Abstract
Objective
Congenital heart block (CHB), a manifestation of neonatal lupus, is associated with maternal anti-Ro/SSA and anti-La/SSB autoantibodies and recurs in ~18% of subsequent pregnancies. This study was undertaken to investigate the effect of the idiotype: antiidiotype (Id:anti-Id) antibody ratio in the ability of intravenous immunoglobulin (IVIG) administered during subsequent pregnancies to prevent CHB.
Methods
We studied 16 anti-Ro/SSA and anti-La/ SSB–positive pregnant women from the Preventive IVIG Therapy for Congenital Heart Block study who had previously given birth to a child with neonatal lupus. In 3 of the mothers, the study pregnancy resulted in the birth of a child with neonatal lupus (2 with CHB and 1 with rash). Sequential serum samples were obtained from all mothers immediately before the administration of IVIG during pregnancy and were evaluated for antibodies against the major B cell epitope 349–364aa of La/SSB (idiotype) and its antiidiotypic antibodies.
Results
Following IVIG treatment, serum titers of anti-La(349–364) (Id antibodies) decreased in 80% of the mothers, and in 60% an increase in anti-Id antibodies against anti-La(349–364) was observed. The Id: anti-Id ratio was significantly higher in mothers whose offspring developed neonatal lupus compared to mothers who gave birth to a healthy child (P < 0.0001). Removal of anti-Id antibodies substantially increased the reactivity against La(349–364) in sera from 5 of 7 mothers tested. All IVIG preparations were examined for Id and anti-Id antibody activity. IVIG from batches administered to mothers who gave birth to a healthy child had an Id:anti-Id activity ratio of <1, in contrast to that given to mothers who gave birth to a child with neonatal lupus. Addition of the IVIG preparations to the maternal sera further enhanced antiidiotypic activity (by up to 4.7-fold) in 11 of 13 patients studied.
Conclusion
This is the first study in humans to demonstrate that IVIG influences the Id–anti-Id network of a specific pathogenic autoantibody. Specifically, we showed that IVIG enhanced the anti-Id antibody response in pregnant women with anti-La/SSB antibodies. A high Id:anti-Id ratio in both the IVIG preparation and the maternal serum may explain the absence of an effect of IVIG in preventing recurrent neonatal lupus in some cases.
Congenital heart block (CHB) is a model of passively acquired autoimmunity that is strongly associated with the transplacental passage of maternal IgG against Ro/SSA and La/SSB ribonucleoproteins (1). CHB is a cardiac manifestation of the so-called neonatal lupus syndromes, a constellation of clinical manifestations found in babies born to mothers with anti-Ro/anti-La antibodies, which may include a cutaneous rash or, very rarely, blood or liver abnormalities that improve over time (2,3).
CHB is the most serious manifestation of neonatal lupus and can cause permanent and often life-threatening damage to the fetal heart. The incidence of CHB in the offspring of mothers with the pathologic autoantibodies ranges between 1% and 2% (4,5), but the recurrence rate in subsequent pregnancies following the birth of a child with neonatal lupus is ~18% (6–8). It appears that the presence of maternal antibodies is a necessary, but not sufficient, condition for the development of CHB. The uterine environment, fetal factors (e.g., genes, viral infections), and maternal factors (e.g., specific autoantibody profile) (9) or the absence of an active antiidiotypic network targeting pathogenic autoantibodies (10) have been proposed as possible cofactors in the development of CHB. In a previous study, in an attempt to define the role of this network in the development of neonatal lupus, we evaluated the idiotypic–antiidiotypic (Id–anti-Id) network of antibodies targeting the dominant epitopes of La/SSB (10); we concluded that the presence of anti-Id antibodies to autoantibodies against La/SSB may protect the fetus by blocking pathogenic maternal autoantibodies.
In the present study of mothers who were enrolled in the Preventive IVIG Therapy for Congenital Heart Block (PITCH) study, we evaluated the effects of intravenous immunoglobulin (IVIG) therapy on the Id–anti-Id network of anti-La/SSB antibodies. We found that, although administration of intravenous immunoglobulin (IVIG) at doses consistent with replacement does not prevent the recurrence of CHB overall (11), it significantly alters the Id–anti-Id network of pathogenic autoantibodies, increasing the anti-Id response against them. We also showed that in the pregnancies resulting in the birth of a healthy child, the anti-La/SSB antibody Id:anti-Id ratio in maternal serum was significantly lower compared to the ratio observed in pregnancies resulting in the birth of a child with neonatal lupus.
