Abstract
Acute liver failure is a remarkably rare syndrome, the result of rapid hepatocyte injury occurring over days or a few weeks, and encompassing multiple etiologies, but all with a remarkably similar clinical picture. The clinical features of coagulopathy and encephalopathy characterize this severe and often fatal condition. To date, transplantation has been the only reliable form of rescue for many patients. Recent developments have included a clearer understanding of the different contributing etiologies, how to build a diagnosis and prognosis based on initial laboratory findings, a more aggressive approach to intensive care management and more detailed understanding of the role of transplantation in this setting. This review will provide an overview of standard practices and new research initiatives and findings for this interesting but vexing orphan disease. Particular attention will be paid to practical matters for clinicians to consider in approaching the ALF patient. Few controlled clinical trials have been possible because of the condition’s rarity. Critical care of these rare patients is key to their survival and decisions must be made decisively, sometimes with inadequate information. Experience is helpful but experienced clinician managers are even rarer than the disease: few hepatologists or intensivists have in-depth experience with ALF patients.
Keywords: acute liver injury, acetaminophen toxicity, coagulopathy, encephalopathy, hepatic necrosis
Introduction
Acute liver failure (ALF) is the most common term applied to an unusual clinical syndrome resulting from rapid loss in hepatocyte function. It occurs infrequently, affecting 2,000 patients annually in the United States, and comprises approximately 7% of liver transplants annually and the annual incidence does not appear to be increasing or decreasing at this time. ALF is the culmination of severe liver cell injury from a variety of different causes, but leads to a relatively uniform clinical syndrome characterized by encephalopathy and coagulopathy, the hallmark features (1). Because of its rarity, research in acute liver failure has been limited to a handful of large units or to collaborative networks such as the NIH-sponsored U.S. Acute Liver Failure Study Group (ALFSG).
Definition
Acute liver failure (sometimes referred to as fulminant hepatic failure) is most commonly defined using an age-old definition of coagulopathy (International Normalized Ratio [INR] ≥1.5 and encephalopathy (any degree of altered mentation) in a patient without pre-existing liver disease or cirrhosis (1). The typical interval from onset of symptoms to onset of encephalopathy is 1–2 weeks, but cases evolving more slowly, up to 6 months, may still be included in the definition. A clinical feature that is virtually unique to ALF is cerebral edema, swelling of the brain that may produce herniation of the uncus through the falx cerebrum, yielding brain stem compression and death (2). The morbidity and mortality of ALF recorded in small case series in the pre-transplant era was extremely high, often exceeding 90%, the causes of death including multi-organ failure, hemorrhage, infection and cerebral edema. Fortunately, these dire outcomes have diminished somewhat due to a change in the causes of ALF to more benign etiologies over the past 40 years as well as to the introduction of liver transplantation (3,4). Patients have been designated as hyperacute (<7 days), acute (7–28 days) and subacute (4–26 weeks) in presentation depending on the interval from onset of symptoms to onset of encephalopathy. Different etiologies typically have a specific time frame. For example, acetaminophen cases are virtually always hyperacute, while viral hepatitis, idiosyncratic drug reactions or indeterminate cause cases demonstrate slower onset and evolution (acute or subacute) (5). Whether there is a distinct difference in outcome based on the length of disease itself remains unclear; however, the etiologic diagnosis per se appears to be the strongest driver of outcome (2).
Diagnosis
The diagnosis of ALF must be considered in anyone presenting with the recent onset of a hepatic illness where the prothrombin time/INR has become prolonged. Mental alterations are part of virtually all definitions and the changes may be subtle, including, initially, agitation and confusion, but usually progression to deeper coma grades. Recently, efforts to develop more robust measures to define early encephalopathy grades have been proposed but are still not widely used, and little has taken the place of the West Haven coma grade (1–4) system (6). Any mental alteration in conjunction with a prolonged international normalized ratio (INR) >1.5, qualifies the patient as having ALF, provided they have had an illness of short duration and do not have cirrhosis. A more recent category of severe liver injury has been deemed acute liver injury (ALI) denoted by INR of ≥ 2.0 in the absence of encephalopathy. With non-descript presenting symptoms, the diagnosis is often missed by the initial medical contact. However, the combination of coagulopathy and encephalopathy is unique and is only seen in this setting. The presence of any degree of encephalopathy indicates a severe, life threatening condition that requires immediate hospitalization. Patients are best managed within an intensive care setting and specialty units devoted to acute liver failure are available in the United Kingdom (3). Rapid evaluation for transfer to a transplantation center and consideration for liver transplantation is mandatory once any degree of mental alteration occurs, since disease progression is often very rapid once cognition is disturbed (7).
Acute liver failure has a common clinical picture regardless of the etiology that represents the final pathway of acute organ failure, different from cirrhosis but specific to one organ, the liver. Patients appear to be relatively hypotensive and vasodilated with low systemic vascular resistance, a picture of multi-system failure that resembles in some ways that of gram-negative sepsis or end stage liver disease patients; however portal hypertension and ascites are usually absent, except in subacute cases. Renal failure resembling hepatorenal syndrome may develop and is reversible with return of hepatic function. Infection is unusually common in ALF, presumably signifying the role of the liver in the host’s innate immune defenses.
Practice Points
ALF often is missed by the initial medical contact but is easy to diagnose if considered.
The clinical scenario resembles septic shock and needs to be managed similarly.
It is important to try to rule out cirrhosis in this setting.
Etiology
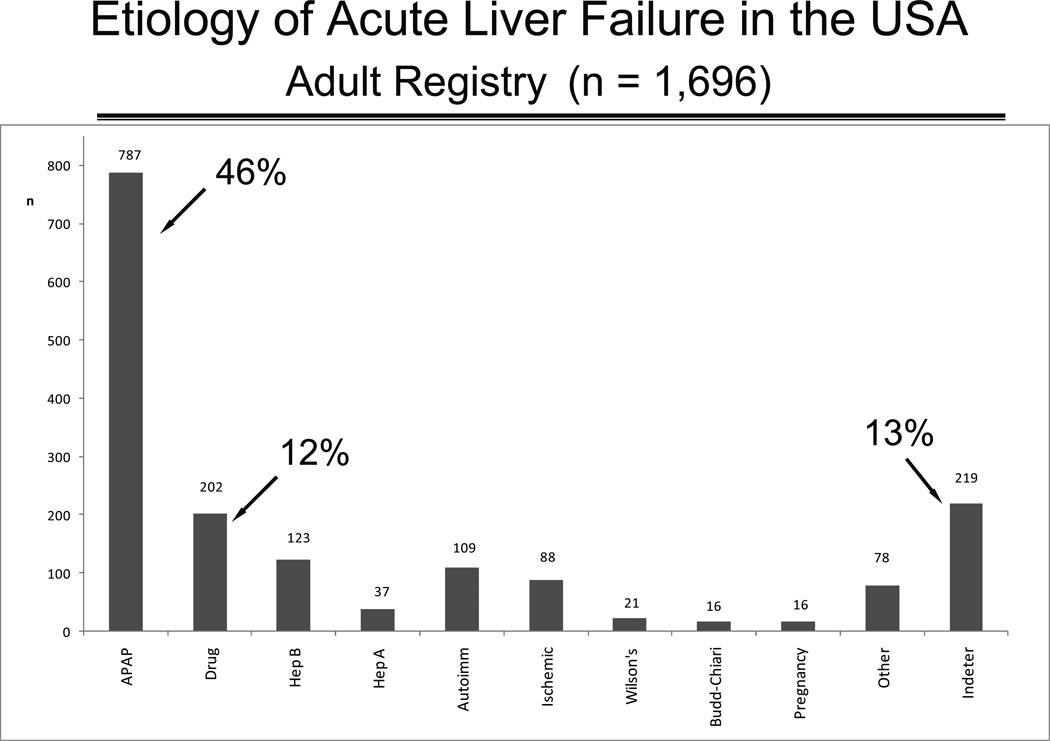
The causes of ALF are many and vary from country to country. In the era prior to transplantation, hepatitis B was very common and accounted for as many as 40–50% of US cases. Prior to 1990, although a well-recognized problem in the United Kingdom, acetaminophen did not account for more than a few US ALF cases. Current US figures provided by the ALFSG now show that acetaminophen-related acute liver failure accounts for nearly 50% of all cases, with hepatitis B responsible for only 7% (4,8,9). The etiologic breakdown in the US (Figure 1) is similar to that found in Europe but is far different in the developing world, such as India, where drug-induced liver injury is limited solely to isoniazid, and acetaminophen injury is virtually unknown (10).
