Abstract
There is abundant evidence showing that connexins form gap junctions. Yet, this does not exclude the possibility that connexins can exert other functions, separate from that of gap junction (or even a permeable hemichannel) formation. Here, we focus on these “non-canonical” functions of Connexin43 (Cx43), particularly in the heart. We describe two specific examples: The importance of Cx43 on intercellular adhesion, and the role of Cx43 in the function of the sodium channel. We propose that these two functions of Cx43 have important repercussions on the propagation of electrical activity in the heart, irrespective of the presence of permeable gap junction channels. Overall, the gap junction-independent functions of Cx43 in cardiac electrophysiology emerge as an exciting area of future research.
Keywords: Connexin43, heart, arrhythmias, cell-cell adhesion
Sixty years ago, Silvio Weidmann published his classic paper on “The electrical constants of Purkinje fibers.” In it, he beautifully demonstrated that electrotonic propagation in cardiac tissue extends well beyond the size of a single cell (Weidmann 1952). His observations provided the physiological evidence that cardiac cells are electrically coupled via low-resistive pathways. Electron microscopic observations followed, culminating with the elegant work of Revel and Karnovsky (Revel and Karnovsky 1967) showing that at the site of close appositional membranes in the cardiac intercalated disc, the membranes were not fused. Instead, the membranes were separated by a gap, traversed by junctions. These findings led Revel to later coin the term “gap junctions.” The demonstration that gap junctions are formed by oligomerization of connexin proteins established gap junction formation as the key function of connexin molecules. Yet, the fundamental importance of connexins in intercellular communication does not exclude the possibility that connexins may exert other functions, separate and independent from that of gap junction formation. This is hardly a novel concept; twenty years ago Ross Johnson and his colleagues reported a very important discovery: Fab fragments of antibodies to the extracellular domain of Connexin43 (Cx43), the most abundant connexin in the heart, inhibits adherens junction assembly in cells in culture (Meyer et al. 1992). This unexpected finding has been followed by others, showing that Cx43 is not only a pore-forming protein that allows ions and small molecules to move between cells (see. e.g. (Danik et al. 2008; Jansen et al. 2012a; Francis et al. 2011; Jansen et al. 2012b; Soder et al. 2009)). Our understanding of the molecular nature of connexins, and their function, has expanded enormously since Ross Johnson, Weidmann, Revel, Bennett, Gilula, Goodenough and many other “giants” of science first paved the way. Yet, a number of interesting questions about the role of connexins in biology remain unanswered, while other concepts that seemed established, are now challenged by novel experimental results. Here, we will dwell on what we call the “non-canonical” functions of Cx43, that is, functions that go beyond that of gap junction formation. While our discussion will center on Cx43 and its role in heart function, these issues likely extend to the functions of other connexins and in other organs. Of the many interesting angles that apply to this topic, we will cover only two aspects: intercellular adhesion and sodium channel function. These functions are described from the point of view of Cx43 as a component of the protein interacting network (the “interactome”) that populates the cardiac intercalated disc.
The intercalated disc as the site of a protein interacting network
Cardiac myocytes are highly differentiated, specialzed and compartmentalized cells. Proteins organize in defined microdomains. Slight changes in the position of a protein within its domain can bring about a major disruption in function (see, e.g. (Nikolaev et al. 2010)). Connexins occupy a subcellular region called “the intercalated disc.” This electron dense structure is located at the point where two cardiac cells meet end-to-end. In its classical definition, the intercalated disc is composed of three electron dense structures: gap junctions, desmosomes and adherens junctions. The latter two are involved in mechanical coupling between cells. Desmosomes couple to intermediate filaments (desmin, in the case of the heart), whereas adherens junctions anchor N-cadherins to the actin cytoskeleton. A “mixed” desmosome/adherens junction structure, dubbed the area composita, is also present in the adult mammalian heart (Franke et al. 2006). Originally, these structures were considered separate and independent from each other. Recent data suggest this not to be the case. Furthermore, the advent of immunofluorescence techniques brought about the demonstration that other molecules, not classically considered junctional, are also present at the cell end and in fact, co-localize with “junctional” molecules. Of particular interest are two ion channel proteins fundamental to normal cardiac electrophysiology: the sodium channel alpha subunit, NaV1.5, and the potassium channel protein KV1.5. For a number of years, each of these channels, and their accessory proteins, were studied as independent entities. Yet, recent data show that there is extensive cross-talk at the intercalated disc, and that this cross-talk extends to interactions between complexes previously seen as being independent (see Figure 1). As such, loss of expression of plakophilin-2 (PKP2), a desmosomal molecule, affects gap junction-mediated coupling (Oxford et al. 2007) as well as sodium channel function (Sato et al. 2009); loss of N-cadherin expression affects gap junctions (Li et al. 2005) and also the function of Kv1.5 channels (Cheng et al. 2011); loss of intercellular contact leads to a decrease in sodium current (Lin et al. 2011); expression of ankyrin-G (AnkG), a protein associated with the sodium channel complex (Lowe et al. 2008), is necessary for proper intercellular adhesion strength and for proper electrical coupling (Sato et al. 2011); finally, expression of Cx43, a protein previously associated only with gap junctions, is in fact required for the normal function of sodium (Jansen et al. 2011) and potassium currents (Danik et al. 2008). When taken together, the evidence suggests that the intercalated disc is not a site where independent molecules reside, but rather the host of an “interactome” – a protein interacting web that involves molecules relevant to excitability, propagation, and mechanical coupling between cells.
