselective NSAIDs that inhibit both COX-1 and COX-2 are organic acids, and their ulcerogenic potential is associated with their pKa and lipophilicity. NSAIDs with pKa values between approximately 2.8 and 4.4 are most likely to cause ulcers, as are lipophilic NSAIDs that interact with phospholipids and disrupt gastric mucosal membranes. In contrast, most selective COX-2 inhibitors are not acidic and have much higher pKa values. Thus, selective COX-2 inhibitors are less likely than nonselective NSAIDs to cause gastrointestinal mucosal irritation [43]. However, the United States Food and Drug Administration does not distinguish between the gastrointestinal safety profiles of selective and nonselective NSAIDs and applies the same package insert warning labels for all NSAIDs.
Figure 2. Mechanisms of NSAID-induced gastric damage [43].
Figure reproduced with permission from Scarpignato et al. Gastroenterol Clin North Am. 2010;39:433–464. ≪Note: permission to be provided upon acceptance≫
NSAIDs cause both epithelial and microvascular injury, which occur early in the series of pathophysiologic events leading to gross gastrointestinal lesion formation. This injury is mainly caused by increased leukotriene mucosal concentration, with consequent increased expression of adhesion molecules, leading to microvascular ischemia and free radical release. Epithelial damage is caused by both topical (direct) and systemic (prostaglandin-mediated) effects of NSAIDs. Reduction in prostaglandin mucosal concentration is followed by impairment of the gastric mucosal barrier and an increase in acid secretion, which tips the balance between aggressive and defensive factors toward the former.
LT, leukotriene; NSAID, nonsteroidal anti-inflammatory drug; PG, prostaglandin; TNF-α, tumor necrosis factor-α.
The comparative incidence of serious gastrointestinal events associated with selective COX-2 inhibitors is roughly half that of nonselective NSAIDs [3]. However, selective COX-2 inhibitors are still associated with the potential to cause serious gastrointestinal events in high-risk patients, as these inhibitors block the synthesis of gastroduodenal epithelial COX-2–dependent prostanoids that accelerate ulcer healing [3]. In addition, when COX pathways are blocked by NSAIDs, some arachidonic acid is diverted through the lipoxygenase (LOX) pathway, which increases leukotriene synthesis, which can further propagate mucosal damage.
Selective COX-2 inhibitors were developed to reduce the risk of gastrointestinal complications associated with nonselective NSAIDs; however, selective COX-2 inhibitors were found to be associated with an increased risk of cardiovascular events Of note, 2 widely used COX-2 inhibitors, rofecoxib and valdecoxib, were withdrawn from the global market due to cardiovascular safety concerns [54]. The cardiovascular risks associated with selective COX-2 inhibitors have been confirmed in several studies [55–57], and these findings have recently been extended to some nonselective NSAIDs [44,54,58]. The cardiovascular risks associated with NSAIDs (including increased risk of recurrent myocardial infarction and death) have been observed even with short-term use (ie, <1 week), as is common for the management of acute gouty arthritis flares [54].
COX-2 inhibition promotes cardiovascular injury through several mechanisms. Selective COX-2 inhibition leads to a loss of the majority of COX-2–derived vascular prostacyclin synthesis without alteration in platelet thromboxane synthesis [51]. Prostacyclin is a potent vasodilator and inhibitor of platelet aggregation, leukocyte adhesion, and vascular smooth muscle cell proliferation in the kidneys, liver, lungs, and heart. Therefore, suppression of COX-2–derived prostacyclin by both nonselective NSAIDs and selective COX-2 inhibitors increases the risk of thrombosis, hypertension, atherosclerosis, and myocardial infarction [3,51].. It has also been hypothesized that the increase in leukotriene biosynthesis that results from the shunting of arachidonic acid to the LOX pathway may increase the risk of atherosclerosis [51,59].
COX-1 and COX-2 are both expressed in the kidneys. The effects of NSAID-induced inhibition of renal prostaglandin E2 can result in sodium retention, edema, and exacerbation of hypertension. Inhibition of prostacyclin expression can decrease renal blood flow and glomerular filtration rate, which can lead to hyperkalemia as a result of decreased potassium excretion. COX-2 inhibition can be especially problematic in elderly patients, in whom COX-2 expression has been detected in the macula [60].
Colchicine
The earliest recorded use of colchicine to treat gouty arthritis dates back to the ancient Greeks and Egyptians (extracts of Colchicum autumnalis), and colchicine has been used extensively for the treatment of acute gouty arthritis flares since the 18th century [61]. Colchicine is an antimitotic alkaloid that binds to specific sites on the cytoskeletal protein tubulin and disrupts microtubule polymerization. This disruption of normal cytoskeletal assembly results in a range of biologic effects on essential cell functions, including inhibition of intracellular vesicle transport, decreased secretion of chemokines and cytokines, impairment of cell migration, and inhibition of cell division [62].
In line with the current understanding of the pathogenesis of gouty arthritis, in vitro studies have shown that high concentrations of colchicine suppress inflammation by blocking IL-1β processing in monocytes stimulated by MSU, but do not affect IL-1β activation by extracellular adenosine triphosphate. This suggests that colchicine acts upstream of NALP3 inflammasome-driven caspase-1 activation [8]. Despite these findings, it is unlikely that this mechanism accounts for colchicine’s therapeutic effects in crystal-driven inflammation, because these effects have only been observed at colchicine concentrations that are 10-fold to 100-fold higher than those achieved in patients during the treatment of acute gouty arthritis and 100-fold to 1000-fold higher than the concentrations required to mediate prophylaxis of chronic gouty arthritis [8,62,63].