Figure 1.
Bar graph depicting the percent of 1696 patients enrolled in the ALFSG according to etiology. The highest number and percentage of patients are those due to acetaminophen.
After acetaminophen, the most common causes are drug-induced liver injury (comprising largely idiosyncratic reactions to prescription drugs), autoimmune hepatitis and a variety of smaller groups. In many instances (15%), the etiology remains unclear despite extensive history taking and laboratory assessment and these are termed indeterminate. Failure to obtain adequate history prior to onset of coma is one reason for missing the cause of illness in this setting. Regardless of the cause, the final common pathway is remarkably similar: worsening coma associated with a propensity for bleeding, infection and renal failure lead to poor overall survival without transplantation.
Acetaminophen-related liver injury
Although acetaminophen was approved for use in the 1950’s, hepatotoxicity leading to liver failure was not recognized in significant numbers in the U.S. prior to 1980. With the linking of aspirin to Reyes syndrome in children around 1980, Americans began turning to acetaminophen as a safer alternative for children and adults. Cases were reported during the 1980’s of ‘therapeutic misadventures’ and the association of unintended acetaminophen poisoning with alcohol was made (11). Although true incidence studies were (and are) not available, no large case series of acute liver failure patients included acetaminophen until a retrospective study from the ALF Study Group, covering the period 1994–96, found that 20% of cases were related to acetaminophen (8). Similar data were reported in 2000, in which 28% of transplant registry cases were believed related to acetaminophen during a 13-year retrospective study from the University of Pittsburgh (12). However, the U.S. ALF Study, in its first prospective analysis, recorded 39% of all ALF cases as due to acetaminophen between 1998 and 2001 (3), increasing to 51% in 2004. These figures represent the proportion of cases due to acetaminophen and may not be equated to actual incidence. Nevertheless, the increases are striking.
It is important to distinguish between considering all cases entering the hospital with presumed acetaminophen overdose and that smaller subset that experiences acute liver failure. Of 71 acetaminophen overdose patients admitted to Parkland Hospital over a 39-month period, 50 were considered suicidal with only 1 of 50 resulting in ALF and death, whereas 6 deaths occurred among 21 unintentional overdoses (13). Only the 7 patients who died had developed acute hepatic failure, and only 10 of 50 suicidal patients had aminotransferase levels ≥ 1000 IU/L. These data confirmed that most suicidal patients typically receive medical care within 4 hours of ingestion, and are therefore reliably protected by the acetaminophen antidote, N-acetylcysteine (NAC). By contrast, overdoses that are called accidental, or more accurately unintentional, are associated with ingestion over several days, a specific cause of pain and denial of suicidal intent. Late presentation is characteristic of the unintentional group since there is no understanding of possible harm; medical attention only is sought after symptoms of toxicity have developed.
Unintentional acetaminophen-related acute liver failure
The U.S. ALF Study Group has provided a more detailed snapshot of all acetaminophen-related ALF cases (14), with particular attention to the unintentional group. Thirty eight percent of these patients were simultaneously taking more than one acetaminophen-containing preparation and 62% were taking a opioid combination such as hydrocodone and acetaminophen. In many instances, individuals are using alcohol, hypnotics or illicit drugs in combination, undoubtedly clouding judgment and often delaying hospitalization. In a small number of cases, habituation to the opioid compounds, (up to 40 or more tablets per day) may develop over days or weeks prior to onset of liver injury, suggesting addiction to the narcotic component with development of tolerance to the narcotic and to the acetaminophen. Those entering with severe hepatotoxicity have apparently exhausted their compensatory mechanisms. Tolerance has been observed in animals fed increasing acetaminophen doses (15). The events prior to presentation are often unclear but the biochemical picture (AST/ALT elevations/ bilirubin levels) in these ‘chronic’ patients is just as acute, suggesting that patients may tolerate both drugs, then experience ‘breakthrough’ for unclear reasons. It is possible that fasting due to an inter-current illness or simply increasing the dose once too often brings this about. Because patients with unintentional toxicity frequently exhibit signs of poly-substance abuse they may come to hospital later, and are less likely to receive a liver transplant.
As might be expected, suicidal ingestions that eventuate in acute liver failure are associated with late presentation, alcohol or other concomitant drugs that may cloud the sensorium, delaying presentation and larger total doses, indicating more serious intent rather than a gesture. Even with late presentation, N-acetylcysteine orally (and now available as an intravenous preparation, Acetadote®) is used and provides reliable protection against fatal injury preventing a large number of deaths when given within 12 hours of ingestion (16). Use after 24 hours is recommended but efficacy cannot be proven for these late ingestions, since injury is developed by 36 hours, peaking by 72 hours.
Traditionally, acetaminophen poisoning carries a very good prognosis, even if hepatic failure has developed. However, one third of those reaching the threshold of encephalopathy still die and only 7–9% undergo transplantation (4). The spontaneous survival of the acetaminophen patients who develop encephalopathy (64%) exceeds that for most other forms of acute liver failure, such as idiosyncratic drug toxicity, where survival without transplantation is only ~20% (14). Nevertheless, because of the sheer number of cases, deaths due to acetaminophen toxicity constitute the most frequent cause of death in our study. Suicidal predilection or the history of previous suicide attempts, like substance abuse, will often preclude transplant consideration. Once acute liver failure develops, the outcome for either type of overdose, suicidal or unintentional, is similar.
Other issues associated with acetaminophen
Acetaminophen cases are all considered hyperacute, with time from onset of jaundice to encephalopathy of 1 day versus idiosyncratic drugs or viral hepatitis where longer intervals are seen. Table 1 compares presenting and biochemical features of acetaminophen versus idiosyncratic drug reactions and other etiologic categories. As shown, acetaminophen ALF is characterized by very high aminotransferase levels and low bilirubins, while much lower aminotransferases and higher bilirubin levels characterize the more slowly evolving pictures.
Table 1. Comparison of Different ALF Etiology Groups.