PATIENTS AND METHODS
Study subjects
Between January 2007 and January 2009, 21 women were enrolled in the PITCH study, a US-based multicenter, prospective, open-label clinical trial, after providing informed consent. All of the following inclusion criteria were required for study enrollment: 1) documentation of anti-Ro/SSA and/or anti-La/SSB antibody positivity; 2) a previous child with CHB (any degree, documented by electrocardiography if a live birth and/or by echocardiography and/or histologic findings if fetal death) and/or characteristic neonatal lupus rash (confirmed by dermatologic assessment and/or biopsy findings); and 3) current intrauterine pregnancy of <12 weeks’ gestation, with normal fetal heartbeat and heart structure. Exclusion criteria included 1) current prednisone dosage > 20 mg/day or current use of dexamethasone at any dosage; 2) IgA levels below normal values for the laboratory conducting the test; or 3) presence of any structural abnormalities of the fetal heart that could cause CHB, such as L-transposition of the great arteries, atrioventricular (AV) septal defect, or heterotaxia. Of the 21 women enrolled, there was 1 screening failure: this patient had a spontaneous miscarriage at 9 weeks, prior to initiation of the study protocol.
Sequential serum samples were obtained from the 20 mothers who completed the PITCH study. Three fetuses were diagnosed as having advanced heart block (at weeks 19, 20, and 25 of gestation). One of them was from an anti-La/SSB–negative mother. Of the 17 children without CHB, 1 neonate (from an anti-La/SSB–positive mother) presented with a neonatal lupus rash and Sweet’s syndrome. Sera from 4 mothers were found to be negative for anti-La/SSB antibodies (using a commercial assay) and displayed minimal reactivity against the major epitope of La/SSB, and therefore were not included in further experiments. Therefore, the final study population consisted of 16 women, 3 of whom gave birth to children with neonatal lupus (2 with CHB, 1 with characteristic rash). All sera were positive for antibodies against Ro 60 and Ro 52 autoantigens.
IVIG administration and followup
IVIG infusions (400 mg/kg) were administered over periods of 3–4 hours at 12, 15, 18, 21, and 24 weeks’ gestation. Blood samples were obtained before each infusion, at 28 weeks, at 34 weeks, and at the time of delivery, and analyzed under blinded conditions. Fetal echocardiograms were obtained weekly between week 16 and week 26 of gestation and then every 2 weeks thereafter until week 34.
Peptide synthesis and definitions
Synthetic peptides corresponding to the major B cell epitope of La/SSB, G349SGKGKVQFQGKKTKF364 (La[349–364]), as well as its complementary epitope, K364FRFLALKLYFSFTRP349 (cpep[349–364]), were purchased from Biosynthesis. We have previously shown that antibodies against complementary epitope La(349–364) are antiidiotypic to antibodies against La(349–364) (10,12,13); thus, antibodies against epitope 349–364 were considered as idiotypic and antibodies against complementary epitope 349–364 as antiidiotypic.
Antipeptide and anti–complementary peptide enzyme-linked immunosorbent assays (ELISAs)
Antipeptide and anti–complementary peptide antibodies were detected with an ELISA that was optimized for each synthetic peptide, according to a previously described protocol (10).
Isolation of anti-La(349–364) IgG antibodies
Specific CNBr-activated Sepharose 4B immunoaffinity columns were generated by standard methods, using 15 mg of La(349–364) peptide. IgG from 4 sera that strongly recognized the La/SSB epitope 349–364 on ELISA were purified with protein A–Sepharose, concentrated, dialyzed against phosphate buffered saline (PBS), and passed through the peptide immunoaffinity column. Subsequently, the column was washed with PBS and eluted with 8M urea. The eluates were dialyzed against PBS, concentrated, and redialyzed. Antipeptide IgG concentrations were evaluated by the Lowry method (DC Protein Assay; Bio-Rad).