Other studies have reported that pharmacologically relevant concentrations of colchicine directly inhibit intracellular signaling molecules (eg, tyrosine kinases and phospholipases) in neutrophils that inhibit some, but not all, of the inflammatory actions of these cells (eg, chemotaxis, superoxide anion production, adhesion to cellular substrata, and mobilization and release of lysosomal enzymes during phagocytosis) [6,64–70]. Evidence also shows that colchicine inhibits neutrophil migration following crystal activation without changes in production of the chemokine IL-8 [71]. Colchicine induces COX-1 and COX-2 gene expression and does not inhibit COX-1 or COX-2 in neutrophils [72].
Previous observations have shown that concentrations of colchicine that are similar to those achieved during prophylaxis of acute attacks (ie, nanomolar concentrations) alter the expression of endothelial adhesion molecules (E-selectin) on cells required for the recruitment of neutrophils [73], thereby suppressing the development of acute gouty arthritis. At higher concentrations, colchicine induces shedding of neutrophil adhesion molecules (L-selectin), thus preventing further neutrophil recruitment [73]. All of the actions of colchicine in this setting have been attributed to the capacity of colchicine to disrupt microtubules.
Safety of Colchicine
Colchicine has a narrow therapeutic index between efficacy and treatment-limiting gastrointestinal side effects, including diarrhea and abdominal pain caused by increased peristaltic activity [42,62]. The pharmacokinetics and safety of colchicine are driven in large part by its binding to tubulin. Because tubulin-bound colchicine has a slow dissociation rate, the half-life of this complex is approximately 20 to 30 hours. After colchicine therapy is discontinued, its terminal elimination half-life is roughly 16 hours and the biologic effects of colchicine require 24 to 48 hours to dissipate [62]. Colchicine’s long half-life may contribute to its narrow therapeutic margin (particularly in patients with renal or hepatic impairment), as colchicine is predominantly metabolized in the liver and up to 20% of an administered dose is cleared by the kidneys. The half-life of colchicine in patients with severe renal impairment is 2-fold to 3-fold longer compared with patients with normal renal function, which significantly increases the risk of colchicine accumulation and toxicity [62,74].
Colchicine is metabolized in the liver by the cytochrome P450 enzyme CYP3A4. Therefore, interactions between colchicine and drugs with CYP3A4 inhibitory activity have the potential to cause colchicine accumulation, increase colchicine’s pharmacologic effects, and predispose patients to colchicine toxicity. Drug-drug interactions have been observed between colchicine and CYP3A4 inhibitors including cimetidine, clarithromycin, erythromycin, fluoxetine, paroxetine, nefazodone, indinavir and other protease inhibitors, tolbutamide, and azole antifungals [62,63]. For example, cimetidine decreases the hepatic clearance of colchicine by roughly 30%, which prolongs the plasma elimination half-life [62], and clarithromycin markedly increases colchicine exposure, which has been associated with fatalities in patients with renal insufficiency [75].
Colchicine also binds to P-glycoprotein (P-gp), an adenosine triphosphate-binding protein widely distributed in cell membranes in the intestinal epithelium, biliary tract, blood-brain barrier, and renal proximal tubules. P-gp influences absorption, bioavailability, and elimination of its substrates [62]. These substrates include chemotherapeutic agents, macrolide antibiotics, protease inhibitors, and glucocorticoids, as well as some statins (eg, simvastatin and fluvastatin) and calcium-channel blockers (eg, verapamil) that are commonly prescribed in patients with cardiovascular comorbidities associated with gouty arthritis [62]. Similar to CYP3A4 inhibitors, the use of P-gp modulating agents in combination with colchicine can lead to intracellular colchicine accumulation, with increases in pharmacologic effects or toxicity [62].
Colchicine’s interactions with microtubules can cause accumulation of lysosomes and autophagic vacuoles in the cytoskeleton, resulting in pathologic alterations in skeletal muscle and induction of significant axonal neuropathy. These adverse consequences may present as myopathy (eg, rhabdomyolysis), neuropathy, or bone marrow suppression [76]. Although the likelihood of serious AEs associated with colchicine is generally considered to be dose-dependent (ie, mortality rates are estimated at 10% at doses >0.5 mg/kg) [42,62], an analysis by Wilbur and Makowsky [76] identified cases of colchicine-induced myotoxicity at lower doses. In the 75 cases identified, the mean (standard deviation) cumulative daily dose of colchicine was only 1.4 (0.96) mg [76] (for comparison, standard therapeutic doses of colchicine for acute gouty arthritis attacks are 1.2 mg at the first sign of a flare followed by 0.6 mg 1 hour later) [63]. In many of the cases, patients had been receiving standard doses of colchicine for long periods of time but experienced recent declines in renal function or other underlying conditions [76].
Glucocorticoids
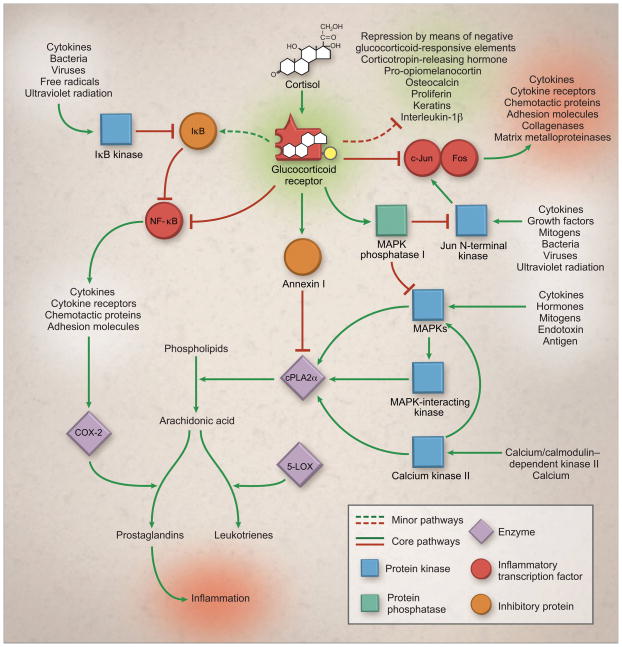
Glucocorticoids have long been used to treat acute gouty arthritis attacks, particularly in patients who are intolerant to NSAIDs or colchicines [48]. This class of agents has many well-described anti-inflammatory actions that are mediated through binding interactions with glucocorticoid receptors, which are localized in the cytoplasm of target cells found in almost all tissues in the human body [77,78]. The complex molecular architecture of glucocorticoid-induced antagonism of inflammation is outlined in Figure 3.