Clinical and demographic features of acute liver failure according to etiologic groups. Acetaminophen-related ALF is considered hyperacute and demonstrates the very high aminotransferase and low bilirubin levels, compared to drug-induced liver injury which is more indolent.
| APAP n=787 |
Drug n=202 |
Indeterminate n=219 |
HepA/HepB n=37/123 |
All Others N=328 |
|
|---|---|---|---|---|---|
| Age (median) | 37 | 47 | 38 | 48/43 | 45 |
| Sex (% F) | 76 | 66 | 60 | 46/45 | 73 |
| Jaundice (Days) (median) |
0 | 8 | 8 | 3/5 | 4 |
| Coma ≥3 (%) | 53 | 37 | 50 | 51/55 | 43 |
| ALT (median) | 3846 | 685 | 849 | 2124/1702 | 677 |
| Bili (median) | 4.4 | 19.8 | 22.0 | 12.5/19.1 | 14.6 |
| Tx (%) | 9 | 40 | 45 | 32/41 | 30 |
| Spontaneous Survival (%) |
67 | 31 | 27 | 54/24 | 38 |
| Overall Survival (%) |
75 | 68 | 69 | 84/61 | 65 |
Recent studies from ALFSG and others highlight more subtle issues surrounding acetaminophen. Using a recently developed assay that reliably detects acetaminophen–containing protein adducts released into the plasma by dying hepatocytes, 20% of both adult and pediatric ALF patients with indeterminate etiology (no cause discerned after extensive investigation and testing) were found to be due to unrecognized acetaminophen poisoning (17,18). Adducts are not found with acetaminophen use unless there is toxicity Elevated adduct levels strongly implicate acetaminophen as the primary cause of the injury (Figure 2). The acetaminophen adducts assay provides the smoking gun, evidence of specific hepatocyte damage due to acetaminophen, when historical data is lacking; approximately 19% of the indeterminate group have levels of adducts comparable to those observed in suicidal cases, suggesting that a lack of history makes the case indeterminate (19). when the biochemical patterns of these indeterminate cases were analyzed, all cases fit the profile of high ALT, low bilirubin observed in the known acetaminophen cases. Insufficient data may result from patient obtundation, or denial, whether purposeful or out of ignorance.
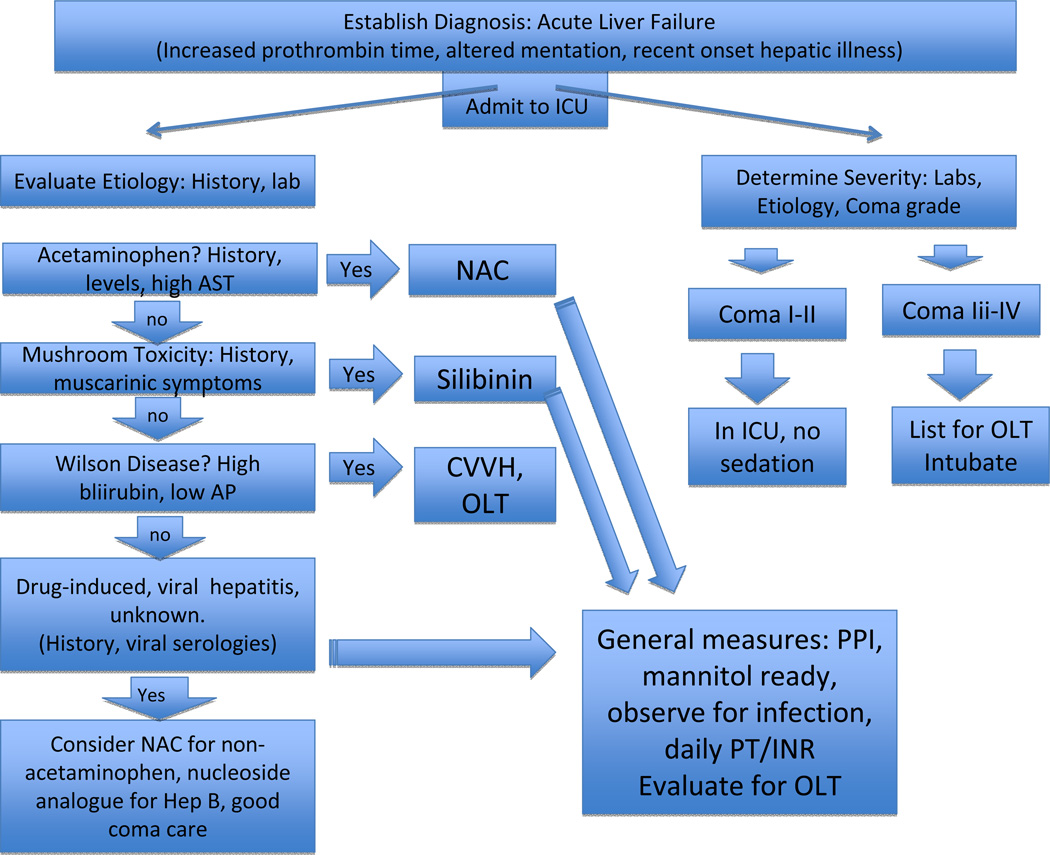
Figure 2.
Algorithm for triaging ALF patients. The initial step is to establish the diagnosis as ALF, then the etiology and then the severity. Time is of the essence if transplantation is an option. Patients may rapidly deteriorate so frequent neuro checks and consideration of intubation is indicated for any significant degree of drowsiness (Coma grade 2+ or higher). Sedation is avoided in early coma grades to allow for continued observation of CNS function.
Whether acetaminophen dosing should be limited in the presence of chronic liver disease or cirrhosis remains unclear. A recent paper has questioned the use of 4 grams/day as the standard cutoff for acetaminophen maximum dosing (20), and a recent FDA Advisory Panel recommended lowering the recommended daily dose of 4 gm and ‘unbundling’ the opioid-acetaminophen compounds. To date, FDA has implemented a change to lower to 325 mg the quantity of acetaminophen allowed in opioid combinations and industry is beginning to recommend lower total daily doses (21,22).
Practice Points
Acetaminophen injury is very common but still can be missed when the history is obscured or the patient comatose on arrival.
The characteristic high AST or ALT and low bilirubin is often the hint that this is acetaminophen toxicity.
Use of acetaminophen adducts can be helpful in puzzling cases to confirm or exclude the diagnosis.
Idiosyncratic drug reactions
The developed world is particularly subject to rare acute liver failure due to idiosyncratic drug induced liver injury (DILI), because of the large quantity of drugs we ingest. Still, prescription drugs are remarkably safe; the combined number of idiosyncratic drug cases thought to cause ALF in our series is dwarfed by the single agent, acetaminophen. The presentation of DILI is more subacute as shown in Table 1, with lower aminotransferases and higher bilirubin levels. The likelihood of survival in this setting is less than 30%, and they more often undergo liver transplantation (23,24). Most frequently implicated drugs include antibiotics (most commonly anti-tuberculous medications, but also sulfa drugs and others). Next most common are non-steroidal anti-inflammatory agents and anti-convulsants.
Viral hepatitis
Cases of viral hepatitis that develop hepatic failure represent a small fraction of all cases (~1%) and are largely comprised of hepatitis B, with hepatitis A less frequent and hepatitis E quite prevalent in areas with poor sanitation. Hepatitis B that presents with a fulminant picture may be due to new acute infection or an acute exacerbation of chronic hepatitis B (25).
Other causes
A variety of other etiologies have been implicated from autoimmune hepatitis (which may be difficult to diagnose, 26), Budd-Chiari syndrome (hepatic vein thrombosis), Wilson disease (27), pregnancy-associated liver failure and the rapid evolution of metastatic or lymphomatous hepatic infiltration (28).