Preparation of F(ab)2 anti-La(349–364) fragments and assay for direct detection of anti-Id antibodies
Purified anti-La(349–364) IgG antibodies were subjected to enzymatic degradation with pepsin and purified to be free of traces of intact IgG, as described previously (12). Purified F(ab)2 fragments of anti-La(349–364) antibodies (5 μg/ml in carbonate buffer [pH 9.6]) were applied in Costar high-binding microtiter plates for 1 hour at 25°C. Subsequently, the plates were blocked and washed, and sera were added at a dilution of 1:150–1:300 and incubated overnight at 4°C. The wells were then washed and alkaline phosphatase–conjugated goat anti-human IgG (1:2,000 in blocking buffer; Jackson ImmunoResearch) was added. The plates were incubated for 1 hour at room temperature. The wells were then washed and an ELISA amplification substrate system (Invitrogen) was applied. Absorbance was measured at 495 nm.
Anti-La(349–364) ELISA after removal of anti-Id antibodies
Anti-La(349–364) reactivity was evaluated after dissociation of Id–anti-Id immune complexes (by heating the samples at 53°C) and subsequent removal of anti-Id antibodies using anti-La(349–364) purified antibodies coupled to protein A–Sepharose beads. Normal IgG coupled to protein A–Sepharose beads was used as a negative control.
RESULTS
Augmentation of the anti-Id response by IVIG administration
Id and anti-Id antibody titers in serial serum samples from 15 of 16 anti-La/SSB–positive mothers, including 2 who gave birth to children with neonatal lupus, were assessed under blinded conditions. Serial samples were not available from 1 of the anti-La/ SSB–positive mothers, who also gave birth to a child with neonatal lupus; thus, this mother was not included in the analyses involving serial samples. We found that IVIG administration augmented the anti-Id response and in parallel attenuated the Id response, in the majority of sera tested. More specifically, after therapy the titer of anti-Id antibodies was increased (by 14–620%) in 9 mothers (60%), anti-Id activity remained the same as at study entry in 2 mothers, and the titer of anti-Id antibodies was decreased (by 13–78%) in 4 mothers. In contrast, the titer of Id antibodies was decreased (by 14–61%) in 12 mothers (80%), Id activity remained the same as at study entry in 1 mother, and the titer of Id antibodies was increased (by 38–53%) in 2 mothers.
The Id:anti-Id ratio of antibodies against the major epitope of La/SSB predicts the appearance of neonatal lupus in anti-La–positive sera
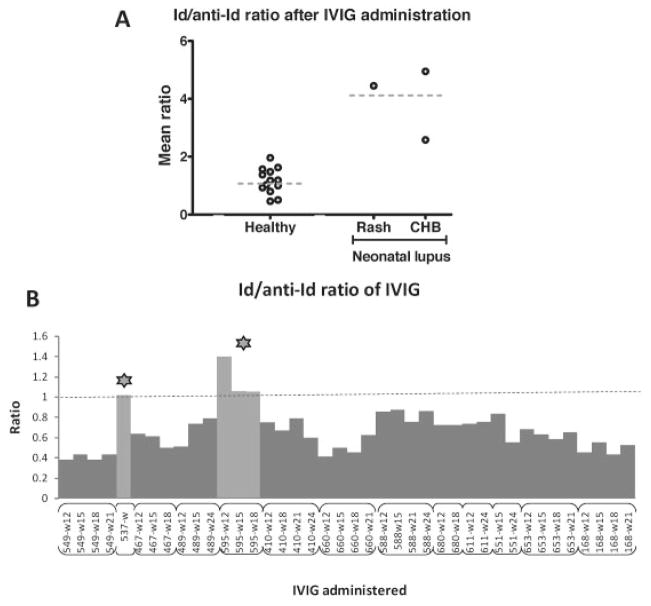
In the 2 mothers whose study pregnancy resulted in the birth of a child with neonatal lupus and for whom sequential sera were available (patients 595 and 575), anti-Id antibody titers did not increase. Although the Id antibody titers in these 2 mothers were decreased by 32% and 42%, respectively, these titers remained 4.96- and 4.45-fold higher than the anti-Id antibody titers. In contrast, mothers who gave birth to a healthy child had either a lower titer of Id antibodies than anti-Id antibodies or a robust increase in anti-Id antibodies during the period of IVIG treatment, bringing their levels very close to the levels of Id antibodies by the end of therapy (t = 6.81, P < 0.0001, average difference between Id and anti-Id titer in patients 595 and 575 versus the other 13 mothers). Furthermore, all 3 anti-La–positive mothers who gave birth to a child with neonatal lupus exhibited significantly higher Id:anti-Id ratios than those in mothers whose study pregnancy did not result in the birth of a child with neonatal lupus (t = 7.09, P < 0.0001) (Figure 1A).