Figure 3. Partial molecular architecture underlying the glucocorticoid-induced antagonism of inflammation [80].
Figure reproduced with permission from Rhen et al. N Engl J Med. 2005;353:1711–1723. ≪Note: permission to be provided upon acceptance≫
Inflammatory pathways are characterized by positive feedback loops (ie, cytokines activate NF-κB, which in turn stimulates the synthesis of more cytokines) and by redundancy (ie, cytokines also activate c-Jun–Fos). The glucocorticoid receptor inhibits these pathways at multiple points by directly blocking the transcription of inflammatory proteins by NF-κB and activator protein 1 and by inducing the expression of anti-inflammatory proteins such as IκB, annexin I, and MAPK phosphatase I. Red lines denote inhibition, and black arrows activation.
COX-2 cyclooxygenase 2; cPLA2α, cytosolic phospholipase A2α; IκB, inhibitor of κB; 5-LOX, 5-lipoxygenase; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-κB.
The clinical success of glucocorticoids as effective anti-inflammatory agents is largely attributed to their ability to reduce the expression of pro-inflammatory genes via activation of the glucocorticoid receptor and the concomitant inhibition pro-inflammatory transcription factors (eg, NFκB and activating protein-1 [AP-1]) through a mechanism known as transrepression [78]. In acute gouty arthritis, the most notable anti-inflammatory action of glucocorticoids is the capacity of these agents to prevent activation of NFκB by either TNF-α or IL-1β. Glucocorticoids increase the expression of the inhibitor of κB (IκB), which is the cytoplasmic chaperone that prevents translocation of NFκB to the nucleus [79]. Thus, glucocorticoids prevent IL-1β from stimulating the inflammatory cascade.
However, much of the anti-inflammatory efficacy of glucocorticoids results from pleiotropic effects of the glucocorticoid receptor on diverse signaling pathways through direct and indirect genomic effects. This pleiotropy, along with known nongenomic effects involving second-messenger and membrane-associated receptor signaling, can result in a broad range of side effects [80]. In addition to NFκB and AP-1, other transcription factors that are negatively regulated by the glucocorticoid receptor via transrepression include cyclic adenosine monophosphate (cAMP) response element-binding protein, interferon regulatory factor 3, nuclear factor of activated T cells, signal transducer and activator of transcription, T-box expressed in T cells, and GATA-binding protein 3. Target genes involved in transrepression include those encoding for a broad range of inflammatory cytokines, enzymes, receptors, and adhesion molecules, notably, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-12, COX-2, E-selectin, and TNF-α [77,78]. In addition, glucocorticoids can suppress inflammation through transactivation of genes that are associated with increased synthesis of anti-inflammatory proteins, including lipocortin-1, IL-1 receptor agonist, IκB, and β2-adrenoceptor [77,80].
Safety of Glucocorticoids
Side effects associated with glucocorticoid therapy mainly arise from the ability of the steroid-activated glucocorticoid receptor to activate target genes involved in the metabolism of sugar, protein, fat, muscle, and bone via transactivation and to suppress the hypothalamic-pituitary-adrenal axis via transrepression [78,81]. The most common AEs associated with steroid use for the management of acute gouty arthritis attacks are typically related to the complex effects of glucocorticoids on metabolism and the endocrine system. The most clinically relevant metabolic consequence associated with glucocorticoid therapy is generally considered to be hyperglycemia related to glucocorticoid-induced upregulation in glucose synthesis. This increase in glucose synthesis results from transactivation of a complex network of hepatic enzymes that control gluconeogenesis, mobilization and degradation of proteins, and increased glycogen storage in the liver [78,81].
Glucocorticoid therapy is also associated with adverse cardiovascular effects, most notably, hypertension, dyslipidemia, and reduced fibrinolytic potential [81,82]. These AEs are most common in patients treated with high doses of glucocorticoids and in elderly patients with a family history of essential hypertension [82]. The relationship between glucocorticoids and blood pressure regulation is complex; however, it is thought that the primary mechanisms involved are increases in systemic vascular resistance, extracellular volume, and cardiac contractility [81,82].
Development of psychiatric problems and aggravation of pre-existing psychoses in patients receiving acute glucocorticoid therapy have also been reported; moreover, when acute treatment is discontinued, patients may experience psychiatric withdrawal symptoms, including fatigue and depression [80,81]. These symptoms are more common in women and usually develop within 2 weeks of beginning treatment, particularly with doses of prednisolone that are above 40 mg/day. Glucocorticoids can also have reversible adverse effects on memory and cognition. The underlying mechanisms associated with steroid-induced psychoses are thought to be related to hippocampal damage caused by direct glucocorticoid exposure, including decreased dendritic branching, altered synaptic structures, neuron loss, and inhibition of neuronal regeneration. Glucocorticoids can also cause hypothalamic-pituitary-adrenal axis function abnormalities and dysregulation of the serotonin (5-HT) system. One of the most important mechanisms associated with the pathophysiology of depression is suppression of the 5-HT1A receptor, which occurs via glucocorticoid receptor-mediated transrepression of NFκB [81].