Indeterminate cases
Despite our best efforts, in 15% of patients the diagnosis eludes us. To date, we have found little evidence for other viruses such as parvovirus B19, hepatitis E, herpesviruses (there are a few cases, but not many), occult hepatitis B or other previously unrecognized viruses (29,30). Unrecognized acetaminophen toxicity is clearly implicated and there may be unrecognized autoimmune disease as noted above (19,26).
Practice Points
Determining etiology of ALF quickly is essential to management and understanding prognosis.
Clinical distinctions between acetaminophen and most other causes are readily made.
The frequency of different etiologies causing ALF has evolved over time with viral hepatitis (A and B) on the decline.
Research Agenda
A better understanding of pathogenesis is vital to identify new therapies.
Future strategies to identify at risk individuals might help prevent acetaminophen toxicity.
Better understanding of hepatitis B-related ALF might help prevent such cases.
Clinical Management
The hallmark of management of the ALF patient is good coma care (5,6,31). Rapid evaluation and initiation of antidotes where feasible is needed: N-acetylcysteine for acetaminophen poisoning, penicillin G and silybinin for mushroom poisoning and delivery of the fetus in the cases of pregnancy induced ALF are standard of care. Early consideration should be made to use N-acetylcysteine (NAC) in non-acetaminophen settings. A multi-center double blind, placebo controlled study of its use showed no benefit in overall survival but improved outcomes at 3 weeks and at one year for those with early coma grades (32). All such trials are compromised by the variety of etiologies and presentations and the rescue provided by hepatic transplantation so that survival of a large number of patients (those receiving liver grafts) is greatly improved but their true survival without grafting is never known. Only those with acute liver failure are given the most urgent listing by the United Network for Organ Sharing (UNOS). In the NAC clinical trial, 50% of those who were considered to have stage 3 or 4 encephalopathy on admission to study reached an outcome (death or transplant) by study day 4, emphasizing the severity of their condition and the availability (or lack) of transplantation for many patients—40% ultimately were transplanted. Once the diagnosis of ALF is confirmed (by the finding of coagulopathy and encephalopathy in the setting of acute hepatitis) and a careful search for etiology is undertaken (Figure 3), attention should be turned to consideration of the need to list for transplantation. Controversial management issues that are commonly of concern include managing the coagulopathy, whether intracranial pressure (ICP) monitoring is used, and the treatment of cerebral edema. By establishing that the diagnosis is ALF, and then determining the etiology and severity appropriate triage to an ICU or transfer to a transplant center can be rapidly accomplished. Determining eligibility and listing for transplantation must be an early consideration as time is of the essence.
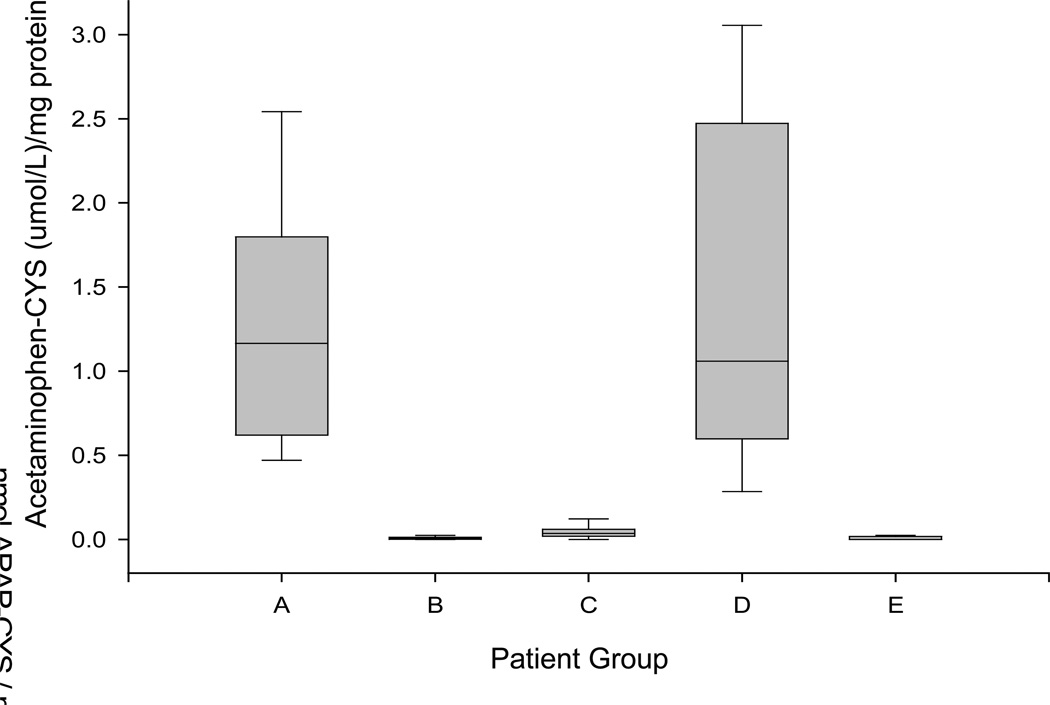
Figure 3.
Serum levels of acetaminophen–CYS adducts in patient groups. (A) Patients with ALF secondary to known acetaminophen overdose. (B) Patients with ALF owing to nonacetaminophen causes. (C) Patients with acetaminophen overdose but no ALF. (D) Patients with ALF of indeterminate etiology and detectable serum adducts. (E) Patients ALF of indeterminate etiology and negative adducts. The boxes represent the 25th–75th IQR and the horizontal line represents the median. The extremes of the population are represented by the endmarks. (Reprinted with permission from reference 17).
Specific therapies
The few therapies that are specific to an etiology emphasize the need for determination of the cause of the illness as soon as is possible (Figure 3). One all-to-frequent scenario is the reactivation of chronic hepatitis B during cancer treatment or immunosuppression (25). Very poor outcomes result since overwhelming infection occurs with massive necrosis of hepatocytes in the setting of previously unrecognized hepatitis B (33). Nucleoside analogues have been suggested as treatment for acute liver failure due to hepatitis B. In the setting of planned chemotherapy, they are mandated and will completely protect against reactivation. Although effective as prophylaxis, there is no strong evidence that their use, once acute liver failure develops, improves outcomes. Still, since transplantation is frequently required, nucleoside analogues are routinely used for their post-transplant prophylaxis effect (34).
Cerebral edema and the role of intracranial pressure monitoring
Cerebral edema is the cause of death in a number of patients with acute liver failure but the actual percentage is unclear. Development of brain swelling due to osmotic changes within the brain and loss of capillary integrity occurs more commonly in hyperacute settings and in young people, where adaptation to rapid changes in brain glutamine levels cannot take place. Thus, acetaminophen patients, typically under age 35, are at highest risk for brain edema. High levels of arterial ammonia have been shown to correlate well with development of cerebral edema, further reinforcing the glutamine hypothesis (35–38). A retrospective study of the impact of ICP monitoring in 332 ALF patients suggested that use of ICP monitoring was center dependent, reflecting, among other things, the confidence level of the neurosurgeon, since a burr hole in the skull is needed and may be hazardous in the presence of severe coagulopathy. Patients in whom a monitor was placed generally received more aggressive mannitol and other treatments when evidence was available clinically of increased ICP levels, and were more likely to be listed for transplantation, but outcomes did not appear to differ in the two groups (39).
