Abstract
One of the strongest associations with autoantibodies directed to components of the SSA/Ro-SSB/La ribonucleoprotein complex is the development of congenital heart block (CHB) in an offspring, an alarming prospect facing 2% of primigravid mothers with these reactivities. This risk is 10-fold higher in women who have had a previously affected child with CHB. Anti-Ro/La antibodies are necessary but insufficient to cause disease. In vitro and in vivo experiments suggest that the pathogenesis involves exaggerated apoptosis, macrophage/myfibroblast crosstalk, TGFβ expression and extensive fibrosis in the conducting system and in some cases surrounding myocardium. A disturbing observation is the rapidity of disease progression, with advanced heart block and life-threatening cardiomyopathy observed <2 weeks from normal sinus rhythm. Once 3rd degree (complete) block is identified, reversal has never been achieved, despite dexamethasone. Current strategies include the evaluation of an early echocardiographic marker of injury, such as a prolonged PR interval and the use of IVIG as a preventative measure for pregnancies of mothers with previously affected children.
Keywords: anti-Ro/La antibodies, congenital heart block, PR interval
With regard to the role of human autoantibodies in tissue injury, one of the most powerful lessons relates to the anti-SSA/Ro-SSB/La system. Indeed, congenital heart block (CHB) absent structural abnormalities and diagnosed before 26 weeks of gestation predicts exposure to anti-SSA/Ro-SSB/La antibodies in greater than 85% of cases independent of maternal health status. CHB is a manifestation of the so called neonatal lupus syndromes [1]. It is considered a pathologic readout of passively acquired autoimmunity consequent to FcRn-mediated placental transport of antibodies reactive with protein components of the SSA/Ro-SSB/La ribonucleoprotein complex [2, 3]. Third degree heart block is the prototypic cardiac lesion and carries a significant mortality (20–30%, primarily foetal and neonatal) and morbidity (67% of surviving affected children require permanent pacing before adulthood) [4–8]. Evidence is emerging that in addition to conduction disease (which can be less advanced), 10–15% of affected offspring will have a life-threatening cardiomyopathy [9–12]. This more extensive injury can occur postnatally, even as late as 10 years of age. Other manifestations of neonatal lupus include cutaneous, liver and haematological disease, all of which generally disappear coincident with the clearance of maternal antibodies from the neonatal circulation by approximately 8 months (reviewed in [13]).
The relative rarity of autoimmune-associated CHB has posed a challenge to clinical and epidemiologic researchers. The establishment in 1994 of the Research Registry for Neonatal Lupus [4], a rare disease registry funded by the US National Institutes of Health, has enabled the acquisition of larger and thus more statistically reliable series. As of October 2008, the Registry has enrolled 416 families in which 449 children have a manifestation of neonatal lupus and the maternal sera has documented antibodies to at least SSA/Ro. In total, 260 children have CHB, 36 have CHB and rash, 139 have rash only, six an isolated cardiomyopathy in the absence of any conduction defects and eight isolated haematological or hepatic manifestations alone. Several important clinical facts regarding CHB have been established based on the Registry, which include the morbitity and mortality cited above. The most common time of detection of disease is between 20 and 24 weeks of gestation, which is relevant for timing of foetal surveillance as discussed below.
Whilst one normally considers translational research as moving from bench to bedside, the wealth of clinical information regarding CHB facilitates the application of ‘reverse’ translation. The following clinical clues have guided the basic research efforts. If isolated CHB is diagnosed before 26 weeks, the majority of mothers will be identified to have the candidate autoantibodies. However, if a mother is known to have anti-SSA/Ro antibodies and no prior child with CHB or rash, the risk of CHB is only 2%. If the mother has both anti-SSA/Ro and SSB/La antibodies, the risk is slightly higher at 5%. If the mother has antibodies and a prior child with CHB or rash, the risk of CHB in a subsequent pregnancy rises to nearly 20%. The maternal heart is almost never affected. Finally, serial echocardiographic follow up of at risk foetuses has revealed that normal sinus rythymn can progress to third degree block in 7 days. The research implications are obvious. The maternal antibody is necessary but not sufficient to cause disease. This suggests the contribution of foetal and environmental factors. Thus, the pathologic cascade to scarring is rapid but variable. In 98% of cases, the injury is forestalled and kept in check with no overt or even subtle abnormality apparent. In some cases, there may be subclinical injury such as first degree block. In very rare cases, the cascade is fully executed with advanced block (2nd or 3rd). To further challenge the research task, the target antigens are located intracellullary in healthy cells.