Despite the use of glucocorticoids in clinical practice, the actual incidence of AEs when they are used as a short-term course in gouty arthritis is not known. Recently, the European League Against Rheumatism (EULAR) developed recommendations to monitor AEs associated with low-dose glucocorticoid therapy for rheumatoid arthritis. Per these recommendations, patients treated with glucocorticoid therapy should be monitored for diabetes/glucose intolerance, hypertension, electrolyte shifts, infections, mood changes, and mental problems, which may occur as possible side effects of steroid treatment [83]. Whether similar recommendations need to be followed while using steroids during an acute attack of gouty arthritis is not clear; however, since gouty arthritis is often associated with comorbidities, the occurrence of some of these side effects cannot be ruled out. Also, some of these AEs can occur following single injections of intra-articular or intramuscular corticosteroids. Therefore, patients receiving glucocorticoids for the management of acute gouty arthritis must be observed carefully.
Biologic Therapies: Interleukin-1β Antagonists
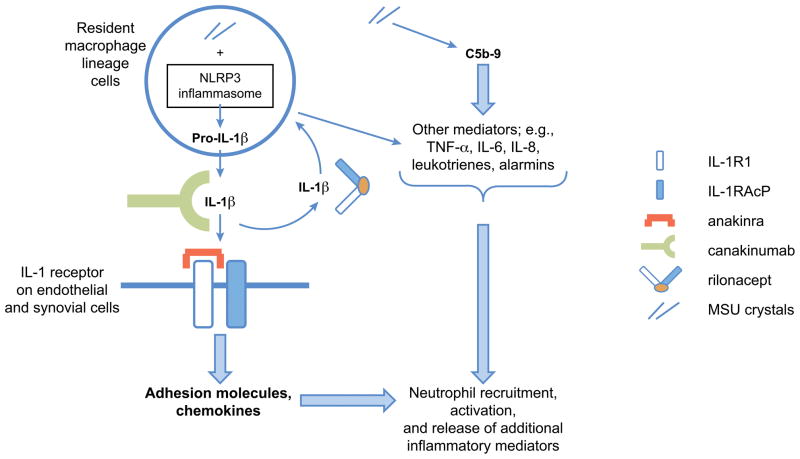
From the earlier discussion of the role of IL-1β in inflammation, it is clear that agents that target IL-1β or prevent the actions of IL-1β on cells are likely to be useful therapies for the treatment or prevention of acute gouty attacks. As noted above, binding of IL-1β by either rilonacept or canakinumab or binding of IL-1R1 by anakinra may be effective mechanisms for treating or preventing acute gouty arthritis attacks (Figure 4) [33].
Figure 4. Mechanisms of inflammation and interleukin-1 antagonism in acute gouty arthritis [33].
Figure reproduced with permission from Neogi. Arthritis Rheum. 2010;62:2845–2849. ≪Note: permission to be provided upon acceptance≫
In acute gouty arthritis, phagocytosed MSU crystals activate the NALP3 inflammasome, leading to caspase-1 activation, which in turn leads to cleavage of proIL-1β and secretion of mature IL-1β. IL-1β must bind to both IL-1R1 and IL-1RAcP for signal transduction to occur. In endothelial cells, IL-1β appears to be a major trigger for altered adhesion molecule and chemokine expression, which, together with other inflammatory events, results in neutrophil recruitment that drives the initiation of gouty inflammation. Anakinra binds to IL-1R1, blocking IL-1β and IL-1α; rilonacept acts as a soluble receptor, comprising both IL-1R1 and IL-1RAcP fused to the Fc portion of immunoglobulin G1, neutralizing IL-1β as well as IL-1α; and canakinumab, a monoclonal antibody with IL-1β specificity, neutralizes IL-1β only.
IL, interleukin; IL-1R1, IL-1 receptor type 1; IL-1RAcP, IL-1 receptor accessory protein; MSU, monosodium urate; TNF-α, tumor necrosis factor-α.
Although all 3 agents target IL-1β, their MOAs are different. Anakinra is an IL-1R antagonist that binds to IL-1R1 and blocks IL-1β and IL-1α [84]. Rilonacept is a recombinant dimeric fusion protein consisting of portions of IL-1R and the IL-1R accessory protein linked to the Fc portion of IgG1. Rilonacept acts as a receptor to neutralize both IL-1β and IL-1α and as a soluble decoy receptor [85]. Canakinumab is a fully human monoclonal antibody that binds to human IL-1β and neutralizes its activity by blocking its interaction with IL-1 receptors. However, it does not bind IL-1α or IL-1 receptor antagonist (IL-1RA) [86]. The specificity of these agents in targeting IL-1β may have positive implications for safety and tolerability.
Published studies in gout include two phase III trials for canakinumab [89] and one phase III trial for rilonacept [90]. Results suggest that these biologic therapies may provide improvement in pain and inflammation associated with acute gouty arthritis attacks. There are no published trials evaluating anakinra in patients with gouty arthritis.
Safety of Interleukin-1β Antagonists
Early safety findings from phase II trials of canakinumab and rilonacept (8–16 weeks’ duration) and a pilot study of anakinra (4-weeks’ duration) demonstrated that IL-1β antagonists are generally well tolerated in patients with gouty arthritis [29,32,88,91], although infrequent but serious AEs, including infections and administration reactions, have been reported. Safety results of recently published phase III trials (of short duration of 16–24 weeks) for canakinumab and rilonacept are consistent with these earlier phase II results [89,90]. In the canakinumab phase III trials [89], the most common AEs were back pain, hypertension, and headache. In the rilonacept phase III trial [90], the most common AEs were injection-site reactions, upper respiratory tract infections, and headache. As mentioned above, there are no published phase II or III trials evaluating anakinra in patients with gouty arthritis.
As with other biologics, the potential for increased bacterial infections is of concern with IL-1 antagonist [92], with the immunosuppressant effect of these agents likely the mechanism for the increased risk [93]. In the phase III trials, infections were reported in 20.4% of patients receiving canakinumab and 12.2% of patients receiving the active comparator (triamcinolone acetonide) [89], and in 18.0% of patients receiving rilonacept and 22.8% of patients receiving placebo [90]. No opportunistic infections were reported with either of these agents. As there are no head-to-head comparative gouty arthritis studies between rilonacept, canakinumab, and anakinra, no safety comparisons can be made. Of note, none of these agents are yet approved by the FDA for gouty arthritis. A long-term study of rilonacept is ongoing (NCT01459796) and a long-term study of canakinumab has recently been completed (NCT00927810), both in gouty arthritis. Results, when available, will provide information on the long-term safety of these agents in this patient population.