Cardiovascular tone and its management
Most patients with ALF demonstrate ‘warm shock’, that is, profound vasodilation and low mean arterial pressures. In addition, due to poor oral intake and encephalopathy prior to admission, most are volume depleted and require initial fluid resuscitation and possibly central venous pressure determinations to aid in this process. Use of pulmonary artery catheters are seldom recommended. The renal hemodynamics resemble those of the patient with hepato-renal syndrome in cirrhosis: low systemic vascular resistance and renal artery vasoconstriction that is potentially reversible with improvement in liver function. Patients with acetaminophen poisoning may demonstrate acute kidney injury due to direct toxic effects of acetaminophen with oliguria requiring continuous venovenous hemodialysis (CVVHD). The initial aim should be volume resuscitation and, if the mean arterial pressure is < 80 mmHg, support with pressor agents, the most effective of which appears to be norepinephrine (6). In the setting of multi-organ failure, plasma troponin I levels are frequently elevated, but the significance of this finding is unclear (40).
Role of coagulopathy in ALF
Severe alterations in nearly all clotting parameters are seen in ALF in part due to failure of synthesis as well as factor consumption. The classic interpretation has been that severe bleeding is a likely outcome. Overall, however, there are abnormalities in both the coagulation and fibrinolytic pathways and recent data suggest that the defects are balanced, that is, there is a relative preservation of hemostasis unless the platelet count is very low (41). The reason for low platelet counts has not been elucidated—consumption may play a role whereas thrombopoietin is also made within the liver. Thrombopoietin levels are not uniformly low and do not appear to correlate with platelet levels (42). In general, fresh frozen plasma (FFP), cryoprecipitate or platelets is reserved for active bleeding or if an invasive procedure is planned. There is little support for the use of recombinant factor VIIa in this setting. Withholding FFP allows for the use of the INR has a continuously available and reliable measure of hepatic synthetic function and outcome.
Infection in ALF
The milieu of inflammation and necrosis in acute liver failure (ALF) is presumed to predispose patients to the development of infection. Proposed mechanisms of susceptibility include severe complement deficiency as well as impaired polymorphonuclear and Kupffer cell function. Over the past 30 years, reports of ALF-related infections have varied considerably. The incidence of bacteremia has been cited between 22% and 80%, with fungemia noted in 32%. No recent studies have directly considered the role of infection in worsening outcomes, or whether prophylactic antibiotics are of value in this setting (43,44).
Practice Points
Consideration should be given to the use of NAC for non-acetaminophen ALF, given its good safety profile and evidence of efficacy in early coma grade patients.
Do not replace clotting factors unless bleeding is actually occurring—use INR as a prognostic tool.
Cerebral edema may be on the decline for unclear reasons.
ICP monitoring remains in use in some centers but is controversial.
Research Agenda
New therapies are sorely needed; however, many patients present late, when rescue short of transplantation is highly unlikely.
Study should be made of the use of checklists in the ICU setting.
Further studies of the role of infection in ALF are needed.
Prognosis
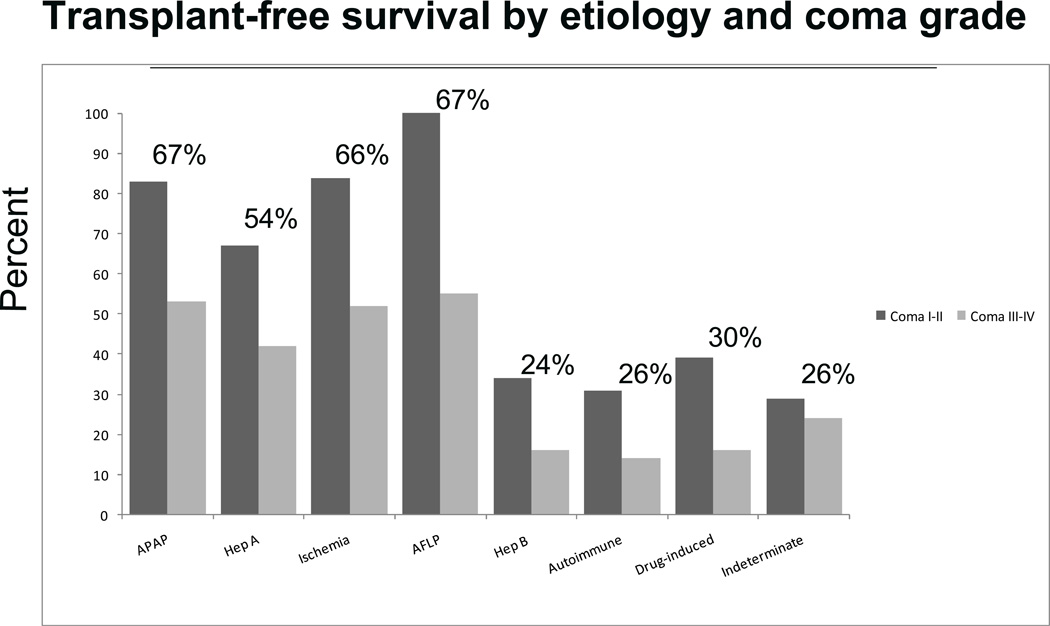
Determining prognosis in ALF is vital. Unlike cirrhosis, use of a transplant in ALF would be unnecessary if the patient can fully recover without a graft. If transplanted, life long immunosuppression is also the dividend. Prognostic scores specific for ALF have been limited in efficacy; the most commonly utilized is the King College Criteria (KCC, 45). These and other scoring systems to date have been relatively sensitive but not specific with fairly robust prediction of death but inability to reliably determine who will survive. This may be because more patients could recover if complications such as infection did not occur. Two key factors involved in determining outcome are etiology and coma grade on admission (Figures 3 and 4). Acetaminophen, hepatitis A, ischemia and pregnancy are all associated with at least 60% short-term survival without transplantation, while DILI, hepatitis B, autoimmune hepatitis and indeterminate causes are around 30% spontaneous survival. Presenting with early coma grade allows for a much more favorable outcome prediction across all etiologies (Figure 4). The Model for End-stage Liver Disease (MELD) has been used in the ALF setting but is not clearly superior to existing prognostic scoring methods (46,47).
Figure 4.
Outcomes according to etiology and coma grade. The bars for each etiology indicate the percent survival without transplantation according to coma grade (I,II vs. III,IV). The percent above each pair indicates the overall percentage of spontaneous survival for that etiology. There is a disparity between the etiologies with good survival and those with poor survival but much poorer in all categories if patient is admitted with an advanced coma grade.