Figure 1.
A, Idiotype:antiidiotype (Id:anti-Id) ratio of antibodies against the major epitope of La/SSB after intravenous immunoglobulin (IVIG) administration, in mothers who gave birth to a child with neonatal lupus and mothers who gave birth to a healthy child. Horizontal dashed bars show the mean. The mean Id:anti-Id ratio was significantly higher in the mothers who gave birth to a child with neonatal lupus than in those who gave birth to a healthy child (P < 0.0001). CHB = congenital heart block. B, Id:anti-Id ratios in the IVIG preparations received by 13 of the 16 mothers. The Id:anti-Id ratio in the IVIG received by the mothers who gave birth to a child with neonatal lupus (stars) was ≥1, and the mean Id:anti-Id ratio in the IVIG received by these mothers was higher than that in the IVIG received by mothers who gave birth to a healthy child (P < 0.0001). Labels below the bars show the patient number and week of IVIG administration.
Confirmatory detection of anti-Id antibodies using highly purified F(ab)2 fragments of anti-La(349–364) antibodies
Although previous studies in our laboratory revealed that complementary peptides could be efficiently used for detection of anti-La/SSB anti-Id antibodies (12), we further confirmed detection of anti-Id antibodies using highly purified F(ab)2 fragments of anti-La(349–364) (Id) antibodies. Sequential sera from 7 anti-La/SSB–positive mothers were used in this assay. The reactivity patterns obtained by the direct detection of anti-Id antibodies were found to be almost identical to those obtained using complementary peptides, for all series of sera tested.
Removal of anti-Id antibodies enhances the reactivity of antibodies against the major epitope of La/SSB, La(349–364)
Our previous work showed that anti-Id antibodies are able to mask the anti-La/SSB response; and dissociation of Id–anti-Id immune complexes followed by blocking of anti-Id antibodies disclosed the hidden autoantibody activity (12). In the present study we used a similar approach, dissociating Id–anti-Id complexes by heating and removing the anti-Id antibodies with beads coated with anti-La(349–364) antibodies. We found that removal of anti-Id antibodies increased the idiotypic reactivity pattern in sequential sera from 5 of 7 mothers tested. None of these 5 mothers gave birth to a child with neonatal lupus during the study. In the serially tested sera from the remaining 2 mothers, there was no significant alteration of idiotypic activity; 1 of these 2 mothers gave birth to a child with neonatal lupus.
Inadequate content of anti-Id antibodies in IVIG preparations administered to the mothers of children with neonatal lupus
The increase in anti-Id activity after IVIG administration observed in the majority of mothers could be attributed to the exogenous addition of anti-Id antibodies contained in the IVIG preparation. Thus, we examined the Id and anti-Id content of the IVIG preparations given to 13 anti-La/SSB–positive mothers. IVIG preparations were found to contain anti-Id antibodies, but the titers fluctuated between batches. Moreover, the ratios of Id:anti-Id activity in the IVIG administered to mothers who gave birth to a child with neonatal lupus were ≥1, which was a higher ratio than the ratios found in the IVIG from batches administered to mothers giving birth to a healthy child (0.41–0.84) (t = 6.43, P < 0.0001). Accordingly, mothers who gave birth to a healthy child had received IVIG containing higher anti-Id activity in relation to Id activity compared to mothers who gave birth to a child with neonatal lupus (Figure 1B).