Conclusion
The NALP3 inflammasome and IL-1β are crucial players in the inflammatory pathway associated with acute gouty arthritis attacks [8]. While the traditional agents, NSAIDs, colchicine, and glucocorticoids, may act on this pathway at some level, their MOAs are not specific. The anti-inflammatory actions of these agents are broad-ranging and biologically complex. In many cases, these agents target pathways that are high upstream in the inflammatory process [3,8]. Although inhibition of these upstream pathways produces a net benefit in reducing pain and inflammation for most patients with acute gouty arthritis, the benefit/risk margin of these agents is generally low and varies substantially from one individual to another. The therapeutic margin for colchicine is always very low, with the potential for serious AEs in patients with renal or hepatic comorbidities and in patients taking other drugs that inhibit CYP3A4 or P-gp [62]. The therapeutic margin for NSAIDs may be particularly low in elderly patients and in patients with comorbidities [3]. The incidence of AEs associated with short courses of glucocorticoid therapy for the treatment of acute gouty arthritis is not known; however, glucocorticoids act on many body systems and based on recent guidelines, patients treated with glucocorticoids should be monitored for diabetes/glucose intolerance, hypertension, electrolyte shifts, infections, mood changes, and mental problems [78,81,83]. NSAIDs, colchicine, and glucocorticoids may have contrasting effects in different body systems, which may contribute to the wide array of AEs associated with these drugs. For these reasons, it has been suggested that more specific, downstream targeting of the inflammation pathway may result in safer and more effective therapies [3].
The IL-1β antagonists being studied for the management of acute gouty arthritis attacks (anakinra, rilonacept, and canakinumab) act on very specific targets of inflammation [33]. Because these agents target an early event immediately downstream from NALP3 inflammasome activation by MSU crystals, it is possible that they will provide effective alternatives to the less specific traditional agents without the associated systemic side effects. Data from long-term studies will further elucidate the potential role of IL-1β antagonism in the management of acute gouty arthritis.
Acknowledgments
Source of funding: Certain sections of the manuscript, including the “Introduction” and the sections titled “Inflammation, an Overview,” “Monosodium Urate Crystals and Inflammation,” and “Termination of Acute Gouty Arthritis,” were written by Dr. Cronstein. Sections focusing on the clinical management of gouty arthritis were prepared by Dr. Sunkureddi, with editorial assistance provided by Cherie Koch, PhD, of Oxford PharmaGenesis Inc. Each author’s contribution was merged together by Dr. Koch who provided editorial assistance to improve the flow and eliminate any redundancies. Dr. Koch also provided assistance with figure preparation and styling of the manuscript for submission. Funding for this assistance was provided by Novartis Pharmaceuticals Corporation. The authors were fully responsible for all content and editorial decisions and received no financial support or other form of compensation related to the development of this manuscript. The opinions expressed in the manuscript are those of the authors, and Novartis Pharmaceuticals Corporation had no influence on the contents.
Footnotes
Conflicts of Interest:
BN Cronstein, MD: Consultant to: Bristol-Myers Squibb, Novartis, CanFite Biopharmaceuticals, Cypress Laboratories, Regeneron (Westat, DSMB), Endocyte, Protalex, Allos, Inc., Savient, Gismo Therapeutics, Antares Pharmaceutical, and Medivector (all < $10,000). Grants received from: King Pharmaceuticals, NIH, Vilcek Foundation, OSI Pharmaceuticals, URL Pharmaceuticals, Inc., and Gilead Pharmaceuticals. Board member of: Vilcek Foundation. Stock held in: CanFite Biopharmaceuticals (received for membership in Scientific Advisory Boards). Four patents held on the use of: adenosine A2A receptor agonists to promote wound healing and to inhibit fibrosis; adenosine A1 receptor antagonists to treat osteoporosis and other diseases of bone; adenosine A1 and A2B receptor antagonists to treat fatty liver; and adenosine A2A receptor agonists to prevent prosthesis loosening.
P. Sunkureddi, MD, PA: Speaker’s Bureau/Consultant to: Novartis, Bristol Myers Squibb, UCB, Pfizer (all >$10,000).
Contributor Information
Bruce N. Cronstein, Email: Bruce.Cronstein@nyumc.org, Paul R. Esserman Professor of Medicine, NYU School of Medicine, 550 First Ave., NBV16N1, New York, NY 10016, USA, Telephone: 1-212-263-6404.
Prashanth Sunkureddi, Email: psunkureddi@gmail.com, Clear Lake Rheumatology, 2060 Space Park Drive, Suite 208, Nassau Bay, TX 77058, USA, Telephone: 1-281-957-9127; Fax: 1-281-957-9157.