Figure 1.
Diagram indicating cross-talk between gap junctions, mechanical junctions and ion channel complexes. Gap junctions refers to Cx43 while mechanical junctions includes desmosomes and adherens junctions (N-cadherin). Ion channel complexes refers primarily to sodium channel complex and Kv1.5. Citations correspond to experimental evidence in cardiac preparations that support the interaction described.
Cx43 and intercellular adhesion
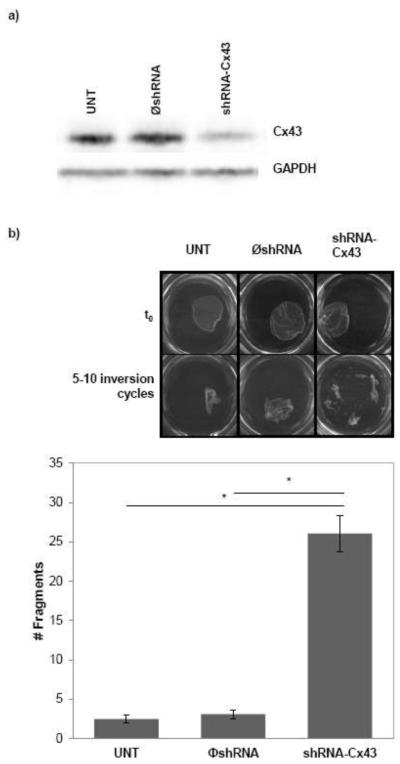
The possible interaction between connexins and mechanical junctions in the heart was highlighted by the findings of Jeff Saffitz and his colleagues. These investigators examined the hearts of patients afflicted with arrhythmogenic right ventricular cardiomyopathy (ARVC), a disease related to mutations in proteins of the desmosome, and demonstrated a consistent loss of gap junction plaques from the intercalated disc (Kaplan et al. 2004). Follow up work showed that loss of expression of the desmosomal protein, PKP2, leads to a loss of Cx43 from the site of intercellular contact, and an ~50% decrease in the extent of dye transfer between cells (Oxford et al. 2007). These and other studies strongly supported the notion that mechanical junctions affect gap junctions. Based on the early work of Meyer et al (Meyer et al. 1992), we have now asked the reciprocal question, is Cx43 expression necessary to maintain intercellular adhesion strength? To address this question, we implemented a “dispase assay,” whereby the contact between the cells and the extracellular matrix is disrupted by the use of dispase. If adhesion between cells is strong, the layer lifts as one sheet (cells remain attached to one another). If cell-cell adhesion is weaker, the sheet fragments. Thus, the more fragments, the weaker the mechanical coupling. Using this method, we recently showed that loss of Ank-G expression (a protein that scaffolds for the sodium channel complex) causes a decrease in mechanical coupling between cells (Sato et al. 2011). Here, we applied this method to compare two cell populations: one, HEK293 cells that endogenously express Cx43 and another one, a stable HEK293 line where we used lentivirus to permanently silence Cx43 expression. Three groups are compared: untreated (UNT), treated with a virus that contains a non-silencing construct ( shRNA-Cx43) and a third group where Cx43 expression was prevented (shRNA-Cx43). The Western blot in panel A shows the loss of Cx43 expression in the corresponding group. As shown in Panel B, loss of Cx43 expression brought about a loss of intercellular adhesion strength, represented by a significant increase in the number of fragments detected 90 minutes after dispase addition. These results show that Cx43 expression is relevant to mechanical coupling. Whether this effect is consequent to gap junctions being a physical element that provides intercellular adhesion, or whether the result involves intermolecular interactions between Cx43 and components of the mechanical junctions, remains to be determined. The results do show that intercellular adhesion strength is a function of Cx43 that extends beyond the formation of a low-resistive pathway between cells.
