Abstract
Activation of TLR by ssRNA after FcγR-mediated phagocytosis of immune complexes (IC) may be relevant in autoimmune-associated congenital heart block (CHB) where the obligate factor is a maternal anti-SSA/Ro Ab and the fetal factors, protein/RNA on an apoptotic cardiocyte and infiltrating macrophages. This study addressed the hypothesis that Ro60-associated ssRNAs link macrophage activation to fibrosis via TLR engagement. Both macrophage transfection with noncoding ssRNA that bind Ro60 and an IC generated by incubation of Ro60-ssRNA with an IgG fraction from a CHB mother or affinity purified anti-Ro60 significantly increased TNF-α secretion, an effect not observed using control RNAs or normal IgG. Dependence on TLR was supported by the significant inhibition of TNF-α release by IRS661 and chloroquine. The requirement for FcγRIIIa-mediated delivery was provided by inhibition with an anti-CD16a Ab. Fibrosis markers were noticeably increased in fetal cardiac fibroblasts after incubation with supernatants generated from macrophages transfected with ssRNA or incubated with the IC. Supernatants generated from macrophages with ssRNA in the presence of IRS661 or chloroquine did not cause fibrosis. In a CHB heart, but not a healthy heart, TLR7 immunostaining was localized to a region near the atrioventricular groove at a site enriched in mononuclear cells and fibrosis. These data support a novel injury model in CHB, whereby endogenous ligand, Ro60-associated ssRNA, forges a nexus between TLR ligation and fibrosis instigated by binding of anti-Ro Abs to the target protein likely accessible via apoptosis.
The association of isolated congenital heart block (CHB) with maternal autoantibodies to SSA/Ro and SSB/La ribonucleoproteins is approaching the predictable, even in mothers who are completely asymptomatic. Only 2% of neonates born to mothers with the candidate Abs have CHB (1), yet these Abs are present in >85% of mothers whose fetuses are identified with conduction abnormalities in a structurally normal heart (2). This disparity implies that the Abs are necessary but insufficient to cause CHB, and that the final pathway to fibrosis may be variable: kept totally in check in most fetuses (normal sinus rhythm), subclinical in others (first-degree block) and fully executed in very few (advanced block). Indeed, the spectrum of conduction abnormalities observed on electrocardiogram includes first-, second-, and third-degree block with the histologic hallmark of advanced block being atrioventricular (AV) nodal replacement by fibrosis (3). Moreover, fibrotic injury can extend to the myocardium and endocardium, in rare cases absent detectable AV nodal dysfunction (4). Immunohistologic evaluation of hearts from fetuses dying with CHB has revealed exaggerated apoptosis, clusters of macrophages in zones of fibrosis that colocalize with IgG and apoptotic cells, TNF-α and TGF-β mRNA expression in these cells, and extensive collagen deposition in the conducting system (5, 6). These in vivo observations are supported by in vitro studies. Specifically, the consideration of exaggerated apoptosis as the initial link between maternal autoantibodies and tissue injury led to the observation that cardiocytes are capable of phagocytosing autologous apoptotic cardiocytes and anti–SSA-Ro/SSB-La Abs inhibit this function (7). Recognizing that this perturbation of physiologic efferocytosis might divert uptake to professional FcγR-bearing phagocytes raised the hypothesis that macrophages engage TLR via binding to the RNA moiety of the target autoantigen.