Reference List
- 1.Garrod AB. Researches on Gout.-Part I. The Urine in the Different Forms of Gout.-Part II. The Influence of Colchicum upon the Urine. Med Chir Trans. 1858;41:325–360. doi: 10.1177/095952875804100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steele AD, McCarty DJ., Jr An experimental model of acute inflammation in man. Arthritis Rheum. 1966;9:430–442. doi: 10.1002/art.1780090308. [DOI] [PubMed] [Google Scholar]
- 3.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Giovine FS, Malawista SE, Nuki G, et al. Interleukin 1 (IL 1) as a mediator of crystal arthritis. Stimulation of T cell and synovial fibroblast mitogenesis by urate crystal-induced IL 1. J Immunol. 1987;138:3213–3218. [PubMed] [Google Scholar]
- 5.Di Giovine FS, Malawista SE, Thornton E, et al. Urate crystals stimulate production of tumor necrosis factor alpha from human blood monocytes and synovial cells. Cytokine mRNA and protein kinetics, and cellular distribution. J Clin Invest. 1991;87:1375–1381. doi: 10.1172/JCI115142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert C, Poubelle PE, Borgeat P, et al. Crystal-induced neutrophil activation: VIII. Immediate production of prostaglandin E2 mediated by constitutive cyclooxygenase 2 in human neutrophils stimulated by urate crystals. Arthritis Rheum. 2003;48:1137–1148. doi: 10.1002/art.10851. [DOI] [PubMed] [Google Scholar]
- 7.Cronstein BN, Weissmann G. The adhesion molecules of inflammation. Arthritis Rheum. 1993;36:147–157. doi: 10.1002/art.1780360204. [DOI] [PubMed] [Google Scholar]
- 8.Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 9.Terkeltaub R, Baird S, Sears P, et al. The murine homolog of the interleukin-8 receptor CXCR-2 is essential for the occurrence of neutrophilic inflammation in the air pouch model of acute urate crystal-induced gouty synovitis. Arthritis Rheum. 1998;41:900–909. doi: 10.1002/1529-0131(199805)41:5<900::AID-ART18>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.Baker JF, Schumacher HR. Update on gout and hyperuricemia. Int J Clin Pract. 2010;64:371–377. doi: 10.1111/j.1742-1241.2009.02188.x. [DOI] [PubMed] [Google Scholar]
- 11.Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364:443–452. doi: 10.1056/NEJMcp1001124. [DOI] [PubMed] [Google Scholar]
- 12.Martin WJ, Walton M, Harper J. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheum. 2009;60:281–289. doi: 10.1002/art.24185. [DOI] [PubMed] [Google Scholar]
- 13.Schiltz C, Lioté F, Prudhommeaux F, et al. Monosodium urate monohydrate crystal-induced inflammation in vivo: quantitative histomorphometric analysis of cellular events. Arthritis Rheum. 2002;46:1643–1650. doi: 10.1002/art.10326. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz-Bravo E, Sieck MS, Schumacher HR., Jr Changes in the proteins coating monosodium urate crystals during active and subsiding inflammation. Immunogold studies of synovial fluid from patients with gout and of fluid obtained using the rat subcutaneous air pouch model. Arthritis Rheum. 1993;36:1274–1285. doi: 10.1002/art.1780360912. [DOI] [PubMed] [Google Scholar]
- 15.Cherian PV, Schumacher HR., Jr Immunochemical and ultrastructural characterization of serum proteins associated with monosodium urate crystals (MSU) in synovial fluid cells from patients with gout. Ultrastruct Pathol. 1986;10:209–219. doi: 10.3109/01913128609032219. [DOI] [PubMed] [Google Scholar]
- 16.Russell IJ, Papaioannou C, McDuffie FC, et al. Effect of IgG and C-reactive protein on complement depletion by monosodium urate crystals. J Rheumatol. 1983;10:425–433. [PubMed] [Google Scholar]
- 17.Hasselbacher P, Schumacher HR. Immunoglobulin in tophi and on the surface of monosodium urate crystals. Arthritis Rheum. 1978;21:353–361. doi: 10.1002/art.1780210311. [DOI] [PubMed] [Google Scholar]
- 18.Naff GB, Byers PH. Complement as a mediator of inflammation in acute gouty arthritis. I. Studies on the reaction between human serum complement and sodium urate crystals. J Lab Clin Med. 1973;81:747–760. [PubMed] [Google Scholar]
- 19.Tramontini N, Huber C, Liu-Bryan R, et al. Central role of complement membrane attack complex in monosodium urate crystal-induced neutrophilic rabbit knee synovitis. Arthritis Rheum. 2004;50:2633–2639. doi: 10.1002/art.20386. [DOI] [PubMed] [Google Scholar]
- 20.Scott P, Ma H, Viriyakosol S, et al. Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals. J Immunol. 2006;177:6370–6378. doi: 10.4049/jimmunol.177.9.6370. [DOI] [PubMed] [Google Scholar]
- 21.Joosten LA, Netea MG, Fantuzzi G, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009;60:3651–3662. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [Epub 2011 Feb 8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinarello CA. How interleukin-1β induces gouty arthritis. Arthritis Rheum. 2010;62:3140–3144. doi: 10.1002/art.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joosten LA, Netea MG, Mylona E, et al. Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1β production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis. Arthritis Rheum. 2010;62:3237–3248. doi: 10.1002/art.27667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez RV, Reval M, Campos MD, et al. Involvement of peripheral cyclooxygenase-1 and cyclooxygenase-2 in inflammatory pain. J Pharm Pharmacol. 2002;54:405–412. doi: 10.1211/0022357021778475. [DOI] [PubMed] [Google Scholar]
- 26.Pouliot M, James MJ, McColl SR, et al. Monosodium urate microcrystals induce cyclooxygenase-2 in human monocytes. Blood. 1998;91:1769–1776. [PubMed] [Google Scholar]
- 27.Hasselbacher P. Stimulation of synovial fibroblasts by calcium oxalate and monosodium urate monohydrate. A mechanism of connective tissue degradation in oxalosis and gout. J Lab Clin Med. 1982;100:977–985. [PubMed] [Google Scholar]
- 28.McMillan RM, Hasselbacher P, Hahn JL, et al. Interactions of murine macrophages with monosodium urate crystals: stimulation of lysosomal enzyme release and prostaglandin synthesis. J Rheumatol. 1981;8:555–562. [PubMed] [Google Scholar]