Role of transplantation
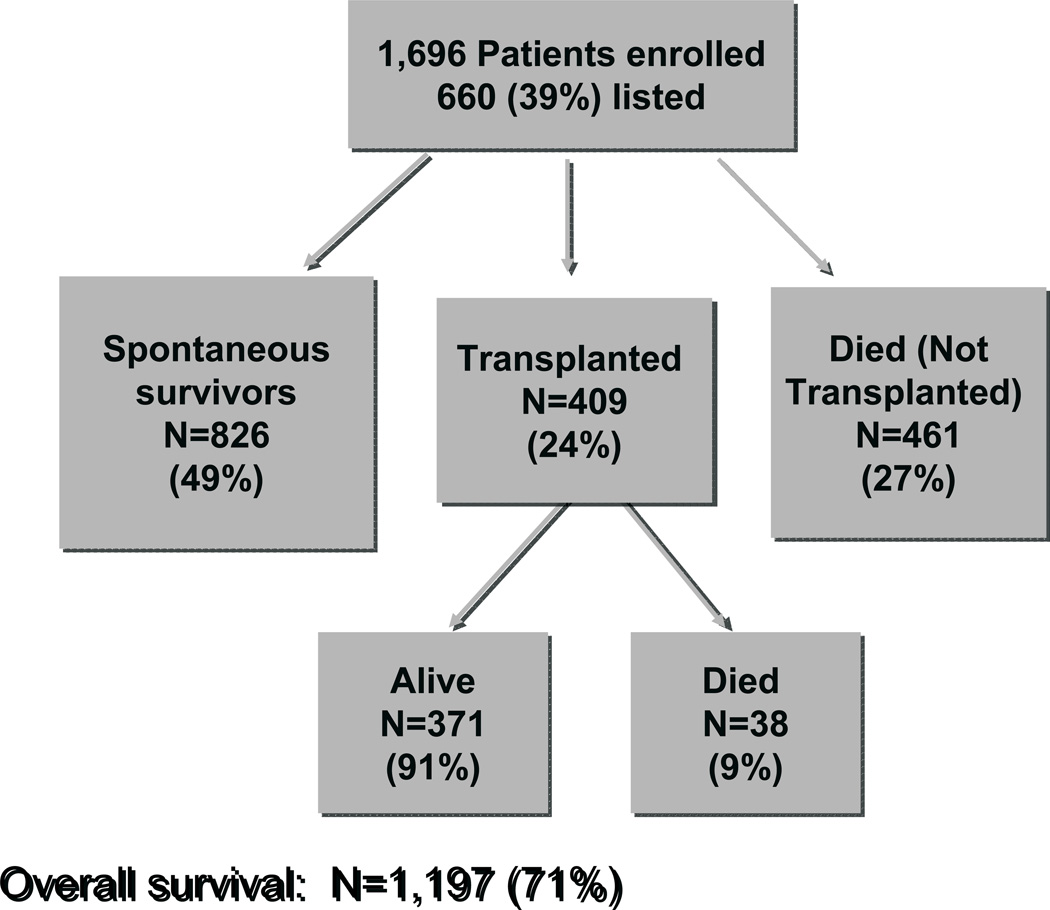
There is no fully proven medical therapy for this devastating clinical syndrome. Prior to 1980, ALF series demonstrated survival in less than 10%. Small series of cases subjected to new experimental treatments (e.g., heparin!) showed that occasionally patients do survive; however, controlled trials were virtually impossible to perform. In this setting, transplantation seemed nothing short of a miracle. In the current era, overall survival is approximately 67%, a considerable improvement in the last 3 decades. However, transplants are only performed on 25–30% of patients admitted to the ALF study (Figure 5). The reasons for the relatively infrequent use of liver grafting includes the difficulties in obtaining organs in urgent fashion, as well as the changing mix of etiologies. The change from hepatitis B to a predominance of acetaminophen makes up much of these differences, since acetaminophen clearly carries a better prognosis than most other etiologies and is often associated with compromising psychosocial issues and substance abuse.
Figure 5.
Outcomes for 1,696 patients enrolled in the ALFSG study. Only 39% are listed for transplantation and 24% receive a graft.
Even in the transplant era, nearly 30% of patients die with ALF. Reasons for the failure to transplant more patients have included lack of available organs, late presentation with rapid deterioration, substance abuse issues, repeated suicidal behavior or other organ system involvement (malignancy, heart failure) that would preclude grafting. Patients undergoing transplantation for ALF are typically young and otherwise healthy and thus should be quite optimal candidates compared to their cirrhosis counterparts. Nevertheless, short term and one year survival figures are below that for cirrhotic patients in part because of the extreme emergency conditions often encountered (48). There is no option for deferring transplant in these patients. Similarly, living related donors might solve the organ shortage; however, it is extremely difficult to prepare a donor adequately and perform the surgery in a timely fashion. Few living related donors have been used to date in adults with ALF although this has gained some traction in pediatric acute liver failure where the graft size needed can be quite small (49). Despite full physical recovery, many patients who have experienced cerebral edema have mental impairment that is life long. To date, little long-term data on these complications are available.
New Therapies Undergoing Current Trial
To date, the NAC trial is one of the very few controlled trials in ALF and its results remain controversial (50). More recent efforts have been directed toward reliable means to lower ammonia levels. Neither lactulose nor rifaximin has been shown to favorably affect outcomes or coma grade in acute liver failure but they are still widely used. Surgeons discourage the use of lactulose since it may lead to gaseous distension of the colon, interfering with access to the surgical field. A blinded, controlled trial performed in India sought to test the value of L-ornithine L-acetate infusions in 203 patients with ALF a substance that would alter the balance of glutamate toward glutamine in the circulation thus trapping ammonia, based on animal studies (51). There appeared to be no benefit in patients either in lowering ammonia or in improving outcomes, perhaps because ammonia is regenerated by gut glutaminases (52). A new compound, ornithine phenyl acetate, is currently under consideration after several trials in experimental animals (53–55).
Hypothermia to protect brain function has also been demonstrated in animals and man to reliably lower intracranial pressure. It is not routinely used at present, in part due to uncertainties regarding its safety. The challenging logistics of performing clinical trials in patients with ALF to evaluate hypothermia has thus far defeated efforts to demonstrate its safety or efficacy.
Bio-artificial liver assist devices
An artificial liver device that would replace the diverse functions of the liver is an unmet need that seems far in the future. A number of groups have designed membrane-containing cartridges filled with hepatocytes from human cell lines or animal donors, allowing plasma to flow by the cells. Some improvement in encephalopathy has been observed but no real improvement could be demonstrated in overall survival in a controlled trial (56,57). A recent meta-analysis further demonstrated the scarcity of data and the lack of convincing efficacy (58). Several centers have experimented with extracorporeal transgenic pig livers or cadaver livers deemed unfit for transplantation but these techniques have not gained general acceptance (59). Finally, early experimentation is underway using infusions of hepatic stem cells that are said to be non-immunogenic (60); this remains highly experimental.
Practice Points
Nothing approaching a perfect prognostic score has been achieved to date.
Listing for transplantation does not preclude removing from the list should an organ become available and the situation either deteriorate or improve significantly.
Ammonia-lowering agents may prolong short-term survival so that patients can be optimized prior to transplantation or so that natural recovery processes can occur.
Research Agenda
An artificial liver is still a long away from reality.
Bioinformatics might help manage the reams of data resulting from measuring multiple analytes.
Perhaps a prognostic score cannot be developed in this setting—patients are too heterogeneous.
Summary
Acute liver failure challenges our best clinical and surgical skills because of its rarity, rapid progression and frequently bad outcomes. The small numbers of patients do not readily lend themselves to controlled trials and are studied only with great difficulty. Evidence-based medicine in this field is virtually non-existent. Nevertheless, descriptive information aids our understanding of what to expect and where small gains might be made in this condition. Patients may be vulnerable to infection, bleeding and cerebral edema and seek medical care typically with very advanced hepatic injury. It is unlikely that there is one overall therapy that will improve hepatic function and restore hepatocyte mass in this condition. Rescue therapies that provide temporary liver support, or other treatments short of transplantation that don’t enhance hepatic regeneration, are likely to fail unless there is reconstitution of functional hepatic stem cells. Most of our efforts in treating ALF in 2011 should be directed toward prevention applied to various etiologic categories. Possible targets might include, for acetaminophen, further education regarding the risk of acetaminophen liver injury, and limitation of over the counter use of this harmful agent.