In formulating hypotheses regarding CHB, one might take the position that not all anti-Ro+ mothers have the truly pathogenic maternal component. Wahren-Herlenius [14, 15] have confirmed and extended our earlier work on epitope mapping of the 52-kDa SSA/Ro protein (Ro52) response and risk of CHB [16]. Based on a study of nine CHB-mothers and 26 anti-Ro+ mothers of healthy children, this group posited that Ab to aa 200–239 of Ro52 (p200) predicted CHB with greater certainty than currently available testing for either Ro60 or Ro52 [15]. Utilizing an extensive serum bank from the RRNL, we identified an equivalent frequency of maternal anti-p200 Ab exposure in affected as well as unaffected children [17]. An international exchange of antisera is ongoing. However, we have had the opportunity to study the anti-Ro52 p200 response in one woman during an affected and unaffected pregnancy. The titre of this specific antibody response remained equivalent despite the foetal outcome.
With regard to accessibility of the target antigens, the issue of cross reactivity has been continuously considered albeit generally fraught with disappointment. Encouraging information was recently presented at the National meeting of the American College of Rheumatology in 2007. Monoclonal antibodies reactive with the peptide p200 of Ro52 recognized α-enolase in a neonatal rat heart library [18]. This is potentially quite exciting because α-enolase is a key glycolytic dimeric protein expressed in rat cardiocytes during foetal development and may be a membrane protein. However, utilizing sera from the Registry, anti-α-enolase reactivity was infrequent (<15% of CHB-mothers) [19]. These data await further study, but again the search for a putative surface reactivity has been frustrating.
If one at least accepts that the maternal autoantibody is responsible for initiating injury, then understanding the mechanism by which this occurs is important. The first challenge is to explain the mechanism of ‘necessity’, i.e. how maternal autoantibodies directed to intracellular antigens (likely involved in transcriptional regulation) bind tissue and perturb cardiac function. Boutjdir et al. extended two previous reports [20, 21] regarding arrhythmogenic effects of anti-SSA/Ro/SSB/La Abs by demonstrating that affinity purified anti-Ro52 Abs induce AV block in an isolated human foetal heart and inhibit inward calcium fluxes through L type calcium channels in human foetal ventriculocytes (whole cell and single channel) [22]. Whilst these observations support that maternal Abs perturb ion flux across the cardiocyte membrane and as such may be a relevant factor in CHB, a molecular basis has yet to be defined (e.g. definitive crossreactivity of anti-Ro/La with calcium channel receptor), particularly, with regard to inflammation and subsequent fibrosis. Ab to the cardiac 5HT4 serotoninergic receptors (hypothesized to be crossreactive with Ro52) were only rarely present in sera from affected children [23].
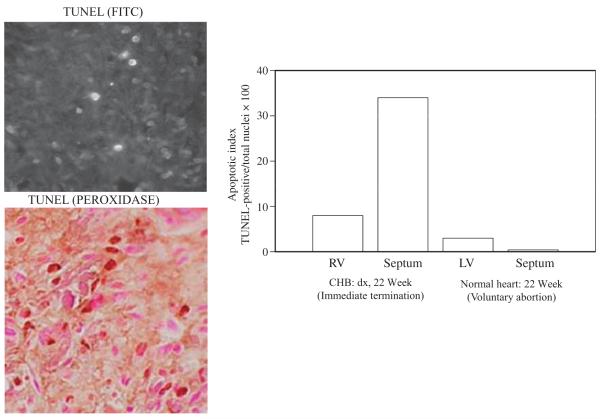
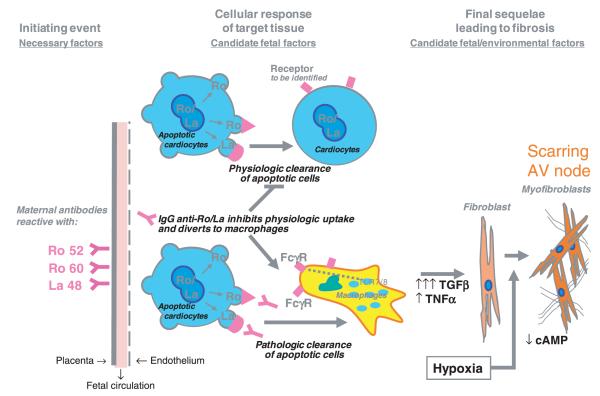
Immunohistologic evaluation of hearts from foetuses dying with CHB has revealed exaggerated apoptosis, clusters of macrophages in zones of fibrosis, which colocalize with IgG and apoptotic cells, TNFα and TGFβ mRNA expression in these cells, and extensive collagen deposition in the conducting system [24]. Figure 1 illustrates apoptosis in the heart of a foetus with CHB. These in vivo observations are supported by in vitro studies. Specifically, the consideration of exaggerated apoptosis as the initial link between maternal autoantibodies and tissue injury led to the observation that cardiocytes are capable of phagocytosing autologous apoptotic cardiocytes and that anti-SSA/Ro-SSB/La Abs inhibit this function [25]. Recognizing that this perturbation of physiologic efferocytosis might divert uptake to professional FcγR-bearing phagocytes fits well with earlier work demonstrating macrophage secretion of pro-inflammatory and fibrosing