- 29.So A, De Smedt T, Revaz S, et al. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.So A, De Meulemeester M, Pikhlak A, et al. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: results of a multicenter, phase II, dose-ranging study. Arthritis Rheum. 2010;62:3064–3076. doi: 10.1002/art.27600. [DOI] [PubMed] [Google Scholar]
- 31.Neogi T. Interleukin-1 antagonism in acute gout: is targeting a single cytokine the answer? Arthritis Rheum. 2010;62:2845–2849. doi: 10.1002/art.27635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CJ, Shi Y, Hearn A, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–2271. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yagnik DR, Evans BJ, Florey O, et al. Macrophage release of transforming growth factor β1 during resolution of monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2004;50:2273–2280. doi: 10.1002/art.20317. [DOI] [PubMed] [Google Scholar]
- 34.Savill JS, Wyllie AH, Henson JE, et al. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sunkureddi P. Gouty arthritis: understanding the disease state and management options in primary care. Adv Ther. 2011;28:748–760. doi: 10.1007/s12325-011-0058-5. [DOI] [PubMed] [Google Scholar]
- 36.Terkeltaub RA, Furst DE, Bennett K, et al. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62:1060–1068. doi: 10.1002/art.27327. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez EB. An update on the pathology and clinical management of gouty arthritis. Clin Rheumatol. 2012;31:13–21. doi: 10.1007/s10067-011-1877-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janssens HJ, Lucassen PL, Van de Laar FA, et al. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):Art. No:CD005521. doi: 10.1002/14651858.CD005521.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2006;65:1312–1324. doi: 10.1136/ard.2006.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richette P, Bardin T. Colchicine for the treatment of gout. Expert Opin Pharmacother. 2010;11:2933–2938. doi: 10.1517/14656566.2010.529432. [DOI] [PubMed] [Google Scholar]
- 41.Scarpignato C, Hunt RH. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol Clin North Am. 2010;39:433–464. doi: 10.1016/j.gtc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Salvo F, Fourrier-Réglat A, Bazin F, et al. Cardiovascular and gastrointestinal safety of NSAIDs: a systematic review of meta-analyses of randomized clinical trials. Clin Pharmacol Ther. 2011;89:855–866. doi: 10.1038/clpt.2011.45. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Doherty M, Pascual E, et al. EULAR evidence based recommendations for gout. Part I: Diagnosis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2006;65:1301–1311. doi: 10.1136/ard.2006.055251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards NL. The role of hyperuricemia and gout in kidney and cardiovascular disease. Cleve Clin J Med. 2008;75(Suppl 5):S13–S16. doi: 10.3949/ccjm.75.suppl_5.s13. [DOI] [PubMed] [Google Scholar]
- 45.Keenan RT, O’Brien WR, Lee KH, et al. Prevalence of contraindications and prescription of pharmacologic therapies for gout. Am J Med. 2011;124:155–163. doi: 10.1016/j.amjmed.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30–38. doi: 10.1038/nrrheum.2009.236. [DOI] [PubMed] [Google Scholar]
- 47.Richette P, Bardin T. Should prednisolone be first-line therapy for acute gout? Lancet. 2008;372:1301–1302. doi: 10.1016/S0140-6736(08)61548-2. [DOI] [PubMed] [Google Scholar]
- 48.Vane JR. Introduction: mechanism of action of NSAIDs. Br J Rheumatol. 1996;35(Suppl 1):1–3. doi: 10.1093/rheumatology/35.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- 49.Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol. 2007;50:470–479. doi: 10.1097/FJC.0b013e318157f72d. [DOI] [PubMed] [Google Scholar]
- 50.Smyth EM, Grosser T, Wang M, et al. Prostanoids in health and disease. J Lipid Res. 2009;50(Suppl):S423–S428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cronstein BN, Terkeltaub R. The inflammatory process of gout and its treatment. Arthritis Res Ther. 2006;8(Suppl 1):S3. doi: 10.1186/ar1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: a nationwide cohort study. Circulation. 2011;123:2226–2235. doi: 10.1161/CIRCULATIONAHA.110.004671. [DOI] [PubMed] [Google Scholar]
- 53.Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 54.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 55.Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081–1091. doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- 56.Fosbol EL, Gislason GH, Jacobsen S, et al. Risk of myocardial infarction and death associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) among healthy individuals: a nationwide cohort study. Clin Pharmacol Ther. 2009;85:190–197. doi: 10.1038/clpt.2008.204. [DOI] [PubMed] [Google Scholar]
- 57.Lötzer K, Funk CD, Habenicht AJ. The 5-lipoxygenase pathway in arterial wall biology and atherosclerosis. Biochim Biophys Acta. 2005;1736:30–37. doi: 10.1016/j.bbalip.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Pham K, Hirschberg R. Global safety of coxibs and NSAIDs. Curr Top Med Chem. 2005;5:465–473. doi: 10.2174/1568026054201640. [DOI] [PubMed] [Google Scholar]
- 59.Cronstein BN. Something old, something new--colchicine in the 21st century. Curr Opin Investig Drugs. 2009;10:1141–1142. [PubMed] [Google Scholar]
- 60.Nuki G. Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Curr Rheumatol Rep. 2008;10:218–227. doi: 10.1007/s11926-008-0036-3. [DOI] [PubMed] [Google Scholar]
- 61.Colcrys (colchicine, USP) Tablets [prescribing information] AR Scientific, Inc; Philadelphia, PA: 2010. [Google Scholar]
- 62.Phelps P. Polymorphonuclear leukocyte motility in vitro. IV. Colchicine inhibition of chemotactic activity formation after phagocytosis of urate crystals. Arthritis Rheum. 1970;13:1–9. doi: 10.1002/art.1780130101. [DOI] [PubMed] [Google Scholar]