Exogenous addition of IVIG increases further anti-Id reactivity in maternal sera
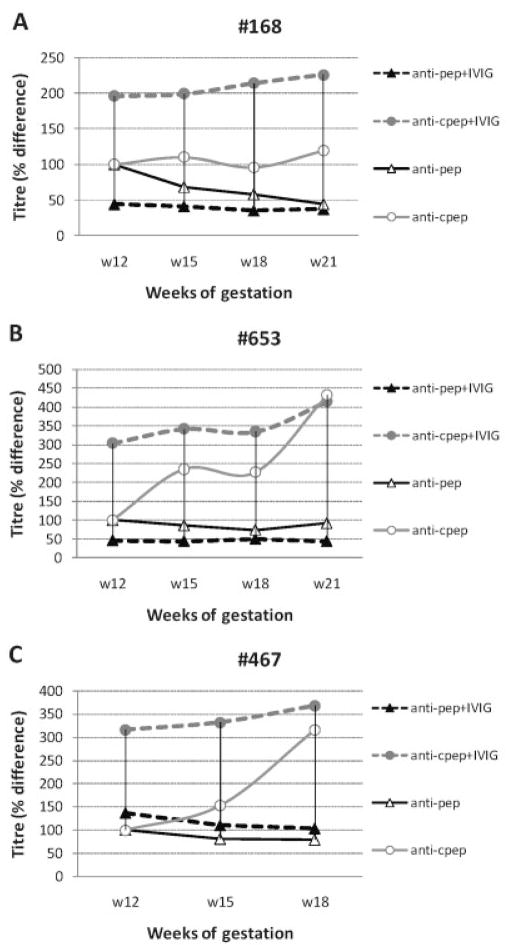
To explore the potential of IVIG to further increase the anti-La/SSB anti-Id activity in recipients’ sera, we added to each mother’s serum (which had been collected just prior to IVIG administration) 5.7 mg/ml of the IVIG preparation that the woman had actually received at each treatment time point. In sera from 11 of 13 women, exogenous IVIG addition enhanced anti-Id activity by 1.20–4.70-fold. Activity in sera from 3 representative patients is shown in Figure 2. No significant alteration in anti-Id activity was observed in sera from the remaining 2 women. In parallel, Id activity was reduced in sera from 9 of 13 women (by 10–53%), remained stable in serum from 1 woman, and increased (by 1.25–1.79-fold) in sera from the remaining 3 women. Overall, in 12 of 13 women, exogenous addition of IVIG led to higher increases of antiidiotypic than of idiotypic activity. Examples of reactivity patterns are available upon request from the corresponding author.
Figure 2.
Direct effect of IVIG on the Id–anti-Id network and the level of pathogenic autoantibodies in 3 representative patients (patients 168 [A], 653 [B], and 467 [C]). Exogenous IVIG preparations (5.7 mg/ml) were added to serum that had been collected during pregnancy just prior to administration of IVIG treatments. Anti-Id activity (anti-cpep) was substantially increased, whereas Id activity (anti-pep) was not significantly affected. See Figure 1 for other definitions.
DISCUSSION
CHB is closely associated with the presence of anti-Ro and anti-La antibodies (2). It has been hypothesized that maternal autoantibodies trigger an inflammatory cascade that leads to irreversible fibrotic replacement of the fetal AV node (3,14). Therefore, once (third-degree) CHB develops in the fetus, it cannot be reversed with any available treatment. The condition is fatal in ~20% of cases. The incidence of CHB is 10-fold higher in the offspring of mothers who are positive for anti-Ro and anti-La antibodies and have had a child with neonatal lupus (8), comprising the group of high-risk pregnancies. In these cases, prophylactic therapy would be particularly beneficial.
Proposed mechanisms to explain how IVIG prevents tissue damage have included the following: 1) it increases elimination of maternal anti-Ro and anti-La; 2) it reduces transplacental transport of antibodies; and 3) it modulates the inhibitory signals on macrophages, with consequent reduction of the inflammatory response and fibrosis in the fetal heart (15–17). In the PITCH study, IVIG was given to prevent development of CHB in the fetuses of 20 high-risk pregnant women (11). In parallel, a study using an identical treatment protocol in 15 high-risk pregnant women was conducted in Europe (18). Both studies were discontinued after reaching the stopping rule, i.e., detection of 3 cases of CHB among the first patients who were enrolled (11,18). Despite the finding that administration of IVIG at low doses does not prevent the recurrence of CHB, it was of particular interest to study how IVIG alters the Id–anti-Id network of pathogenic autoantibodies in the sera of mothers enrolled in the PITCH study.
Our previous work suggested that the presence of anti-Id antibodies to autoantibodies against La/SSB may protect the fetus by blocking the pathogenic maternal autoantibodies (10). This finding prompted us to investigate in detail the Id–anti-Id network of anti-La/SSB antibodies among mothers enrolled in the PITCH study. Sixteen of 20 mothers who completed the PITCH study were anti-La/SSB positive, and 3 gave birth to a child with neonatal lupus (2 with CHB and 1 with rash). The sera of the mothers of all 3 infants with neonatal lupus had a significantly higher Id:anti-Id antibody ratio than those of the mothers who gave birth to a healthy child, suggesting an inadequate antiidiotypic response after IVIG administration.
