Abstract
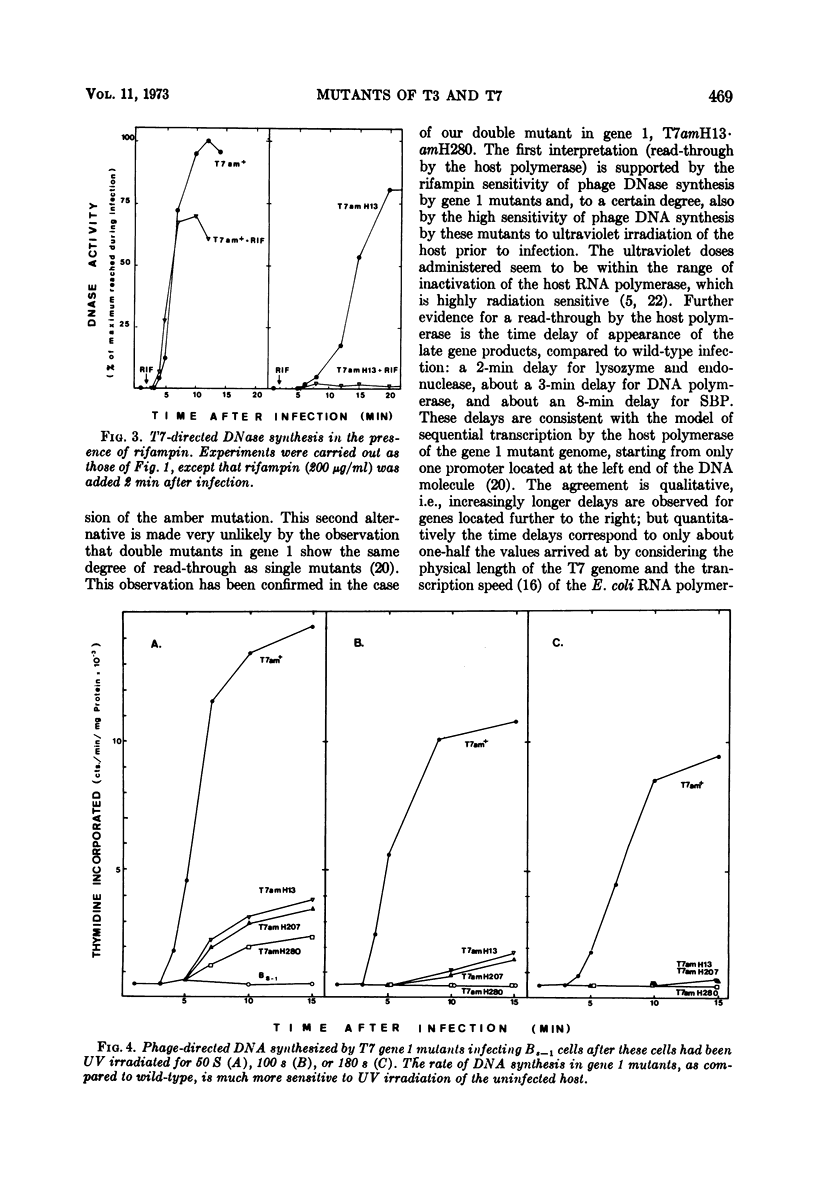
During nonpermissive infection by a T7 amber mutant in gene 1 (phage RNA polymerase-deficient), synthesis of the products of the phage genes 3 (endonuclease), 3, 5 (lysozyme), 5 (DNA polymerase), and 17 (serum blocking power) was shown to occur at about half the rate as during wild-type infection. This relatively high rate of expression of “late” genes (transcribed normally by the phage RNA polymerase) seems to be a general feature of all T7 mutants in gene 1 from our collection. In contrast, T3 gene 1 mutants and a T7 gene 1 mutant from another collection showed late protein synthesis at very reduced rates. Synthesis of the gene 3 endonuclease by T7 gene 1 mutants was very sensitive to the addition of rifampin 2 min after infection, conditions under which there was very little inhibition during wild-type infection. This supports the notion that late gene expression during nonpermissive infection by gene 1 mutants is dependent on the transcription of the T7 genome by the host RNA polymerase. In contrast to T3 gene 1 mutants, the T7 gene 1 mutants of our collection directed the synthesis of phage DNA during nonpermissive infection. This DNA accumulated as a material sedimenting faster than mature T7 DNA.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DE MARS R. I. The production of phage-related materials when bacteriophage development in interrupted by proflavine. Virology. 1955 May;1(1):83–99. doi: 10.1016/0042-6822(55)90007-6. [DOI] [PubMed] [Google Scholar]
- Goddard J. P., Weiss J. J., Wheeler C. M. Error frequency during in vitro transcription of poly U is increased with gamma-irradiated RNA polymerase. Nature. 1969 May 17;222(5194):670–671. doi: 10.1038/222670a0. [DOI] [PubMed] [Google Scholar]
- Grippo P., Richardson C. C. Deoxyribonucleic acid polymerase of bacteriophage T7. J Biol Chem. 1971 Nov 25;246(22):6867–6873. [PubMed] [Google Scholar]
- Hausmann R., Gomez B. Amber mutants of bacteriophages T3 and T7 defective in phage-directed deoxyribonucleic acid synthesis. J Virol. 1967 Aug;1(4):779–792. doi: 10.1128/jvi.1.4.779-792.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R., LaRue K. Variations in sedimentation patterns among deoxyribonucleic acids synthesized after infection of Escherichia coli by different amber mutants of bacteriophage T7. J Virol. 1969 Feb;3(2):278–281. doi: 10.1128/jvi.3.2.278-281.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlich P., Schweiger M., Sauerbier W. Host- and phage-RNA polymerase mediated synthesis of T 7 lysozyme in vivo. Mol Gen Genet. 1971;112(2):152–160. doi: 10.1007/BF00267492. [DOI] [PubMed] [Google Scholar]