Cx43 is necessary for sodium channel function
Figure 1 also shows an arrow connecting Cx43 to the sodium channel complex. This is a very exciting, novel finding regarding the non-canonical functions of Cx43 in the heart. Indeed, in the classical description, sodium channels provide the current that is necessary for the generation of an action potential in most cardiac cells, whereas Cx43 forms gap junctions, the channels that allow for that electrical charge to move between one cell and the next. This description then separates the type of channel, with its function: sodium channels are responsible for cell excitability; gap junctions allow cell-cell passage of charge. In a recent study, however, we reported that loss of Cx43 expression brings about a loss of sodium current amplitude (Jansen et al. 2012a). In other words, the molecule necessary for making the gap junctions is actually necessary to maintain the complex in charge of generating the action potential. This means that loss of Cx43 expression is in fact a double-edge sword: not only will the path between cells be disrupted but also, the amount of charge that is generating by the excited cell will decrease. Loss of Cx43 expression, as it happens in a number of pathological cardiac conditions (Desplantez et al. 2007; Akar et al. 2007; Chkourko et al. 2012; Kalcheva et al. 2007; Qu et al. 2009), can have complex deleterious effects on propagation.
Novel roles of Cx43 in cardiac propagation: sodium current and the intercellular space
These findings come at a time when the classical description of cardiac propagation deserves to be reviewed. Early models, based on the principles of continuous cable theory, represented gap junctions as passive resistors providing the only means for passage of charge between cells. When incorporated into a model of cardiac propagation, these equations predicted that reductions in gap junction-mediated communication would bring about a concomitant reduction in conduction velocity. The first serious challenge to this notion came from experiments demonstrating that 50-80% loss of Cx43 expression in the heart causes a decrease in junctional conductance between cells (Yao et al. 2003), but not a decrease in conduction velocity (Morley et al. 2000; Danik et al. 2004) . Are gap junctions, then, the only path for transfer of charge between cardiac cells? What is the missing element (missing from the equations) that preserves propagation when gap junction-mediated conductance decreases?
While a conclusive answer is yet to come about, it seems pertinent to remain open to models that, though less conventional, may better represent what happens at the site of contact between cells. We refer in particular to the proposed “electrical field mechanism” of cardiac propagation. This model postulates that the large inward sodium current in the proximal side of an intercellular cleft can generate a large negative extracellular potential within the cleft, effectively depolarizing the membrane of the distal cell, activating its sodium channels and allowing for propagation to continue downstream, even in the absence of functional gap junctions (Sperelakis 2002; Hand and Peskin 2010; Mori et al. 2008). While this mechanism would play an insignificant role when gap junctions are present and functional, it would become crucial to maintain propagation when gap junctions close, or when connexins are lost.
The effect of electric fields, also known as ephaptic interactions, has been extensively investigated in the nervous system. These nonsynaptic mechanisms play a significant role in neuronal function and can mediate neuronal synchrony on a fast scale compared to ionic and chemical mechanisms that operate on a much slower scale (Jefferys 1995). In this context, recent studies of the presence of electrical synapses in the mammalian central nervous system have described the chemical transmission through Cx36 in neurons of the mesencephalic trigeminal nucleus. Although in these cells the fraction of opened channels is small, the sodium and potassium conductances enhances the electrical coupling leading to a strong synchronization of these neurons (Curti et al. 2012).
Under the concept of the electric field mechanism, propagation is dependent on the subcellular distribution of sodium channels, as their density specifically at the intercalated disc is critical to the generation of the electrical field in the intercellular cleft (Tsumoto et al. 2011). In this context, it is important to mention our recent results (Lin et al. 2011) showing that a) sodium current amplitude is larger in the area of the intercalated disc, b) steady-state inactivation of sodium channels located in the middle of the cell is shifted toward more negative values; as such, the burden of excitation during propagation falls on the channels at the intercalated disc, and c) sodium current density is larger in cells that remain paired. These results are complementary to those of Petiprez et al (Petitprez et al. 2011), showing that there are two separate pools of Nav1.5 in the heart: one at the intercalated disc, and a separate one associated with the costameres. Our data demonstrated that together with the segregation of channels by subcellular regions, there is also a segregation of function; here we further propose that the function of Nav1.5 at the intercalated disc is determined, at least in part, by Cx43.