cytokines when coincubated with apoptotic cardiocytes bound by SSA/Ro-SSB/La Abs [26, 27]. Those macrophages engage Toll-like receptors (TLR) via binding to the RNA moiety of the target autoantigen is clearly an area, which may provide an additional clue to pathogenesis. Finally, building on the premise that foetal genetics contribute to injury, continued genotyping of anti-Ro exposed affected and unaffected siblings revealed significant skewing in the frequency of polymorphisms in two genes, FcγR3a and TGFβ [24, 28], whose expressed proteins potentially relate to increased IgG binding to macrophages and fibrosis respectively. The discordance of disease in monozygotic twins prompted the novel line of research into the role of hypoxia as an amplification factor on the distal fibrosing component. In vitro studies suggest a role of hypoxia in modulating cyclic AMP (cAMP) and promoting a myofibroblast phenotype. Footprints of hypoxic injury comprised expression of hypoxia-inducible factor (HIF) 1α in affected hearts and increased erythropoietin levels in several cord blood samples of surviving foetuses [29]. A schematic representation of the proposed cascade linking maternal autoantibody to atrioventricular fibrosis and cardiomyopathy is shown in Fig. 2.
Fig. 1.
Histological evidence of increased apoptosis in conduction tissue from a foetus with congenital heart block (CHB). Shown are longitudinal sections through septum of a 22-week-old foetus with CHB. Apoptotic cells were identified by TUNEL fluorescein isothiocyanate staining (Left, upper) or TUNEL peroxidase (Left, lower). Apoptotic cells (brown nuclei) are interspersed with nonapoptotic cells (purple nuclei). For the 22 week foetus and a matched healthy heart, values (right) on the Y axis are the mean apoptotic index (AI), expressed as AI = (TUNEL-positive nuclei/total nuclei) × 100, where the total number of nuclei is the number of nonapoptotic nuclei plus the number of apoptotic (TUNEL-positive) nuclei. One hundred cells were counted in 3–5 fields for each cardiac section. RV, right ventricle; LV, left ventricle; CHB, congenital heart block. Reprinted from Rheum Dis Clin North Am, Vol. 30, Robert M. Clancy & Jill P. Buyon, More to death than dying: apoptosis in the pathogenesis of SSA/Ro-SSB/La-associated congenital heart block, pages 589–602, 2004, with permission from Elsevier.
Fig. 2.
Proposed pathologic cascade from inflammation to fibrosis whereby maternal antibodies initiate events that lead to a persistent myofibroblast, a phenotype that is associated with scarring.
The substantial morbidity and mortality associated with CHB [4–8] and the readily available technology for identification of CHB in utero have prompted the search for effective therapies. Ideally, as CHB is most often identified from 18 to 24 weeks of gestation [4, 5], intrauterine therapy should be possible. Firm guidelines for the obstetric and rheumatologic management of the foetus identified with CHB and the foetus with a normal heartbeat but at high risk of developing CHB, are not established. To address the former, it needs to be known whether the presence of bradycardia represents an irreversible fibrotic process and if continued, autoimmune tissue injury will result in progressive damage. McCue has reported a neonate with 1st degree block at birth, which resolved at 6 months [30]. In contrast, Geggel et al. report an infant born with 2nd degree block who progressed to 3rd degree by 9 weeks of age [31]. Perhaps, most disturbing is our own published observation on the postnatal progression of block. Nine neonates in the Research Registry for Neonatal Lupus (RRNL) [4] had a prolonged PR interval on EKG at birth, four of whom progressed to more advanced AV block. Two children diagnosed in utero with 2nd degree block were treated with dexamethasone and reverted to normal sinus rhythm by birth, but ultimately progressed to 3rd degree block. Four children had 2nd degree block at birth: of these, two progressed to 3rd degree block [10].