- 63.Phelps P. Polymorphonuclear leukocyte motility in vitro. II. Stimulatory effect of monosodium urate crystals and urate in solution; partial inhibition by colchicine and indomethacin. Arthritis Rheum. 1969;12:189–196. doi: 10.1002/art.1780120305. [DOI] [PubMed] [Google Scholar]
- 64.Abramson S, Hoffstein ST, Weissmann G. Superoxide anion generation by human neutrophils exposed to monosodium urate. Arthritis Rheum. 1982;25:174–180. doi: 10.1002/art.1780250210. [DOI] [PubMed] [Google Scholar]
- 65.Reinhardt PH, Naccache PH, Poubelle PE, et al. Monosodium urate crystals promote neutrophil adhesion via a CD18-independent and selectin-independent mechanism. Am J Physiol. 1996;270(1 Pt 1):C31–C39. doi: 10.1152/ajpcell.1996.270.1.C31. [DOI] [PubMed] [Google Scholar]
- 66.Naccache PH, Bourgoin S, Plante E, et al. Crystal-induced neutrophil activation. II. Evidence for the activation of a phosphatidylcholine-specific phospholipase D. Arthritis Rheum. 1993;36:117–125. doi: 10.1002/art.1780360119. [DOI] [PubMed] [Google Scholar]
- 67.Naccache PH, Grimard M, Roberge CJ, et al. Crystal-induced neutrophil activation. I. Initiation and modulation of calcium mobilization and superoxide production by microcrystals. Arthritis Rheum. 1991;34:333–342. doi: 10.1002/art.1780340311. [DOI] [PubMed] [Google Scholar]
- 68.Popa-Nita O, Proulx S, Paré G, et al. Crystal-induced neutrophil activation: XI. Implication and novel roles of classical protein kinase C. J Immunol. 2009;183:2104–2114. doi: 10.4049/jimmunol.0900906. [DOI] [PubMed] [Google Scholar]
- 69.Matsukawa A, Yoshimura T, Maeda T, et al. Analysis of the cytokine network among tumor necrosis factor α, interleukin-1β, interleukin-8, and interleukin-1 receptor antagonist in monosodium urate crystal-induced rabbit arthritis. Lab Invest. 1998;78:559–569. [PubMed] [Google Scholar]
- 70.Ben Chetrit E, Fischel R, Hinz B, et al. The effects of colchicine and hydroxychloroquine on the cyclo-oxygenases COX-1 and COX-2. Rheumatol Int. 2005;25:332–335. doi: 10.1007/s00296-004-0442-4. [DOI] [PubMed] [Google Scholar]
- 71.Cronstein BN, Molad Y, Reibman J, et al. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest. 1995;96:994–1002. doi: 10.1172/JCI118147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swarup A, Sachdeva N, Schumacher HR., Jr Dosing of antirheumatic drugs in renal disease and dialysis. J Clin Rheumatol. 2004;10:190–204. doi: 10.1097/01.rhu.0000135555.83088.a2. [DOI] [PubMed] [Google Scholar]
- 73.Hung IF, Wu AK, Cheng VC, et al. Fatal interaction between clarithromycin and colchicine in patients with renal insufficiency: a retrospective study. Clin Infect Dis. 2005;41:291–300. doi: 10.1086/431592. [DOI] [PubMed] [Google Scholar]
- 74.Wilbur K, Makowsky M. Colchicine myotoxicity: case reports and literature review. Pharmacotherapy. 2004;24:1784–1792. doi: 10.1592/phco.24.17.1784.52334. [DOI] [PubMed] [Google Scholar]
- 75.Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 76.De Bosscher K, Haegeman G. Minireview: latest perspectives on antiinflammatory actions of glucocorticoids. Mol Endocrinol. 2009;23:281–291. doi: 10.1210/me.2008-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Auphan N, DiDonato JA, Rosette C, et al. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 78.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 79.Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 80.Sholter DE, Armstrong PW. Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol. 2000;16:505–511. [PubMed] [Google Scholar]
- 81.van der Goes MC, Jacobs JW, Boers M, et al. Monitoring adverse events of low-dose glucocorticoid therapy: EULAR recommendations for clinical trials and daily practice. Ann Rheum Dis. 2010;69:1913–1919. doi: 10.1136/ard.2009.124958. [DOI] [PubMed] [Google Scholar]
- 82.Kineret (anakinra) [prescribing information] Biovitrum AB; Stockholm, Sweden: 2009. [Google Scholar]
- 83.Arcalyst (rilonacept) Injection for subcutaneous use [prescribing information] Regeneron Pharmaceuticals, Inc; Tarrytown, NY: 2009. [Google Scholar]
- 84.Ilaris (canakinumab) Injection for subcutaneous use [prescribing information] Novartis Pharmaceuticals Corporation; East Hanover, NJ: 2011. [Google Scholar]
- 85.Chen K, Fields T, Mancuso CA, et al. Anakinra’s efficacy is variable in refractory gout: report of ten cases. Semin Arthritis Rheum. 2010;40:210–214. doi: 10.1016/j.semarthrit.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 86.Schlesinger N, De Meulemeester M, Pikhlak A, et al. Canakinumab relieves symptoms of acute flares and improves health-related quality of life in patients with difficult-to-treat gouty arthritis by suppressing inflammation: results of a randomized, dose-ranging study. Arthritis Res Ther. 2011;13:R53. doi: 10.1186/ar3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schlesinger N, Alten RE, Bardin T, et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2011-200908. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 88.Schumacher HR, Jr, Evans RR, Saag KG, et al. Rilonacept (Interleukin-1 Trap) for prevention of gout flares during initiation of uric acid-lowering therapy: Results of the presurge-1 trial. Arthritis Care Res. 2012 doi: 10.1002/acr.21690. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 89.Schumacher HR, Jr, Sundy JS, Terkeltaub R, et al. Rilonacept (interleukin-1 trap) in the prevention of acute gout flares during initiation of urate-lowering therapy: results of a phase II randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64:876–884. doi: 10.1002/art.33412. [DOI] [PubMed] [Google Scholar]
- 90.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Furst DE. The risk of infections with biologic therapies for rheumatoid arthritis. Semin Arthritis Rheum. 2010;39:327–346. doi: 10.1016/j.semarthrit.2008.10.002. [DOI] [PubMed] [Google Scholar]