The electric field mechanism also requires a preservation of the dimensions of the intercellular space. Experimental values for this variable are less solid. Currently, we are using high-pressure freezing methods, and tomographic electron microscopy to obtain images of the intercellular space with a high level of structural preservation (Delmar and Liang 2011). Using these methods, we have began to collect images that allow us to make accurate measurements of the intercellular space under conditions where Cx43 is reduced, either by genetic manipulation (e.g., in Cx43-deficient hearts) or by disease. In this context, it seems pertinent to remind the reader of the discovery of Ross Johnson and his colleagues, twenty years ago: Connexins are important for intercellular adhesion (see also Figure 2). It would follow that diseases that cause loss or remodeling of Cx43 would lead to an increased separation of the cells at the intercalated disc. From the point of view of electrophysiology, this would become an important challenge to the preservation of propagation between cells.
Figure 2.
Loss of Cx43 induces loss of intercellular adhesion strength. A) Western blot for Cx43 in HEK293 cells untreated (UNT), treated with a virus that contains a non-silencing construct ( shRNA-Cx43) and silenced for Cx43 (shRNA-Cx43). GAPDH was used as loading control. B) Dispase assay. Cells were treated with 2.4U/mL dispase for 90min to disrupt attachment to extracellular matrix. Images were taken before (t0) and after 5-10 inversion cycles. Bars show number of fragments after subjecting monolayers to 5-10 inversion cycles. n=10-20, p<0.001
Conclusions
We have discussed two “non-canonical” functions of Cx43: preservation of cell-cell adhesion, and preservation of sodium current amplitude in cardiac cells. We have also argued in favor of a model of cardiac propagation where cell-cell transfer of charge occurs not only by the flow of current through gap junction channels but also, by an electric field mechanism that relies on a) a tight intercellular gap and b) the accumulation of functional sodium channels at the intercalated disc. Thus, we speculate that under conditions of poor gap junction-mediated electrical coupling, propagation can be maintained via the electrical field mechanism, but only if sodium current properties, and a narrow cleft, are preserved. As such, we propose that the Cx43-dependent loss of sodium current, and perhaps a Cx43-dependent increase in the size of the intercellular gap, are critical to propagation failure resulting from reduced Cx43 abundance. Cx43 may play a key role in intercellular communication not only directly, by forming gap junctions, but also indirectly, by maintaining a high sodium current density at the intercalated disc, and a narrow intercellular cleft for the transfer of activation. The gap junction-independent functions of Cx43 in cardiac electrophysiology emerge as an exciting area of future research.
References
- Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. American journal of physiology Heart and circulatory physiology. 2007;293(2):H1223–1230. doi: 10.1152/ajpheart.00079.2007. doi:10.1152/ajpheart.00079.2007. [DOI] [PubMed] [Google Scholar]
- Cheng L, Yung A, Covarrubias M, Radice GL. Cortactin is required for N-cadherin regulation of Kv1.5 channel function. The Journal of biological chemistry. 2011;286(23):20478–20489. doi: 10.1074/jbc.M111.218560. doi:10.1074/jbc.M111.218560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chkourko HS, Guerrero-Serna G, Lin X, Darwish N, Pohlmann JR, Cook KE, Martens JR, Rothenberg E, Musa H, Delmar M. Remodeling of mechanical junctions and of microtubule-associated proteins accompany cardiac connexin 43 lateralization. Heart rhythm : the official journal of the Heart Rhythm Society. 2012 doi: 10.1016/j.hrthm.2012.03.003. doi:10.1016/j.hrthm.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti S, Hoge G, Nagy JI, Pereda AE. Synergy between electrical coupling and membrane properties promotes strong synchronization of neurons of the mesencephalic trigeminal nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(13):4341–4359. doi: 10.1523/JNEUROSCI.6216-11.2012. doi:10.1523/JNEUROSCI.6216-5 11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circulation research. 2004;95(10):1035–1041. doi: 10.1161/01.RES.0000148664.33695.2a. doi:10.1161/01.RES.0000148664.33695.