Based on the proposed pathogenesis of CHB, treatment approaches would be predicted to focus on reduction of a generalized inflammatory response and prevention of fibrosis. The use of maternal oral dexamethasone therapy has been popularized by several groups, but its scientific merit and risks are questionable. Our own group initially investigated [12], in a retrospective chart review, the use of this medication by evaluating cases from the RRNL. In 28 of 47 mothers identified to be carrying a foetus with CHB, treatment was instituted with a fluorinated steroid (dexamethasone or betamethasone). In 21 treated cases with 3rd degree block at presentation, the block was not reversible. Three treated foetuses with alternating 2nd and 3rd degree block eventually developed permanent 3rd degree block. There were four interesting cases that had 2nd degree block, which reversed to 1st degree block at birth. However, long-term follow-up revealed that two of these subsequently progressed to 2nd degree block [10]. In the group of 22 pregnancies in which maternal steroids were not given, 18 had irreversible 3rd degree heart block, two had 2nd alternating with 3rd degree block which progressed to 3rd degree block, as did the two patients with 2nd degree block [12]. It was concluded from this retrospective study that there was no difference in mortality, prematurity or degree of final block or need for pacemaker for foetuses treated with or without steroids. It was noted that the presence of pericardial or pleural effusions as well as ascites and hydrops seemed to improve with the use of steroids. Additionally, there was a suggestion of reversal of less advanced block which in theory could forestall the need for a pacemaker. Overall, this study, albeit limited in number of patients and based on retrospectively collected data, helped to popularize the use of fluorinated steroids as a treatment of in utero-identified CHB.
Data from a recently completed prospective open label multicentre study, carried out as part of the PRIDE study (PR Interval and Dexamethasone Evaluation), supported similar conclusions regarding dexamethasone for the treatment of CHB. Specifically the study comprised 30 pregnancies treated with dexamethasone (22–3rd degree; 6–2nd degree; 2–1st degree) and 10 untreated (9–3rd degree;1–1st degree). The initial median ventricular rates, age at diagnosis and degree of cardiac dysfunction were similar between groups. Six deaths occurred in the dexamethasone group. There was no reversal of 3rd degree block, with therapy or spontaneously. In foetuses treated with dexamethasone, 1/6 with 2nd degree progressed to 3rd degree and three remained in 2nd degree (one paced, two progressed to 3rd); two reverted to normal sinus rhythm (NSR) (one progressed to 2nd). Dexamethasone reversed both foetuses with 1st degree to NSR by seven days with no regression upon discontinuation. Absent dexamethasone, the one 1st degree detected at 38 weeks had NSR at birth (overall stability or improvement 4/8 dexamethasone versus 1/1 non-dexamethasone). Median gestational birth age was 37 weeks vs. 38 weeks, dexamethasone versus non-dexamethasone, P = 0.019. Prematurity and small for gestational age were restricted to the dexamethasone group. Pacemaker use and growth parameters at birth and 1 year were similar between groups. Thus, dexamethasone should not be used to reverse established complete block, but might hold some promise for less advanced blocks and associated cardiomyopathy.
As foetuses presenting with third-degree block may not benefit from treatment, the critical times to intervene would be: (i) when the PR interval is prolonged but atrial signals continue to reach the ventricles (1st or 2nd degree block); or (ii) when signs of myocardial dysfunction alone are present. From the clinical perspective, there is a clear need to identify an early marker of CHB. From a basic science perspective, the knowledge that antibodies can induce lesser degrees of injury would be important. Accordingly, a US-based observational study of pregnant women known to have anti-Ro antibodies was initiated in which echocardiograms were performed serially beginning at 16 weeks of gestation, (PR Interval and Dexamethasone Evaluation (PRIDE) study) [32]. The primary outcome measure was the mechanical PR interval, defined using the gated-pulsed Doppler technique as the time interval from the onset of the mitral A wave (atrial systole) to the onset of the aortic pulsed Doppler tracing (ventricular systole) within the same left ventricular cardiac cycle. Secondary outcomes included evaluation of myocardial function. The goal was to determine the earliest noninvasive echocardio-graphic marker of injury.