Therefore, unbalanced idiotypic antibodies, targeting the major epitope of La/SSB by antiidiotypic antibodies, may be an important determinant of neonatal lupus occurrence in the offspring of mothers treated with IVIG. However, it was unclear how each dose of IVIG alters the anti-Id network and influences Id and anti-Id antibody levels. To address this question, we carried out detailed analyses of Id and anti-Id reactivity in maternal sera at each time point of IVIG administration. Repeated IVIG administration led to a gradual increase of anti-Id activity and a decrease of Id activity in the majority of cases, bringing the levels of anti-Id antibodies close to the levels of Id antibodies. However, this was not the case for the mothers of the affected children with neonatal lupus; in sera from these mothers, anti-Id antibody titers did not approach those of the Id antibodies.
Inadequate anti-Id response has been recently correlated with the appearance of other autoimmune diseases, such as type 1 diabetes mellitus (DM) (19). Type 1 DM is characterized by the presence of autoantibodies to glutamate decarboxylase 65 (GAD65) (20). GAD65 antibodies often herald the onset of type 1 DM by months or years and, together with other autoantibodies to islet cells, are used to predict disease (21). Recently, Oak et al demonstrated that masked GAD65 antibodies are present in the healthy population and the absence of a particular anti-Id antibody, rather than GAD65 antibodies per se, is a characteristic of type 1 DM (19). Consistent with this concept are our results, which suggest the existence of masked Id antibodies (by anti-Id antibodies) in the mothers who gave birth to a healthy child. More specifically, we found that elimination of anti-Id antibodies from the sera of mothers treated with IVIG substantially enhanced the autoantibody (Id) activity in 5 of 7 series of sera tested (but not in the sera of 1 mother who gave birth to a child with neonatal lupus).
The increase of anti-Id activity and decrease of Id activity observed in many of the maternal sera following IVIG administration could be attributed to direct addition of anti-Id antibody to the mothers’ sera. This hypothesis was confirmed by examining the Id and anti-Id antibody content of the different IVIG preparations administered to 13 of the 16 anti-La/SSB–positive mothers enrolled in the PITCH study. These experiments showed that all but 2 of the mothers had received IVIG preparations with higher anti-Id activity than Id activity. The 2 mothers in whom this was not the case each gave birth to a child with CHB. Thus, it is plausible that the reason IVIG did not prevent CHB in 2 mothers was that they received IVIG with a high Id:anti-Id activity ratio, and thus anti-Id antibodies were not effectively increased.
Previous studies have indicated that IVIG exerts reactivity against different autoantigens, as well as antiidiotypic antibodies (22–25). This reactivity is likely due to the presence of polyreactive IgG antibodies and anti-Id antibodies in the pooled plasma used for IVIG fractionation (26,27). However, it can be augmented by various chemical treatments to which the IgG molecules have been subjected during the fractionation process (22,28). More specifically, the production process of commercial IVIG preparations (which differs among brands) involves fractionation and virus inactivation steps that include, in some cases, treatment at extreme physical conditions. These procedures significantly affect the autoreactivity of IVIG (22). Therefore, it is not surprising that different brands and batches of commercial IVIG (as used in the PITCH study) do not have identical Id and anti-Id antibody content.
Exogenous addition, to individual women’s serum, of the IVIG preparation that they had received at each study time point during pregnancy led to a large increase in anti-Id activity and a small decrease in Id activity in the majority of sera tested. In this respect, higher doses of IVIG (up to 2 mg/kg), which are presumed to be safe in pregnancy (29,30), might be more efficient for the prevention of neonatal lupus. Moreover, given that the IVIG preparations administered to mothers who gave birth to a child with CHB had an Id:anti-Id activity ratio of ≥1, pretesting of IVIG and selecting preparations with low Id–anti-Id potential for administration to high-risk women might be more efficacious in preventing neonatal lupus and CHB.
In conclusion, our study demonstrates that IVIG preparations contain anti-Id antibodies, and their administration can enhance the antiidiotypic antibody response in pregnant women who are positive for anti-La/SSB. The success of IVIG therapy may be a function of the anti-Id potential of each IVIG preparation as well as the Id:anti-Id ratio achieved in maternal sera after administration of IVIG.