- Herrlich P., Schweiger M. T3 and T7 bacteriophage deoxyribonucleic acid-directed enzyme synthesis in vitro. J Virol. 1970 Dec;6(6):750–753. doi: 10.1128/jvi.6.6.750-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryme I. F., Berentsen S. A. Lysozyme synthesis in Escherichia coli B cells infected with Phage T7. Biochim Biophys Acta. 1970 Apr 15;204(2):630–632. doi: 10.1016/0005-2787(70)90185-1. [DOI] [PubMed] [Google Scholar]
- Ritchie D. A., Malcolm F. E. Heat-stable and density mutants of phages T1, T3 and T7. J Gen Virol. 1970 Oct;9(1):35–43. doi: 10.1099/0022-1317-9-1-35. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Mosteller R. D., Yanofsky C. Tryptophan messenger ribonucleic acid elongation rates and steady-state levels of tryptophan operon enzymes under various growth conditions. J Mol Biol. 1970 Aug;51(3):541–550. doi: 10.1016/0022-2836(70)90007-0. [DOI] [PubMed] [Google Scholar]
- Scherzinger E., Herrlich P., Schweiger M., Schuster H. The early region of the DNA of bacteriophage T7. Eur J Biochem. 1972 Feb 15;25(2):341–348. doi: 10.1111/j.1432-1033.1972.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A., Thomas C. A., Jr Some special structural features of intracellular bacteriophage T7 concatemers. J Mol Biol. 1972 Jul 21;68(2):319–345. doi: 10.1016/0022-2836(72)90216-1. [DOI] [PubMed] [Google Scholar]
- Schweiger M., Herrlich P., Millette R. L. Gene expression in vitro from deoxyribonucleic acid of bacteriophage T7. J Biol Chem. 1971 Nov 25;246(22):6707–6712. [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Hausmann R. Integration of two sets of T7 mutants. Virology. 1969 Nov;39(3):587–588. doi: 10.1016/0042-6822(69)90106-8. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Jakes K. Phage T7 lysozyme mRNA transcription and translation in vivo and in vitro. Biochem Biophys Res Commun. 1971 Oct 15;45(2):315–320. doi: 10.1016/0006-291x(71)90820-5. [DOI] [PubMed] [Google Scholar]
- Sümegi J., Sanner T., Pihl A. Inactivation of DNA-dependent RNA polymerase from Escherichia coli by x-rays in solution. Biochim Biophys Acta. 1972 Mar 14;262(2):145–153. doi: 10.1016/0005-2787(72)90227-4. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., KORN D. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI K12-LAMBDA. J Biol Chem. 1963 Oct;238:3383–3389. [PubMed] [Google Scholar]



