Abstract
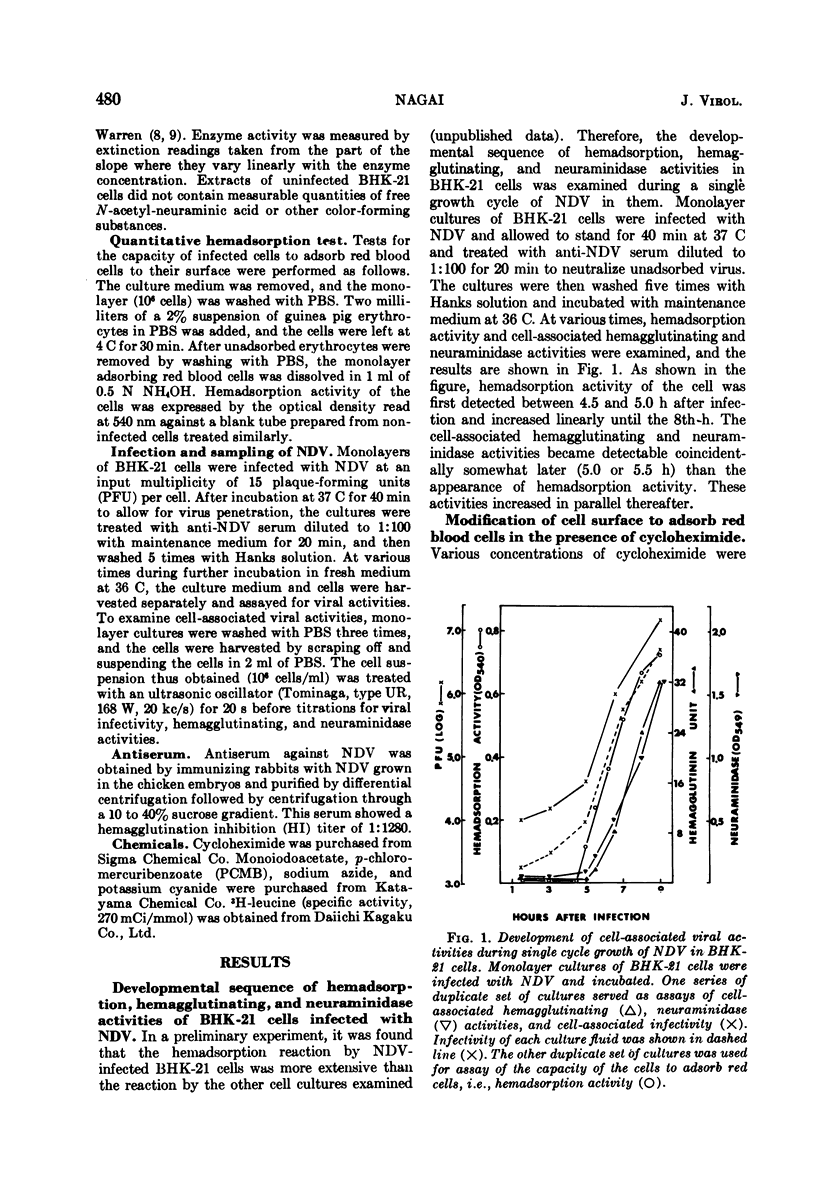
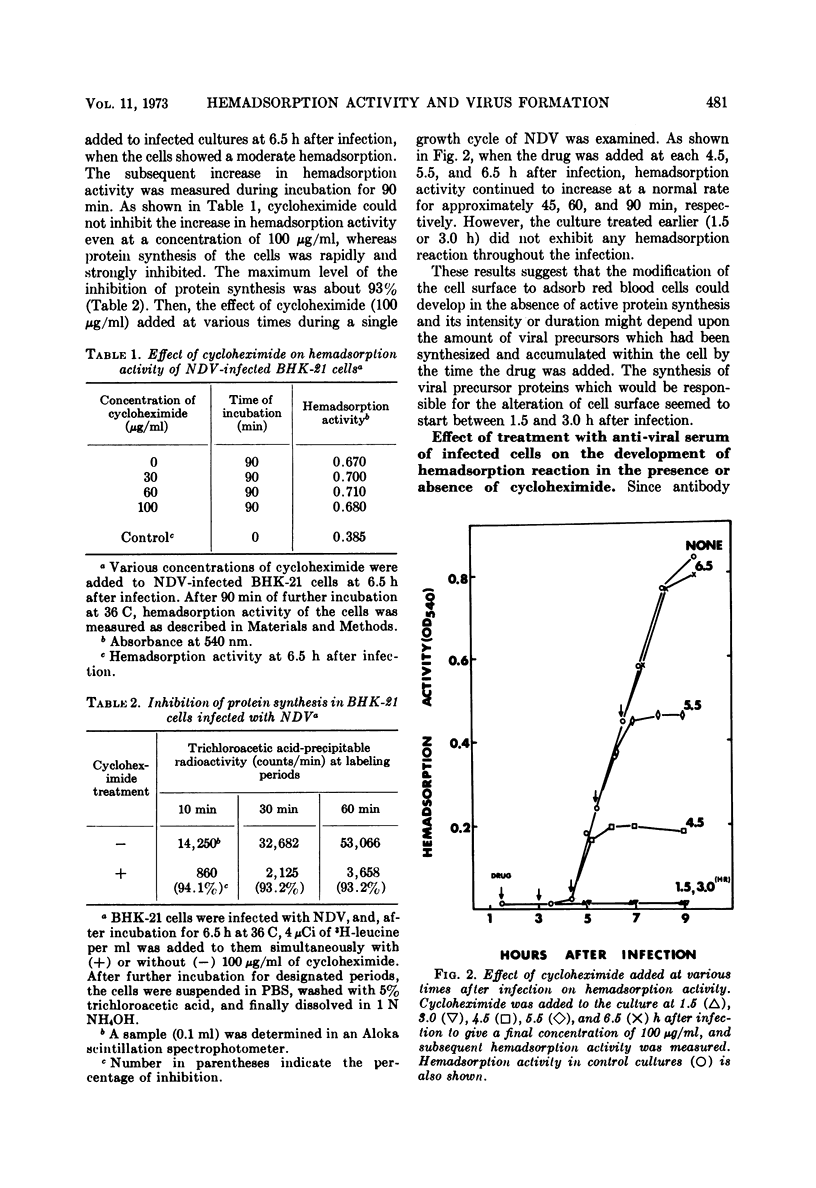
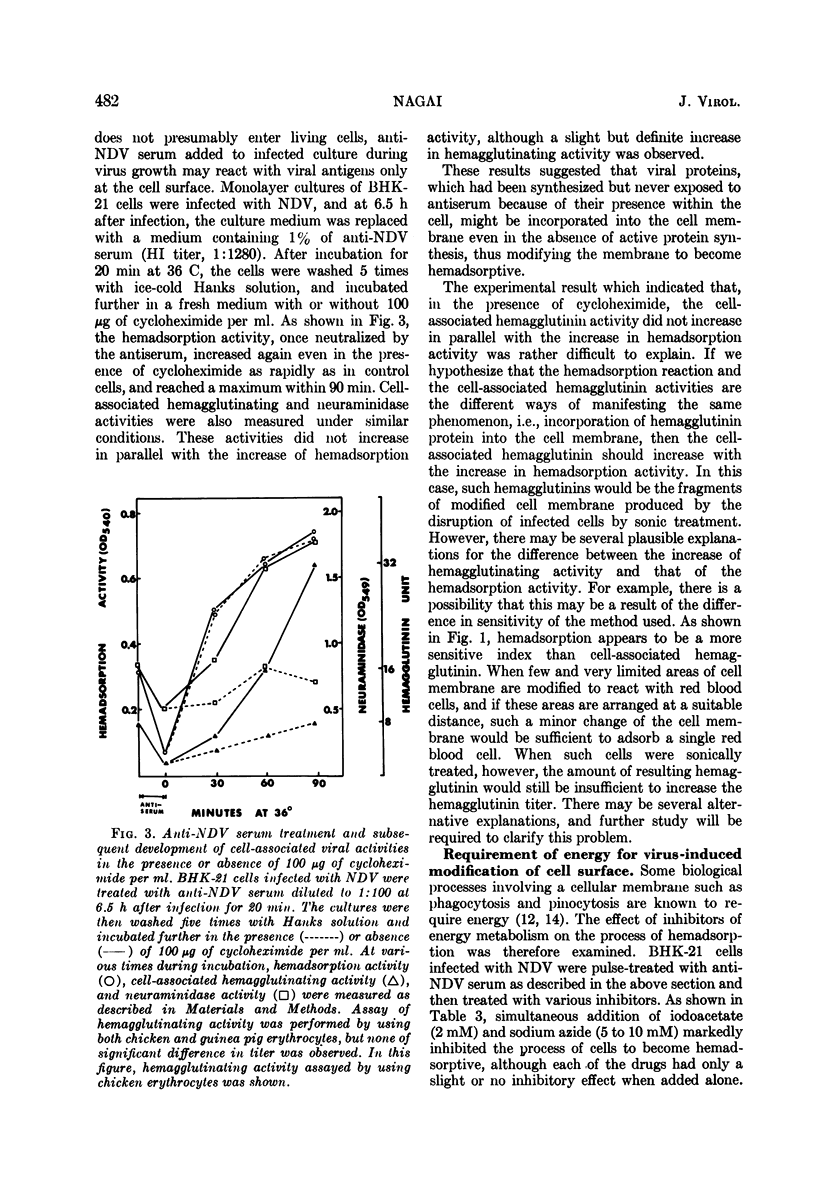
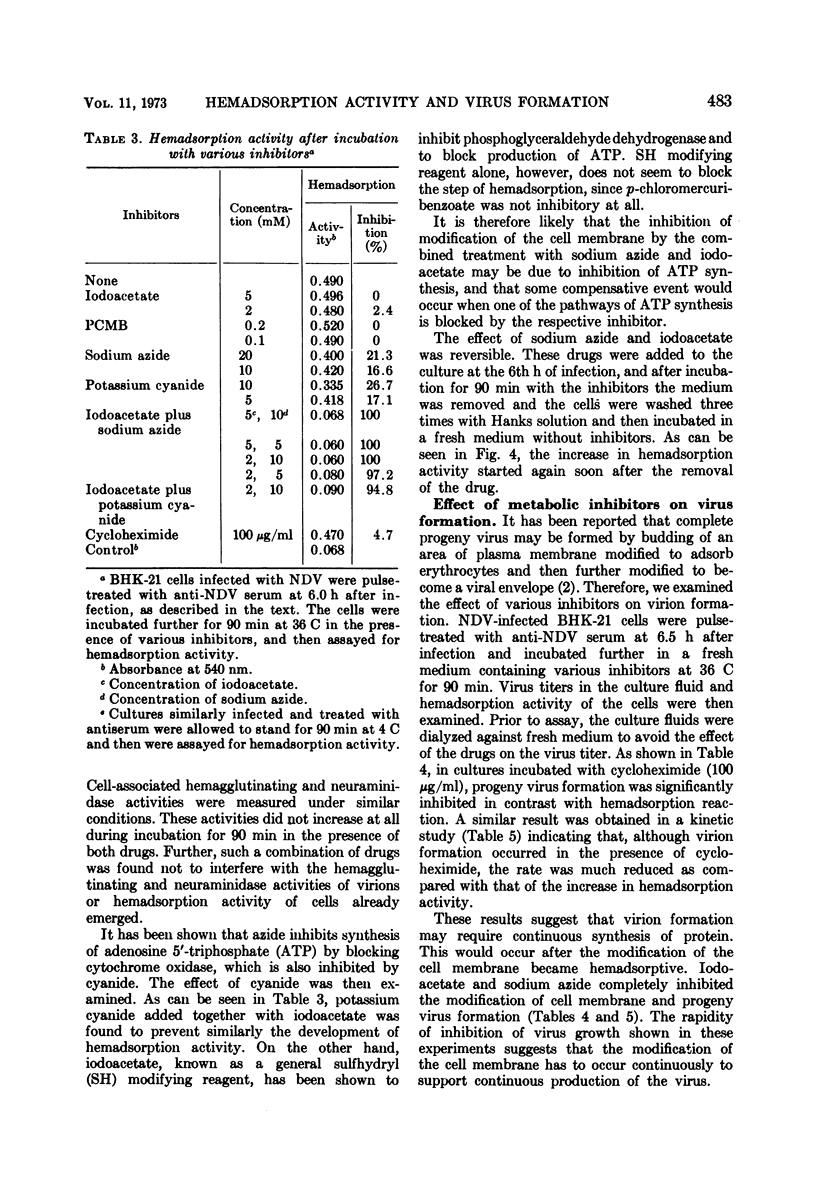
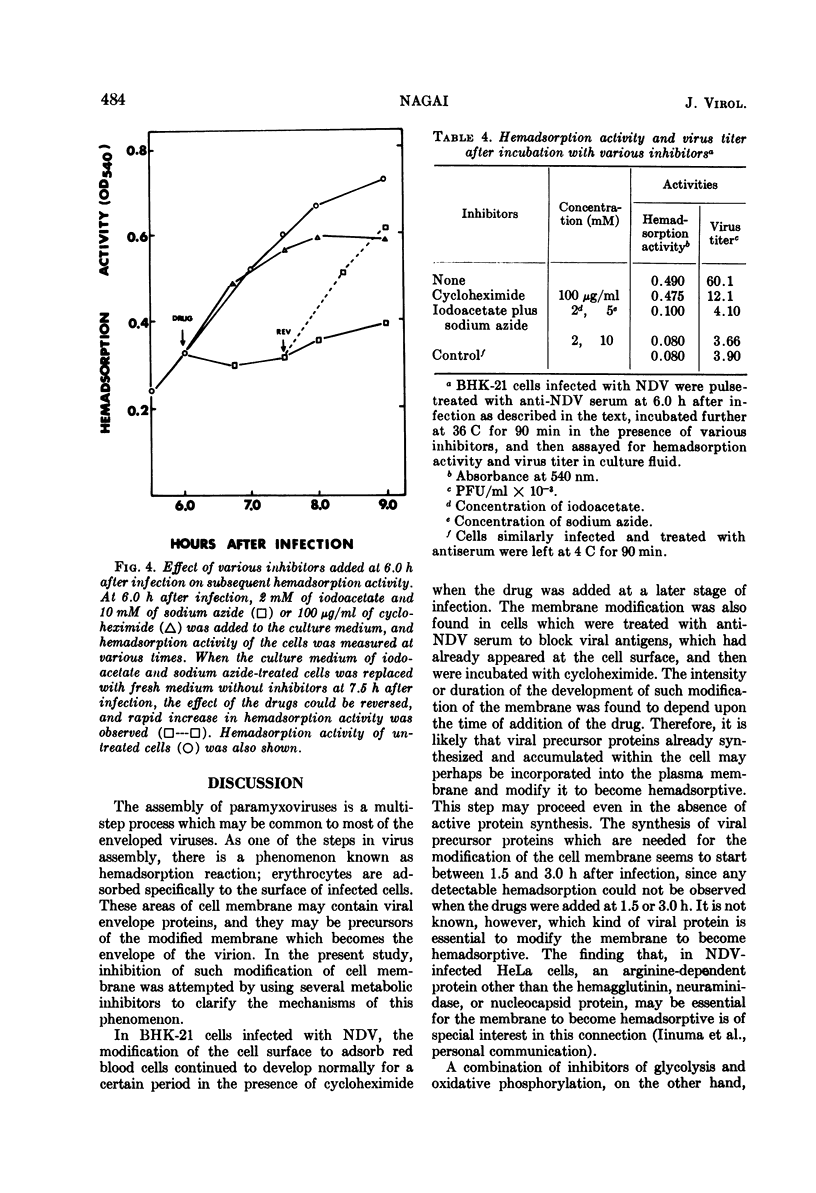
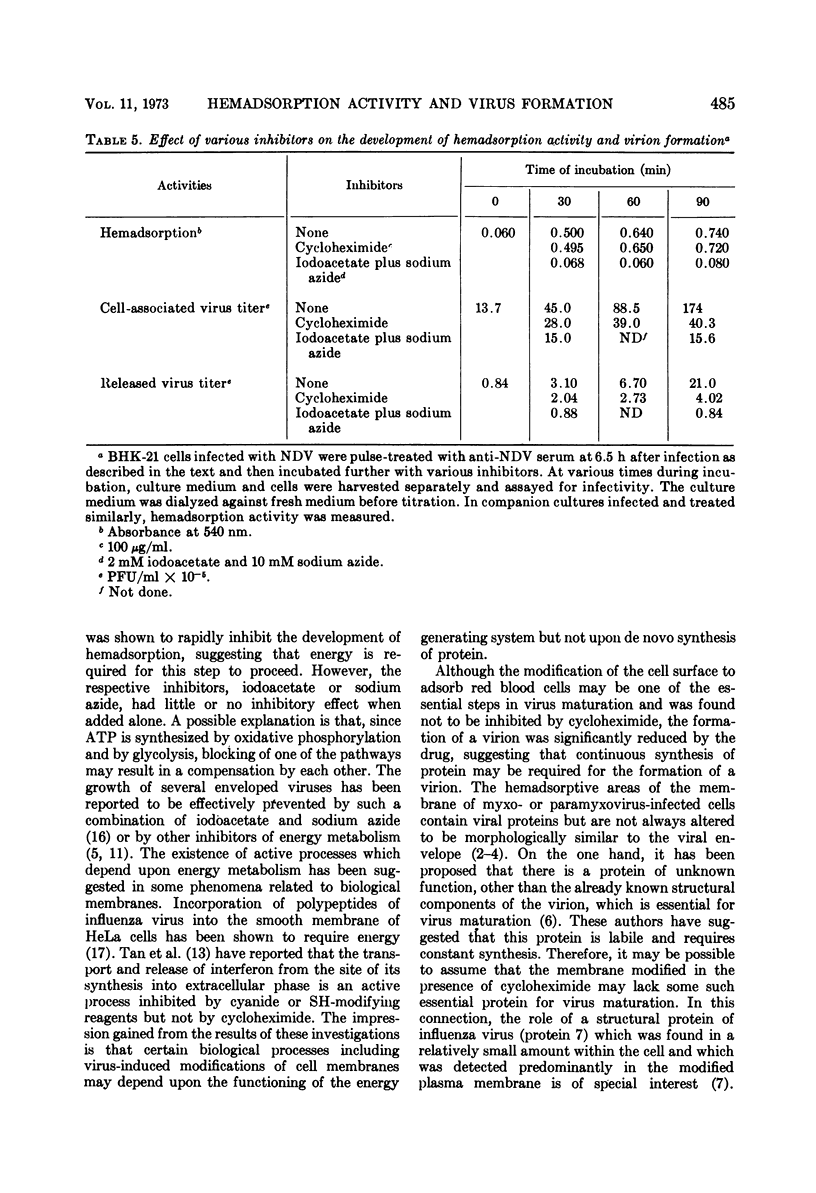
Differential effect of various metabolic inhibitors on the development of hemadsorption activity and virus formation in cells infected with Newcastle disease virus (NDV) was investigated. It was found that, in BHK-21 cells infected with NDV, cycloheximide did not prevent the development of hemadsorption activity, whereas protein synthesis and virus formation by the cell were rapidly inhibited by the drug. When the drug was added to the culture at 4.5 h after infection or later, hemadsorption