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danik SB, Rosner G, Lader J, Gutstein DE, Fishman GI, Morley GE. Electrical remodeling contributes to complex tachyarrhythmias in connexin43-deficient mouse hearts. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22(4):1204–1212. doi: 10.1096/fj.07-8974com. doi:10.1096/fj.07-8974com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmar M, Liang FX. Connexin43 and the regulation of intercalated disc function. Heart rhythm : the official journal of the Heart Rhythm Society. 2011 doi: 10.1016/j.hrthm.2011.10.028. doi:10.1016/j.hrthm.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplantez T, Dupont E, Severs NJ, Weingart R. Gap junction channels and cardiac impulse propagation. The Journal of membrane biology. 2007;218(1-3):13–28. doi: 10.1007/s00232-007-9046-8. doi:10.1007/s00232-007-9046-8. [DOI] [PubMed] [Google Scholar]
- Francis R, Xu X, Park H, Wei CJ, Chang S, Chatterjee B, Lo C. Connexin43 modulates cell polarity and directional cell migration by regulating microtubule dynamics. PloS one. 2011;6(10):e26379. doi: 10.1371/journal.pone.0026379. doi:10.1371/journal.pone.0026379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW, Borrmann CM, Grund C, Pieperhoff S. The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. European journal of cell biology. 2006;85(2):69–82. doi: 10.1016/j.ejcb.2005.11.003. doi:10.1016/j.ejcb.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Hand PE, Peskin CS. Homogenization of an electrophysiological model for a strand of cardiac myocytes with gap-junctional and electric-field coupling. Bulletin of mathematical biology. 2010;72(6):1408–1424. doi: 10.1007/s11538-009-9499-2. doi:10.1007/s11538-009-9499-2. [DOI] [PubMed] [Google Scholar]
- Jansen JA, Noorman M, Musa H, Stein M, de Jong S, van der Nagel R, Hund TJ, Mohler PJ, Vos MA, van Veen TA, de Bakker JM, Delmar M, van Rijen HV. Reduced Heterogeneous Expression Of Cx43 Results In Decreased Nav1.5 Expression And Reduced Sodium Current Which Accounts For Arrhythmia Vulnerability In Conditional Cx43 Knockout Mice. Heart Rhythm. 2011 doi: 10.1016/j.hrthm.2011.11.025. doi:S1547-5271(11)01360-9 [pii] 10.1016/j.hrthm.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JA, Noorman M, Musa H, Stein M, de Jong S, van der Nagel R, Hund TJ, Mohler PJ, Vos MA, van Veen TA, de Bakker JM, Delmar M, van Rijen HV. Reduced heterogeneous expression of Cx43 results in decreased Nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart rhythm : the official journal of the Heart Rhythm Society. 2012a;9(4):600–607. doi: 10.1016/j.hrthm.2011.11.025. doi:10.1016/j.hrthm.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JA, van Veen TA, de Jong S, van der Nagel R, van Stuijvenberg L, Driessen H, Labzowski R, Oefner CM, Bosch AA, Nguyen TQ, Goldschmeding R, Vos MA, de Bakker JM, van Rijen HV. Reduced cx43 expression triggers increased fibrosis due to enhanced fibroblast activity. Circulation Arrhythmia and electrophysiology. 2012b;5(2):380–390. doi: 10.1161/CIRCEP.111.966580. doi:10.1161/CIRCEP.111.966580. [DOI] [PubMed] [Google Scholar]
- Jefferys JG. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiological reviews. 1995;75(4):689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- Kalcheva N, Qu J, Sandeep N, Garcia L, Zhang J, Wang Z, Lampe PD, Suadicani SO, Spray DC, Fishman GI. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(51):20512–20516. doi: 10.1073/pnas.0705472105. doi:10.1073/pnas.0705472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan SR, Gard JJ, Protonotarios N, Tsatsopoulou A, Spiliopoulou C, Anastasakis A, Squarcioni CP, McKenna WJ, Thiene G, Basso C, Brousse N, Fontaine G, Saffitz JE. Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (Naxos disease) Heart rhythm : the official journal of the Heart Rhythm Society. 2004;1(1):3–11. doi: 10.1016/j.hrthm.2004.01.001. doi:10.1016/j.hrthm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Li J, Patel VV, Kostetskii I, Xiong Y, Chu AF, Jacobson JT, Yu C, Morley GE, Molkentin JD, Radice GL. Cardiac-specific loss of N-cadherin leads to alteration in connexins with conduction slowing and arrhythmogenesis. Circulation research. 2005;97(5):474–481. doi: 10.1161/01.RES.0000181132.11393.18. doi:10.1161/01.RES.0000181132.11393.18. [DOI] [PubMed] [Google Scholar]