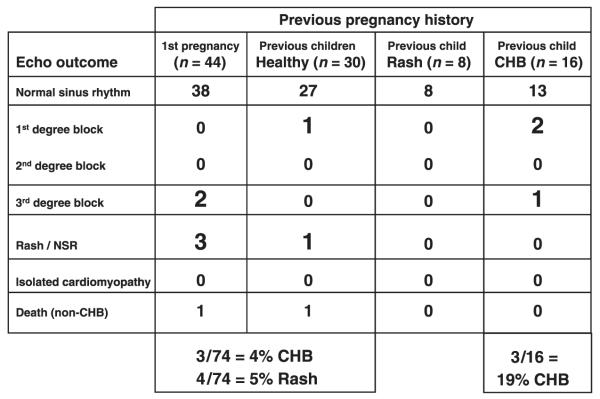
One hundred and eighteen pregnant women with anti-SSA/Ro antibodies were enrolled, with 98 completing an evaluable course (Summarized in Fig. 3). The protocol entailed foetal echocardiograms weekly from 16–26 weeks’ gestation and biweekly from 26–34 weeks. PR intervals >150 ms (mean + 3 SD) were considered abnormally prolonged, consistent with first-degree block. Ninety-two foetuses had normal PR intervals throughout the study. Neonatal lupus developed in 10 cases, four of which were rash only. Three foetuses had 3rd degree block, none of whom had a preceding abnormal PR interval, albeit in two more than one week elapsed between echocardiographic evaluations. Tricuspid regurgitation preceded complete block in one foetus and an atrial echodensity preceded the block in a second foetus. Three foetuses had a PR interval >150 msec. Two, each detected before 21 weeks, reversed within 1 week after institution of 4 mg of dexamethasone. Whether dexamethasone was curative or incidental cannot be assigned. A third case developed 1st degree block at 32 weeks after normal PR intervals in utero, as demonstrated on the ECG performed at birth; the block persists at age 3 years. No cases of any block developed after a normal EKG at birth. Overall, heart block occurred in three (19%) of 16 pregnancies in mothers with a previous CHB child and three (5%) of 56 with no previously affected children once again reinforcing the 10-fold increase in recurrence.
Fig. 3.
Outcome versus history of pregnancy for the PR Interval and Dexamethasone Evaluation (PRIDE) study (98 patients). The study was initiated to test the hypothesis that the mechanical PR interval serves as a biomarker to detect early injury in CHB.
The two critical issues raised by the PRIDE study are: (i) the clinical significance of a prolonged PR interval, and (ii) the biologic implication with regard to tissue injury. An isolated prolongation of the PR interval may be transient, related to vagal tone or medication use or reversible injury, or it may be permanent or progress to more marked delay because of physical injury to the specialized electrical pathway, e.g. because of inflammation or scarring. It may be that PR prolongation represents a variant of normal and only in retrospect does it have clinical significance if it is either sustained after birth or progresses to more advanced block. A PR interval that exceeds the expected 95% confidence interval of a normal population can be transient, sustained or progressive. Perhaps, the final outcome depends on the influence of foetal and environmental factors. These permissive factors might be present in certain foetuses and not others, thus accounting for the rarity of clinical disease. If prolongation of the PR interval does represent tissue injury, regardless of how minimal, it might be so rapid as to go unnoticed. Accurate identification of the foetus in whom 1st degree unambiguously represents a warning sign would be a major advance, since early disease may be reversible. This identification requires a definition that is acceptable to the managing physicians and that has a reasonable prediction of being sustained or progressive if left untreated. This task is particularly challenging as dexamethasone and betamethasone have both maternal and foetal risks.
Given the identification of advanced block and severe cardiomyopathy within 1 week of a normal echocardiogram and its usual occurrence before 24 weeks of gestation, it would seem appropriate to perform weekly monitoring between 16 and 24 weeks. The goal of this monitoring would be to identify a biomarker of reversible injury, such as a PR interval prolongation above 150 ms, moderate/severe tricuspid regurgitation and/or an atrial echodensity.
The significant morbidity and mortality of third degree block and the informative histological data demonstrating extensive fibrosis of the AV node suggests the need for development of a new prophylactic therapy, other than dexamethasone, to be given early in pregnancy before the onset of disease, perhaps targeted to the highest risk pregnancies such as in women with prior affected offspring. The opportunity is present since risk is identifiable, a database is available to predict outcomes and properly power a study and research clues should be translatable. Therapy should be targeted to either eliminate the ‘necessary’ factor (no antibody, no disease) or modify the inflammatory component before it provokes an irreversible scarring phenotype of the fibroblast [27]. IgG pooled from the plasma of healthy donors (immune globulin therapy, also know as IVIG) is a promising agent, which might have an effect at several levels of the proposed pathologic cascade. In a study comprised of eight pregnancies in mothers with anti-Ro antibodies and a previous child with CHB, treatment with 1 g kg−1 of IVIG at the 14th and 18th week of gestation prevented CHB in seven cases [33]. Although even one case of CHB is disappointing, it is less than the predicted recurrence rate of one in five. However, the dosing schedule of IVIG may not have been optimal. Arguably, initiation of IVIG at 14 weeks might be too late since it is at least 2 weeks after maternal antibody transfer has become effective and these 2 weeks might be a critical time for prevention. Furthermore, discontinuation at 18 weeks may have been premature.