activity of the cell continued to develop normally for about 1 h. Similar increase in hemadsorption activity was found in cells which were treated with anti-NDV serum (to neutralize their hemadsorption activity) and then washed and incubated with cycloheximide. However, when cells were treated with the drug early in the infection (1.5 or 3.0 h), they did not show any detectable hemadsorption reaction throughout the infection. In contrast to cycloheximide, iodoacetate added to the culture together with sodium azide inhibited completely both the development of hemadsorption activity and the formation of progeny virus. These results suggest that the change of cell surface to become hemadsorptive may depend upon the energy generating system but not upon de novo synthesis of protein, whereas production of infectious virus may require continuous synthesis of protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R. F., Gordon I., Stevenson D. Electron microscope study of hemadsorption in measles virus infection. Virology. 1965 Nov;27(3):441–445. doi: 10.1016/0042-6822(65)90129-7. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Dimmock N. J. An electron microscopic study of single-cycle infection of chick embryo fibroblasts by influenza virus. Virology. 1969 Nov;39(3):499–515. doi: 10.1016/0042-6822(69)90098-1. [DOI] [PubMed] [Google Scholar]
- Duc-Nguyen H. Hemadsorption of mumps virus examined by light and electron microscopy. J Virol. 1968 May;2(5):494–506. doi: 10.1128/jvi.2.5.494-506.