- Lin X, Liu N, Lu J, Zhang J, Anumonwo JM, Isom LL, Fishman GI, Delmar M. Subcellular heterogeneity of sodium current properties in adult cardiac ventricular myocytes. Heart rhythm : the official journal of the Heart Rhythm Society. 2011;8(12):1923–1930. doi: 10.1016/j.hrthm.2011.07.016. doi:10.1016/j.hrthm.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe JS, Palygin O, Bhasin N, Hund TJ, Boyden PA, Shibata E, Anderson ME, Mohler PJ. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. The Journal of cell biology. 2008;180(1):173–186. doi: 10.1083/jcb.200710107. doi:10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA, Laird DW, Revel JP, Johnson RG. Inhibition of gap junction and adherens junction assembly by connexin and A-CAM antibodies. The Journal of cell biology. 1992;119(1):179–189. doi: 10.1083/jcb.119.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Fishman GI, Peskin CS. Ephaptic conduction in a cardiac strand model with 3D electrodiffusion. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(17):6463–6468. doi: 10.1073/pnas.0801089105. doi:10.1073/pnas.0801089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley GE, Vaidya D, Jalife J. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. Journal of cardiovascular electrophysiology. 2000;11(3):375–377. doi: 10.1111/j.1540-8167.2000.tb01811.x. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327(5973):1653–1657. doi: 10.1126/science.1185988. doi:10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circulation research. 2007;101(7):703–711. doi: 10.1161/CIRCRESAHA.107.154252. doi:10.1161/CIRCRESAHA.107.154252. [DOI] [PubMed] [Google Scholar]
- Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, Albesa M, Bittihn P, Luther S, Lehnart SE, Hatem SN, Coulombe A, Abriel H. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circulation research. 2011;108(3):294–304. doi: 10.1161/CIRCRESAHA.110.228312. doi:10.1161/CIRCRESAHA.110.228312. [DOI] [PubMed] [Google Scholar]
- Qu J, Volpicelli FM, Garcia LI, Sandeep N, Zhang J, Marquez-Rosado L, Lampe PD, Fishman GI. Gap junction remodeling and spironolactone-dependent reverse remodeling in the hypertrophied heart. Circulation research. 2009;104(3):365–371. doi: 10.1161/CIRCRESAHA.108.184044. doi:10.1161/CIRCRESAHA.108.184044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel JP, Karnovsky MJ. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. The Journal of cell biology. 1967;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato PY, Coombs W, Lin X, Nekrasova O, Green KJ, Isom LL, Taffet SM, Delmar M. Interactions between ankyrin-G, Plakophilin-2, and Connexin43 at the cardiac intercalated disc. Circulation research. 2011;109(2):193–201. doi: 10.1161/CIRCRESAHA.111.247023. doi:10.1161/CIRCRESAHA.111.247023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato PY, Musa H, Coombs W, Guerrero-Serna G, Patino GA, Taffet SM, Isom LL, Delmar M. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circulation research. 2009;105(6):523–526. doi: 10.1161/CIRCRESAHA.109.201418. doi:10.1161/CIRCRESAHA.109.201418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soder BL, Propst JT, Brooks TM, Goodwin RL, Friedman HI, Yost MJ, Gourdie RG. The connexin43 carboxyl-terminal peptide ACT1 modulates the biological response to silicone implants. Plastic and reconstructive surgery. 2009;123(5):1440–1451. doi: 10.1097/PRS.0b013e3181a0741d. doi:10.1097/PRS.0b013e3181a0741d. [DOI] [PubMed] [Google Scholar]
- Sperelakis N. An electric field mechanism for transmission of excitation between myocardial cells. Circulation research. 2002;91(11):985–987. doi: 10.1161/01.res.0000045656.34731.6d. [DOI] [PubMed] [Google Scholar]
- Tsumoto K, Ashihara T, Haraguchi R, Nakazawa K, Kurachi Y. Roles of subcellular Na+ channel distributions in the mechanism of cardiac conduction. Biophysical journal. 2011;100(3):554–563. doi: 10.1016/j.bpj.2010.12.3716. doi:10.1016/j.bpj.2010.12.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S. The electrical constants of Purkinje fibres. The Journal of physiology. 1952;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JA, Gutstein DE, Liu F, Fishman GI, Wit AL. Cell coupling between ventricular myocyte pairs from connexin43-deficient murine hearts. Circulation research. 2003;93(8):736–743. doi: 10.1161/01.RES.0000095977.66660.86. doi:10.1161/01.RES.0000095977.66660.86. [DOI] [PubMed] [Google Scholar]