In addition to its efficacy in affording resistance to infections, the intravenous administration of high doses IVIG has been of benefit in a variety of immune-mediated and inflammatory diseases and is FDA approved for primary immunodeficiency, idiopathic thrombocytopenia purpura, Kawasaki disease, B cell-chronic lymphocytic leukaemia with hypo-gammaglobulinaemia, paediatric HIV infection and for allogenic bone marrow transplant in adults [34]. Many of the proposed effects of IVIG may be entirely nonspecific, mediated through the constant regions of IVIG. These include blocking FcRn, which would enhance autoantibody half-life, and blocking of stimulatory FcγR and stimulating inhibitory FcγR, FcγRIIB [35, 36]. Of relevance to CHB, FcRn is the receptor which mediates placental transport of maternal IgG to the foetus [2]. It has recently been emphasized that the sialic rich IgG fraction of IVIG confers enhanced anti-inflammatory activity [37].
Accordingly, IVIG may be particularly effective in prevention of the passively acquired autoimmune disease of CHB. The rationale considers several potential mechanisms, the first two relate to lowering or even eliminating maternal antibody in the foetal circulation (maternal perspective): (i) increased catabolism of maternal antibody, and (ii) decreased placental transport of maternal antibody. By decreasing antibody levels, there would be less antibody available to bind apoptotic cardiocytes. Thus, the initial cascade to injury might be abrogated. The third consideration is an effect of IVIG transported into the foetal circulation where it might act to upregulate surface expression of the inhibitory FccRIIB receptor on foetal macrophages thereby decreasing secretion of TNFa and exaggerated TGFβ (foetal perspective). Highly speculative would be an anti-apoptotic effect of IVIG, which would certainly be relevant to the pathogenesis of CHB, in which we have accumulating evidence that apoptosis of cardiocytes provides an essential link between antibody and fibrosis. Perhaps, an effect amplifying the hypothesized mechanisms proposed is the influence of FcγR polymorphisms on the response to IVIG therapy.
The Preventive IVIG Therapy for Congenital Heart Block (PITCH) study is currently enrolling patients. Sample size calculations utilize Simon’s two-stage optimal design. Based on a two-sided significance level of 0.05, a power of 90% to show a reduction of risk to 5% given the prediction that 18% of untreated subjects will get some degree of heart block, the first stage will enrol 19 mothers who have had a previous child with CHB or rash, to receive IVIG (400 mg kg−1 IVIG every 3 weeks × 5) from 12 to 24 weeks gestational age. If fewer than three mothers have children with 2nd or 3rd degree heart block, then an additional 35 mothers will be enrolled into the second stage of the study for a total of 54 subjects. At the end of the trial, the treatment will be considered efficacious and worthy of further study if fewer than six mothers out of 54 subjects have a child with advanced heart block. The secondary outcomes to be evaluated include 1st degree block, signs of myocardial injury absent any conduction defects, and isolated endocardial fibroelastosis as assessed by echocardiogram and EKG at birth or presence of an abnormal fluid collection as assessed on the final foetal echocardiogram and obstetrical ultrasound before delivery. The rationale for the dose of IVIG, which is traditionally considered a replacement dose and not an –anti-inflammatory dose of 1 g kg−1, was based on the immaturity of placental transport in the early second trimester and the foetus as the targeted patient. Effect of dose on maternal antibody lowering as a potential biomarker related to efficacy will be of interest.
In sum, whilst CHB is an uncommon disease it is one in which mechanisms regarding pathogenesis might be applicable to a broad range of antibody mediated injury. Several elements of the proposed pathologic cascade can be applied to the study of other autoimmune diseases such as lupus nephritis, e.g. dual signalling via immune complex FccR uptake and Toll-like receptor signalling. The rarity of disease suggests that many components must come together for the full expression of CHB and therapies may have benefit at multiple points along the cascade. Currently, the optimal approach employs serial echocardiograms to identify an early marker, which represents potentially progressive injury but is reversible with treatment.
Acknowledgements
This study was funded by NIH-NIAMS grant 1-R01-AR46265 (the PRIDE Study) to Dr Buyon, Alliance for Lupus Research Preventative IVIG Therapy for Congenital Heart Block (PITCH) study to Dr Buyon, NIH-NIAMS RO1 AR42455-01 grant to Dr Buyon, American Heart Association, Heritage Affiliate grant 0655938T to Dr Clancy and a generous gift from the Lee family.
Footnotes
Conflict of interest statement Dr Friedman has received speaking and consulting fees from MedImmune, Inc. Dr Buyon has received a gift from the Lee family.