Acknowledgments
Funding source:
The Acute Liver Failure Study Group is funded by NIDDK U-01 Cooperative Research Agreement DK-58369. Additional support provided by the Rollin and Mary Ella King and the Jeanne Roberts Funds of the Southwestern Medical Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
None
References
- 1.Trey C, Davidson CS. The management of fulminant hepatic failure. In: Popper H, Schaffner F, editors. Progress in liver diseases. New York: Grune & Stratton; 1970. pp. 282–298. [PubMed] [Google Scholar]
- 2.Lee WM, Squires RH, Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute Liver Failure: Summary of a Workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 4.Ostapowicz GA, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 5.Stravitz RT, Kramer AH, Davern T, Shaikh AOS, Caldwell SH, Mehta RL, et al. Intensive care of patients with acute liver failure: Recommendations of the Acute Liver Failure Study Group. Crit Care Med. 2007;35:2498–2508. doi: 10.1097/01.CCM.0000287592.94554.5F. [DOI] [PubMed] [Google Scholar]
- 6.Stravitz RT, Kramer DJ. Management of acute liver failure. Nat Rev Gastroenterol Hepatol. 2009;6:542–553. doi: 10.1038/nrgastro.2009.127. [DOI] [PubMed] [Google Scholar]
- 7.Lee WM, Schiødt FV. Fulminant hepatic failure. In: Schiff ER, Sorrell MF, Maddrey WC, editors. Schiff's Diseases of the Liver. 8th ed. Philadelphia: Lippincott-Raven Publishers; 1999. pp. 879–895. [Google Scholar]
- 8.Schiødt FV, Atillasoy E, Shakil O, Lee WM, et al. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transplant Surg. 1999;5:29–34. doi: 10.1002/lt.500050102. [DOI] [PubMed] [Google Scholar]
- 9.Lee WM, Seremba E. Etiologies of acute liver failure. Curr Opin Crit Care. 2008;14:198–201. doi: 10.1097/MCC.0b013e3282f6a420. [DOI] [PubMed] [Google Scholar]
- 10.Acharya SK, Dasarathy S, Kumer TL, Sushma S, Prasanna KSU, Tandon A, et al. Fulminant hepatitis in a tropical population: clinical course, cause, and early predictors of outcome. Hepatology. 1996;23:1448–1455. doi: 10.1002/hep.510230622. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman HJ, Maddrey WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology. 1995;22:767–773. [PubMed] [Google Scholar]
- 12.Shakil AO, Kramer D, Mazariegos GV, Fung JJ, Rakela J. Acute liver failure: clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transpl. 2000;6:163–169. doi: 10.1002/lt.500060218. [DOI] [PubMed] [Google Scholar]
- 13.Schiodt FV, Rochling FA, Casey DL, Lee WM. Acetaminophen toxicity in an urban county hospital. N Engl J Med. 1997;337:1112–1117. doi: 10.1056/NEJM199710163371602. [DOI] [PubMed] [Google Scholar]
- 14.Larson AM, Fontana RJ, Davern TJ, Polson J, Lalani EK, Hynan LS, Reisch JS, Shakil OA, Schiødt FV, Ostapowicz GA, Lee WM the ALFSG. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology. 2005;42:1367–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 15.Shayiq RM, Roberts DW, Rothstein K, Snawder JE, Benson W, Ma X, et al. Repeat exposure to incremental doses of acetaminophen provides protection against acetaminophen-induced lethality in mice: an explanation for high acetaminophen dosage in humans without hepatic injury. Hepatology. 1999;29:451–463. doi: 10.1002/hep.510290241. [DOI] [PubMed] [Google Scholar]
- 16.Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11:525–548. doi: 10.1016/j.cld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Davern TJ, II, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, et al. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–694. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 18.James LP, Alonso EM, Hynan LS, Hinson JA, Davern TJ, Lee WM, et al. Detection of acetaminophen-protein adducts in children with acute liver failure of indeterminate cause. Pediatrics. 2006;118:e676–e681. doi: 10.1542/peds.2006-0069. [DOI] [PubMed] [Google Scholar]
- 19.Khandelwal N, James LP, Sanders C, Larson AM, Lee WM Acute Liver Failure Study Group. Unrecognized acetaminophen toxicity as a cause of indeterminate acute liver failure. Hepatology. 2011;53:567–576. doi: 10.1002/hep.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, Harris SC. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen per day. JAMA. 2006;296:87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 21.FDA Drug Safety Communication. [accessed August 21, 2011]; http://www.fda.gov/Drugs/DrugSafety/ucm239821.htm.
- 22. [accessed August 21, 2011]; http://www.nytimes.com/2011/01/14/health/policy/14fda.html?scp=1&sq=tylenol%20package&st=cse,
- 23.Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reuben A, Koch DG, Lee WM the Acute Liver Failure Study Group. Drug induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology. 2010;52:2065–2078. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dao DY, Hynan LS, Yuan HJ, Sanders C, Balko J, Attar N, Lok ASF, Lee WM. Two distinct types of hepatitis B-related acute liver failure are separable by quantitative anti-HBc IgM and HBV DNA levels. Hepatology. 2011 doi: 10.1002/hep.24732. Epub ahead of print, October 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stravitz RT, Lefkowitch JH, Fontana RJ, Gershwin ME, Leung PS, Sterling RK, Manns MP, Norman GL, Lee WM the ALFSG. Autoimmune acute liver failure: Proposed clinical and histological criteria. Hepatology. 2011;53:517–526. doi: 10.1002/hep.24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korman JD, Volenberg I, Balko J, Webster J, Schiodt FV, Squires RH, Jr, Lee WM, Schilsky M. Screening for Wilson disease in acute liver failure: A comparison of currently available diagnostic tests. Hepatology. 2008;48:1167–1174. doi: 10.1002/hep.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowbotham D, Wendon J, Williams R. Acute liver failure secondary to hepatic infiltration: a single centre experience of 18 cases. Gut. 1998;42:576–580. doi: 10.1136/gut.42.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee WM, Brown KE, Young NS, Dawson GJ, Schlauder GG, Gutierrez RA, Fontana R, Rossaro L, Davern T, Lalani E the Acute Liver Failure Study Group. Brief Report: No evidence for hepatitis E or parvovirus B19 Infection in patients with acute liver failure. Dig Dis Sci. 2006;51:1712–1715. doi: 10.1007/s10620-005-9061-5. [DOI] [PubMed] [Google Scholar]
- 30.Levitsky J, Thadareddy A, Lakeman FD, Whitley RJ, Luby JP, Lee WM, Fontana RJ, Blei AT, Ison MG. Detection and diagnosis of herpes simplex virus infection in adults with acute liver failure. Liver Transplant. 2008;14:1498–1504. doi: 10.1002/lt.21567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polson J, Lee WM. AASLD Position Paper: Acute Liver Failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 32.Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, Davern TL, III, Murray NG, McCashland T, Reisch J, Robuck PR the ALFSG. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–864. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oketani M, Ido A, Tsubouchi H. Changing etiologies and outcomes of acute liver failure: A perspective from Japan. J Gastroenterol Hepatol. 2011;26(Suppl 1):65–71. doi: 10.1111/j.1440-1746.2010.06574.x. [DOI] [PubMed] [Google Scholar]