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K., Shibuta H., Matumoto M. Inhibitory effect of oligomycin on replication of sendai virus and a bovine enterovirus in cultured cells. Jpn J Exp Med. 1971 Oct;41(5):423–429. [PubMed] [Google Scholar]
- Iinuma M., Nagai Y., Maeno K., Yoshida T., Matsumoto T. Studies on the assembly of Newcastle disease virus: incorporation of structural proteins into virus particles. J Gen Virol. 1971 Sep;12(3):239–247. doi: 10.1099/0022-1317-12-3-239. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Kilbourne E. D. Identification in a recombinant influenza virus of structural proteins derived from both parents. Virology. 1966 Nov;30(3):493–501. doi: 10.1016/0042-6822(66)90125-5. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Maeno K., Kilbourne E. D. Developmental sequence and intracellular sites of synthesis of three structural protein antigens of influenza A2 virus. J Virol. 1970 Feb;5(2):153–164. doi: 10.1128/jvi.5.2.153-164.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno K., Yoshii S., Nagata I., Matsumoto T. Growth of Newcastle disease virus in a HVJ carrier culture of HeLa cells. Virology. 1966 Jun;29(2):255–263. doi: 10.1016/0042-6822(66)90032-8. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Maeno K., Iinuma M., Yoshida T., Matsumoto T. Inhibition of virus growth by ouabain: effect of ouabain on the growth of HVJ in chick embryo cells. J Virol. 1972 Feb;9(2):234–243. doi: 10.1128/jvi.9.2.234-243.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Tan Y. H., Jeng D. K., Ho M. The release of interferon: an active process inhibited by p-hydroxymercuribenzoate. Virology. 1972 Apr;48(1):41–48. doi: 10.1016/0042-6822(72)90112-2. [DOI] [PubMed] [Google Scholar]
- VOGEL J., SHELOKOV A. Adsorption-hemagglutination test for influenza virus in monkey kidney tissue culture. Science. 1957 Aug 23;126(3269):358–359. doi: 10.1126/science.126.3269.358-a. [DOI] [PubMed] [Google Scholar]
- Waite M. R., Pfefferkorn E. R. Inhibition of Sindbis virus production by media of low ionic strength: intracellular events and requirements for reversal. J Virol. 1970 Jan;5(1):60–71. doi: 10.1128/jvi.5.1.60-71.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