References
- 1.Buyon JP, Clancy RM. Neonatal lupus. In: Wallace DJ, Hahn BH, editors. Dubois’ Lupus Erythematosus. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. pp. 1058–80. [Google Scholar]
- 2.Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J Immunol. 1996;157:3317–22. [PubMed] [Google Scholar]
- 3.Lee LA. Neonatal lupus erythematosus. J Invest Dermatol. 1993;100:9S–13S. doi: 10.1111/1523-1747.ep12355173. [DOI] [PubMed] [Google Scholar]
- 4.Buyon JP, Hiebert R, Copel J, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–66. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 5.Waltuck J, Buyon JP. Autoantibody-associated congenital heart block: outcome in mothers and children. Ann Intern Med. 1994;120:544–51. doi: 10.7326/0003-4819-120-7-199404010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Silverman ED. Congenital heart block and neonatal lupus erythematosus: prevention is the goal. J Rheumatol. 1993;20:1101–4. [PubMed] [Google Scholar]
- 7.Julkunen H, Kurki P, Kaaja R, et al. Isolated congenital heart block. Long-term outcome of mothers and characterization of the immune response to SS-A/Ro and to SS-B/La. Arthritis Rheum. 1993;36:1588–98. doi: 10.1002/art.1780361114. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt KG, Ulmer HE, Silverman NH, Kleinman CS, Copel JA. Perinatal outcome of fetal complete atrioventricular block: a multicenter experience. J Am Coll Cardiol. 1991;17:1360–6. doi: 10.1016/s0735-1097(10)80148-2. [DOI] [PubMed] [Google Scholar]
- 9.Moak JP, Barron KS, Hougen TJ, et al. Congenital heart block: development of late-onset cardiomyopathy, a previously under-appreciated sequela. J Am Coll Cardiol. 2001;37:238–42. doi: 10.1016/s0735-1097(00)01048-2. [DOI] [PubMed] [Google Scholar]
- 10.Askanase AD, Friedman DM, Copel J, et al. Spectrum and progression of conduction abnormalities in infants born to mothers with anti-SSA/Ro-SSB/La antibodies. Lupus. 2002;11:145–51. doi: 10.1191/0961203302lu173oa. [DOI] [PubMed] [Google Scholar]
- 11.Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–82. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 12.Saleeb S, Copel J, Friedman D, Buyon JP. Comparison of treatment with fluorinated glucocorticoids to the natural history of autoantibody-associated congenital heart block: retrospective review of the research registry for neonatal lupus. Arthritis Rheum. 1999;42:2335–45. doi: 10.1002/1529-0131(199911)42:11<2335::AID-ANR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Buyon J. Neonatal lupus syndromes. In: Lahita R, editor. Systemic Lupus Erythematosus. Academic Press; San Diego, CA: 1999. p. 337. [Google Scholar]
- 14.Ottosson L, Hennig J, Espinosa A, Brauner S, Wahren-Herlenius M, Sunnerhagen M. Structural, functional and immunologic characterization of folded subdomains in the Ro52 protein targeted in Sjogren’s syndrome. Mol Immunol. 2006;43:588–98. doi: 10.1016/j.molimm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Ottosson L, Salomonsson S, Hennig J, et al. Structurally derived mutations define congenital heart block-related epitopes within the 200–239 amino acid stretch of the Ro52 protein. Scand J Immunol. 2005;61:109–18. doi: 10.1111/j.0300-9475.2005.01542.x. [DOI] [PubMed] [Google Scholar]
- 16.Buyon JP, Slade SG, Reveille JD, Hamel JC, Chan EK. Autoantibody responses to the “native” 52-kDa SS-A/Ro protein in neonatal lupus syndromes, systemic lupus erythematosus, and Sjogren’s syndrome. J Immunol. 1994;152:3675–84. [PubMed] [Google Scholar]
- 17.Clancy RM, Buyon JP, Ikeda K, et al. Maternal antibody responses to the 52-kd SSA/RO p200 peptide and the development of fetal conduction defects. Arthritis Rheum. 2005;52:3079–86. doi: 10.1002/art.21289. [DOI] [PubMed] [Google Scholar]
- 18.Ambrosi A, Strandberg L, Ottosson L, Kampe O, Wahren-Herlenius M. Anti-Ro52 antibodies inducing heart block cross-react with alpha-enolase. Arthritis Rheum. 2007;56:S783. [Google Scholar]
- 19.Llanos C, Chan E, Li S, et al. Antibody reactivity to alpha-enolase in mothers of children with Congenital Heart Block. J Rheumatol. 2008;36:565–9. doi: 10.3899/jrheum.080860. [DOI] [PubMed] [Google Scholar]