- 34.Sorrell MF, Belongia EA, Costa J, Gareen IF, Grem JL, Inadomi JM, et al. National Institutes of Health Consensus Development Conference Statement: management of hepatitis B. Ann Intern Med. 2009;150:104–110. doi: 10.7326/0003-4819-150-2-200901200-00100. [DOI] [PubMed] [Google Scholar]
- 35.Blei AT. Pathogenesis of brain edema in fulminant hepatic failure. Prog Liver Dis. 1995;13:311–330. [PubMed] [Google Scholar]
- 36.Jalan R. Pathophysiological basis of therapy of raised intracranial pressure in acute liver failure. Neurochem Int. 2005 Jul;47(1–2):78–83. doi: 10.1016/j.neuint.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Larsen FS, Wendon J. Prevention and management of brain edema in patients with acute liver failure. Liver Transpl. 2008 Oct;14(Suppl 2):S90–S96. doi: 10.1002/lt.21643. [DOI] [PubMed] [Google Scholar]
- 38.Clemmesen JO, Larsen FS, Kondrup J, Hansen BA, Ott P. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology. 1999;29:648–653. doi: 10.1002/hep.510290309. [DOI] [PubMed] [Google Scholar]
- 39.Vaquero J, Fontana RJ, Larson AM, Bass NM, Davern TJ, Shakil AO, Han S, Harrison ME, Stravitz TR, Muñoz S, Brown R, Lee WM, Blei AT. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl. 2005;11:1581–1589. doi: 10.1002/lt.20625. [DOI] [PubMed] [Google Scholar]
- 40.Parekh NK, Hynan LS, De Lemos J, Lee WM the ALF Study Group. Elevated troponin I levels in acute liver failure: Is myocardial injury an integral part of the acute liver failure? Hepatology. 2007;45:1489–1495. doi: 10.1002/hep.21640. [DOI] [PubMed] [Google Scholar]
- 41.Stravitz RT, Lisman T, Luketic VA, Sterling RK, Puri P, Fuchs M, Ibrahim A, Lee WM, Sanyal AJ. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol. 2011 May 19; doi: 10.1016/j.jhep.2011.04.020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schiodt FV, Balko J, Schilsky MC, Harrison EH, Thornton A, Lee WM the ALFSG. Thrombopoietin in acute liver failure. Hepatology. 2003;37:558–561. doi: 10.1053/jhep.2003.50113. [DOI] [PubMed] [Google Scholar]
- 43.Vaquero J, Polson J, Chung C, Helenowski I, Schiodt FV, Reisch J, Lee WM, Blei AT the Acute Liver Failure Study Group. Infection and the progression to deep hepatic encephalopathy in early fulminant hepatic failure. Gastroenterology. 2003;125:755–764. doi: 10.1016/s0016-5085(03)01051-5. [DOI] [PubMed] [Google Scholar]
- 44.Rolando N, Wade JJ, Stangou A, Gimson AE, Wendon J, Philpott-Howard J, Casewell MW, Williams R. Prospective study comparing the efficacy of prophylactic parenteral antimicrobials, with or without enteral decontamination, in patients with acute liver failure. Liver Transpl Surg. 1996;2:8–13. doi: 10.1002/lt.500020103. [DOI] [PubMed] [Google Scholar]
- 45.O'Grady J, Alexander G, Hayllar K, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 46.Zaman MB, Hoti E, Qasim A, Maguire D, McCormick PA, et al. MELD score as a prognostic model for listing acute liver failure patients for liver transplantation. Transplant Proc. 2006;38:2097–2098. doi: 10.1016/j.transproceed.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Katoonizadeh A, Decaestecker J, Wilmer A, Aerts R, Verslype C, Vansteenbergen W, et al. MELD score to predict outcome in adult patients with non-acetaminophen-induced acute liver failure. Liver Int. 2007;27:329–334. doi: 10.1111/j.1478-3231.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- 48.Lee WM, Squires RH, Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute Liver Failure: Summary of a Workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shouval DS, Mor E, Avitzur Y, Shamir R, Bar-Nathan N, Steinberg R, et al. Living-related donor liver transplantation for children with fulminant hepatic failure in Israel. J Pediatr Gastroenterol Nutr. 2009;48:451–455. doi: 10.1097/MPG.0b013e318196c379. [DOI] [PubMed] [Google Scholar]
- 50.Hanje AJ, Michaels A, Lee WM. Is N-acetylcysteine effective in all non-acetaminophen acute liver failure? In: Jensen Donald M., editor. Controversies in Hepatology. Philadelphia: Slack Incorporated; 2011. [Google Scholar]
- 51.Acharya SK, Bhatia V, Sreenivas V, et al. Efficacy of L-ornithine L-aspartate in acute liver failure: a double-blind, randomized, placebo-controlled study. Gastroenterology. 2009;136:2159–2168. doi: 10.1053/j.gastro.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 52.Lee WM, Jalan RV. Treatment of hyperammonemia in liver failure: A tale of two enzymes. Gastroenterology. 2009;136:2048–2051. doi: 10.1053/j.gastro.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 53.Davies NA, Wright G, Ytrebo LM, Stadlbauer V, Fuskevag OM, Zwingmann C, et al. L-ornithine and phenylacetate synergistically produce sustained reduction in ammonia and brain water in cirrhotic rats. Hepatology. 2009;50:155–164. doi: 10.1002/hep.22897. [DOI] [PubMed] [Google Scholar]
- 54.Ytrebo LM, Kristiansen RG, Maehre H, Fuskevag OM, Kalstad T, Revhaug A, et al. L-ornithine phenylacetate attenuates increased arterial and extracellular brain ammonia and prevents intracranial hypertension in pigs with acute liver failure. Hepatology. 2009;50:165–174. doi: 10.1002/hep.22917. [DOI] [PubMed] [Google Scholar]
- 55.Demetriou A, Brown RS, Busuttil RW, Fair J, McGuire BM, et al. Prospective, Randomized, Multicenter, Controlled Trial of a Bioartificial Liver in Treating Acute Liver Failure. Ann Surg. 2000;239:660–670. doi: 10.1097/01.sla.0000124298.74199.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jalan R, Sen S, Williams R. Prospects for extracorporeal liver support. Gut. 2004;53:890–898. doi: 10.1136/gut.2003.024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J, Khaergard LL, Asl-Nielsen B, Gluud C. Artificial and bioartificial support systems for liver failure: a Cochrane Hepato-Biliary Group Protocol. Liver. 2002;22:433. doi: 10.1034/j.1600-0676.2002.01554.x. [DOI] [PubMed] [Google Scholar]
- 58.Chari RS, Collins BH, Magee JC, DiMaio JM, Kirk AD, Harland RC, et al. Brief report: treatment of hepatic failure with ex vivo pig liver perfusion followed by liver transplantation. N Engl J Med. 1994;331:234–237. doi: 10.1056/NEJM199407283310404. [DOI] [PubMed] [Google Scholar]
- 59.Horslen SP, Hammel JM, Fristoe LW, Kangas JA, Collier DS, Sudan DL, et al. Extracorporeal liver perfusion using human and pig livers for acute liver failure. Transplantation. 2000;70:1472–1478. doi: 10.1097/00007890-200011270-00014. [DOI] [PubMed] [Google Scholar]
- 60.Fukumitsu K, Yagi H, Soto-Gutierrez A. Bioengineering in organ transplantation: targeting the liver. Transplant Proc. 2011;43:2137–2138. doi: 10.1016/j.transproceed.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]