Acknowledgments
Supported in part by the Research Council of the University of Athens. Dr. Buyon’s work was supported by the Alliance for Lupus Research and the NIH (grant AR-042455-16 and National Institute of Arthritis and Musculoskeletal and Skin Diseases contract AR-4-2271).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Tzioufas had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Routsias, Moutsopoulos, Buyon, Tzioufas.
Acquisition of data. Routsias, Kyriakidis, Friedman, Llanos, Clancy, Moutsopoulos, Buyon, Tzioufas.
Analysis and interpretation of data. Routsias, Kyriakidis, Moutsopoulos, Buyon, Tzioufas.
References
- 1.Buyon JP, Clancy RM. Maternal autoantibodies and congenital heart block: mediators, markers, and therapeutic approach. Semin Arthritis Rheum. 2003;33:140–54. doi: 10.1016/j.semarthrit.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Buyon JP, Clancy RM. Neonatal lupus. In: Wallace DJ, Hahn BH, editors. Dubois’ lupus erythematosus. 7. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 1058–80. [Google Scholar]
- 3.Buyon JP, Clancy RM, Friedman DM. Autoimmune associated congenital heart block: integration of clinical and research clues in the management of the maternal/foetal dyad at risk. J Intern Med. 2009;265:653–62. doi: 10.1111/j.1365-2796.2009.02100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cimaz R, Spence DL, Hornberger L, Silverman ED. Incidence and spectrum of neonatal lupus erythematosus: a prospective study of infants born to mothers with anti-Ro autoantibodies. J Pediatr. 2003;142:678–83. doi: 10.1067/mpd.2003.233. [DOI] [PubMed] [Google Scholar]
- 5.Brucato A, Frassi M, Franceschini F, Cimaz R, Faden D, Pisoni MP, et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counter-immunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum. 2001;44:1832–5. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 6.Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–66. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 7.Julkunen H, Eronen M. The rate of recurrence of isolated congenital heart block: a population-based study. Arthritis Rheum. 2001;44:487–8. doi: 10.1002/1529-0131(200102)44:2<487::AID-ANR70>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Llanos C, Izmirly PM, Katholi M, Clancy RM, Friedman DM, Kim MY, et al. Recurrence rates of cardiac manifestations associated with neonatal lupus and maternal/fetal risk factors. Arthritis Rheum. 2009;60:3091–7. doi: 10.1002/art.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strandberg L, Winqvist O, Sonesson SE, Mohseni S, Salomonsson S, Bremme K, et al. Antibodies to amino acid 200–239 (p200) of Ro52 as serological markers for the risk of developing congenital heart block. Clin Exp Immunol. 2008;154:30–7. doi: 10.1111/j.1365-2249.2008.03732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stea EA, Routsias JG, Clancy RM, Buyon JP, Moutsopoulos HM, Tzioufas AG. Anti-La/SSB antiidiotypic antibodies in maternal serum: a marker of low risk for neonatal lupus in an offspring. Arthritis Rheum. 2006;54:2228–34. doi: 10.1002/art.21954. [DOI] [PubMed] [Google Scholar]
- 11.Friedman DM, Llanos C, Izmirly PM, Brock B, Byron J, Copel J, et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum. 2010;62:1138–46. doi: 10.1002/art.27308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Routsias JG, Touloupi E, Dotsika E, Moulia A, Tsikaris V, Sakarellos C, et al. Unmasking the anti-La/SSB response in sera from patients with Sjögren’s syndrome by specific blocking of antiidiotypic antibodies to La/SSB antigenic determinants. Mol Med. 2002;8:293–305. [PMC free article] [PubMed] [Google Scholar]
- 13.Routsias JG, Dotsika E, Touloupi E, Papamattheou M, Sakarellos C, Sakarellos-Daitsiotis M, et al. Idiotype–antiidiotype circuit in non-autoimmune mice after immunization with the epitope and complementary epitope 289–308 aa of La/SSB: implications for the maintenance and perpetuation of the anti-La/SSB response. J Autoimmun. 2003;21:17–26. doi: 10.1016/s0896-8411(03)00063-5. [DOI] [PubMed] [Google Scholar]