- 20.Boutjdir M, Chen L, Zhang ZH, Tseng CE, El-Sherif N, Buyon JP. Serum and immunoglobulin G from the mother of a child with congenital heart block induce conduction abnormalities and inhibit L-type calcium channels in a rat heart model. Pediatr Res. 1998;44:11–9. doi: 10.1203/00006450-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Mazel JA, El-Sherif N, Buyon J, Boutjdir M. Electrocardio-graphic abnormalities in a murine model injected with IgG from mothers of children with congenital heart block. Circulation. 1999;99:1914–8. doi: 10.1161/01.cir.99.14.1914. [DOI] [PubMed] [Google Scholar]
- 22.Qu Y, Xiao GQ, Chen L, Boutjdir M. Autoantibodies from mothers of children with congenital heart block downregulate cardiac L-type Ca channels. J Mol Cell Cardiol. 2001;33:1153–63. doi: 10.1006/jmcc.2001.1379. [DOI] [PubMed] [Google Scholar]
- 23.Buyon JP, Clancy R, Di Donato F, et al. Cardiac 5-HT(4) serotoninergic receptors, 52kD SSA/Ro and autoimmune-associated congenital heart block. J Autoimmun. 2002;19:79–86. doi: 10.1006/jaut.2002.0594. [DOI] [PubMed] [Google Scholar]
- 24.Clancy RM, Backer CB, Yin X, Kapur RP, Molad Y, Buyon JP. Cytokine polymorphisms and histologic expression in autopsy studies: contribution of TNF-alpha and TGF-beta 1 to the pathogenesis of autoimmune-associated congenital heart block. J Immunol. 2003;171:3253–61. doi: 10.4049/jimmunol.171.6.3253. [DOI] [PubMed] [Google Scholar]
- 25.Clancy RM, Neufing PJ, Zheng P, et al. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–22. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miranda-Carus ME, Askanase AD, Clancy RM, et al. Anti-SSA/Ro and anti-SSB/La autoantibodies bind the surface of apoptotic fetal cardiocytes and promote secretion of TNF-alpha by macrophages. J Immunol. 2000;165:5345–51. doi: 10.4049/jimmunol.165.9.5345. [DOI] [PubMed] [Google Scholar]
- 27.Clancy RM, Askanase AD, Kapur RP, et al. Transdifferentiation of cardiac fibroblasts, a fetal factor in anti-SSA/Ro-SSB/La antibody-mediated congenital heart block. J Immunol. 2002;169:2156–63. doi: 10.4049/jimmunol.169.4.2156. [DOI] [PubMed] [Google Scholar]
- 28.Clancy R, Izmirly P, Kimberly RP, et al. Coercion of innate immunity to amplify the adaptive immune response in the cascade to cardiac injury in CHB: implications for prevention with antimalarials. Arthritis Rheum. 2007;56:S810. [Google Scholar]
- 29.Clancy RM, Zheng P, O’Mahony M, et al. Role of hypoxia and cAMP in the transdifferentiation of human fetal cardiac fibroblasts: implications for progression to scarring in autoimmune-associated congenital heart block. Arthritis Rheum. 2007;56:4120–31. doi: 10.1002/art.23061. [DOI] [PubMed] [Google Scholar]
- 30.McCue CM, Mantakas ME, Tingelstad JB, Ruddy S. Congenital heart block in newborns of mothers with connective tissue disease. Circulation. 1977;56:82–90. doi: 10.1161/01.cir.56.1.82. [DOI] [PubMed] [Google Scholar]
- 31.Geggel RL, Tucker L, Szer I. Postnatal progression from second- to third-degree heart block in neonatal lupus syndrome. J Pediatr. 1988;113:1049–52. doi: 10.1016/s0022-3476(88)80581-x. [DOI] [PubMed] [Google Scholar]
- 32.Friedman DM, Kim MY, Copel JA, et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation. 2008;117:485–93. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 33.Kaaja R, Julkunen H, et al. Prevention of recurrence of congenital heart block with intravenous immunoglobulin and corticosteroid therapy: comment on the editorial by Buyon. Arthritis Rheum. 2003;48:280–1. doi: 10.1002/art.10716. author reply 1–2. [DOI] [PubMed] [Google Scholar]
- 34.Looney RJ, Huggins J. Use of intravenous immunoglobulin G (IVIG) Best Pract Res Clin Haematol. 2006;19:3–25. doi: 10.1016/j.beha.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 35.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–6. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 36.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–33. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 37.Nimmerjahn F, Ravetch JV. The antiinflammatory activity of IgG: the intravenous IgG paradox. J Exp Med. 2007;26:513–33. doi: 10.1084/jem.20061788. [DOI] [PMC free article] [PubMed] [Google Scholar]