- 14.Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–82. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 15.Yu Z, Lennon VA. Mechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. N Engl J Med. 1999;340:227–8. doi: 10.1056/NEJM199901213400311. [DOI] [PubMed] [Google Scholar]
- 16.Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J Immunol. 1996;157:3317–22. [PubMed] [Google Scholar]
- 17.Miranda-Carus ME, Askanase AD, Clancy RM, Di Donato F, Chou TM, Libera MR, et al. Anti-SSA/Ro and anti-SSB/La autoantibodies bind the surface of apoptotic fetal cardiocytes and promote secretion of TNF-α by macrophages. J Immunol. 2000;165:5345–51. doi: 10.4049/jimmunol.165.9.5345. [DOI] [PubMed] [Google Scholar]
- 18.Pisoni CN, Brucato A, Ruffatti A, Espinosa G, Cervera R, Belmonte-Serrano M, et al. Failure of intravenous immunoglobulin to prevent congenital heart block: findings of a multicenter, prospective, observational study. Arthritis Rheum. 2010;62:1147–52. doi: 10.1002/art.27350. [DOI] [PubMed] [Google Scholar]
- 19.Oak S, Gilliam LK, Landin-Olsson M, Torn C, Kockum I, Pennington CR, et al. The lack of antiidiotypic antibodies, not the presence of the corresponding autoantibodies to glutamate decarboxylase, defines type 1 diabetes. Proc Natl Acad Sci U S A. 2008;105:5471–6. doi: 10.1073/pnas.0800578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–6. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 21.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, et al. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45:926–33. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 22.Djoumerska I, Tchorbanov A, Pashov A, Vassilev T. The autoreactivity of therapeutic intravenous immunoglobulin (IVIG) preparations depends on the fractionation methods used. Scand J Immunol. 2005;61:357–63. doi: 10.1111/j.1365-3083.2005.01568.x. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich G, Kaveri SV, Kazatchkine MD. A V region-connected autoreactive subfraction of normal human serum immunoglobulin G. Eur J Immunol. 1992;22:1701–6. doi: 10.1002/eji.1830220706. [DOI] [PubMed] [Google Scholar]
- 24.Vassilev TL, Bineva IL, Dietrich G, Kaveri SV, Kazatchkine MD. Variable region-connected, dimeric fraction of intravenous immunoglobulin enriched in natural autoantibodies. J Autoimmun. 1995;8:405–13. doi: 10.1006/jaut.1995.0032. [DOI] [PubMed] [Google Scholar]
- 25.Djoumerska IK, Tchorbanov AI, Donkova-Petrini VD, Pashov AD, Vassilev TL. Serum IgM, IgG and IgA block by F(ab′)2-dependent mechanism the binding of natural IgG autoantibodies from therapeutic immunoglobulin preparations to self-antigens. Eur J Haematol. 2005;74:101–10. doi: 10.1111/j.1600-0609.2004.00350.x. [DOI] [PubMed] [Google Scholar]
- 26.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812–8. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 27.Saenko VA, Kabakov AE, Poverenny AM. Hidden high-avidity anti-DNA antibodies occur in normal human gammaglobulin preparations. Immunol Lett. 1992;34:1–5. doi: 10.1016/0165-2478(92)90019-k. [DOI] [PubMed] [Google Scholar]
- 28.Bouvet JP, Stahl D, Rose S, Quan CP, Kazatchkine MD, Kaveri SV. Induction of natural autoantibody activity following treatment of human immunoglobulin with dissociating agents. J Autoimmun. 2001;16:163–72. doi: 10.1006/jaut.2000.0472. [DOI] [PubMed] [Google Scholar]
- 29.Branch DW, Peaceman AM, Druzin M, Silver RK, El-Sayed Y, Silver RM, et al. for the Pregnancy Loss Study Group. A multicenter, placebo-controlled pilot study of intravenous immune globulin treatment of antiphospholipid syndrome during pregnancy. Am J Obstet Gynecol. 2000;182:122–7. doi: 10.1016/s0002-9378(00)70500-x. [DOI] [PubMed] [Google Scholar]
- 30.Berkowitz RL, Lesser ML, McFarland JG, Wissert M, Primiani A, Hung C, et al. Antepartum treatment without early cordocentesis for standard-risk alloimmune thrombocytopenia: a randomized controlled trial. Obstet Gynecol. 2007;110:249–55. doi: 10.1097/01.AOG.0000270302.80336.dd. [DOI] [PubMed] [Google Scholar]