Pertinent to a focus on the macrophage is the observation that members of the TLR gene family can recognize self-Ags composed of proteins complexed to nucleic acids (reviewed in Ref. 8). It has been posited that self-Ags released from stressed or dying cells complex with preexisting autoantibodies, which are phagocytosed via Fcγ receptor-bearing cells and delivered to the TLR sequestered in an endosomal/lysosomal compartment. Attention to this pathway originated with several independent observations linking the type I IFN system to the etiopathogenesis of systemic lupus erythematosus (SLE) (9). Specificity of the nucleic acid component dictates the TLR engaged. For example, DNA and DNA-associated autoantigens are ligands for TLR9 and ssRNA for TLR7/8. So-called interferogenic immune complexes (ICs) trigger IFN-α synthesis in plasmacytoid dendritic cells as well as cultured PBMCs (10–12). Parallel observations reveal that DNA or RNA-protein macromolecules complexed with cognate Abs are also capable of activating autoreactive B cells (11, 12). This 2-receptor paradigm—binding of FcγR by the respective IgG or BCR by Ag and subsequent intracellular engagement of TLR7/8 by anRNAligand—might be particularly relevant in a disease where the obligate factor is a maternal anti–SSA/Ro-SSB/La Ab, and the candidate fetal factors are the target protein/RNA particles accessible on an apoptotic cardiocyte and the professional FcγR-bearing cells to which uptake has been diverted. Indeed, TLR agonists induce macrophage effector secretion of proinflammatory cytokines such as TNF-α (13). CHB, representing a pathologic consequence of passively acquired autoimmunity, offers a unique opportunity to define the pathogenicity of an autoantibody (a response of the adaptive immune system) in driving end-organ disease in part by co-opting the innate immune system to tip the balance between wound healing and fibrosis.
Accordingly, this study was initiated to evaluate the hypothesis that TLR signaling can result in fibrosis. The specific relevance of this novel paradigm to CHB was addressed by evaluating the individual components required in this cascade, the Ro60-associated ssRNAs (to trigger TLR signaling), Ro60 purified protein (the Ag accessible on apoptotic cardiocytes), and an IgG fraction and affinity purified Abs from a mother whose child had CHB (to provide the source of anti-Ro60 Ab to form the IC taken up by the macrophages) in an in vitro model. Macrophage supernatants generated under conditions to evaluate the dependence of FcγR uptake and TLR7/8 ligation were evaluated for their effects on transdifferentiation (smooth muscle actin staining [SMAc]) of and collagen secretion by cultured human fetal cardiac fibroblasts. Histological evaluation of cardiac tissue from a fetus dying with CHB was assessed to support the in vitro model.
Materials and Methods
Human IgG preparations
Human IgG is routinely isolated using a Protein A-IgG isolation kit (Pierce, Rockford, IL). Samples are processed by application to Detoxi-Gel Endotoxin Removing Gel (Pierce) to remove any contaminating LPS (<1 pg/ml). Protein concentrations of each IgG fraction and affinity purified Ab are assessed by a protein quantification kit (Pierce). The Ab preparations included: IgG fractions (0.3 mg/ml) isolated from: 1) an anti–SSA/Ro-SSB/La-positive mother of a child with CHB (no Abs to dsDNA) and 2) healthy control absent any autoantibodies. In addition, affinity purified Abs to the 60 kDa Ro component were generated from the sera of another SSA/Ro-positive mother of a child with CHB by affinity column chromatography using the Ro60 recombinant protein coupled to cyanogen bromide-activated Sepharose 4B as previously described (7).
Preparation of ssRNAs
For obtaining Ro60-associated ssRNAs, misfolded pre-5S (m-pre5S), and hY3 plasmids (14), kindly provided by Dr. Sandra Wolin (Yale University, New Haven, CT) were digested with HindIII restriction enzyme for linearization. The 1 µg template was subjected to transcription with the TranscriptAid transcription kit (Fermentas Life Sciences, Burlington, Ontario, Canada) using 4 µl 5× reaction buffer, 8 µl equimolar mixture of ATP, CTP, GTP, and UTP, and 2 µl enzyme mix. The reaction mixture was incubated at 37°C for 2 h. After the reaction, 2 µl of RNase-free DNase I was added and the mixture further incubated at 37°C for 15 min. The DNase reaction was stopped by addition of 2 µl EDTA, pH 8.0, and incubation at 65°C for 10 min. The transcripts were purified by phenol/chloroform extraction, resuspended in water at 2.5 mg/ml, and the quality evaluated by RNAQQNANO technologies (Genomics Facility, New York University Medical Center). The ssRNA41/LyoVec control (15) was purchased from InvivoGene (San Diego, CA) and resuspended in water at a concentration of 2.5 mg/ml. hY3 A/U RNA that involves a substitution of the U nucleotides with A nucleotides throughout the entire sequence of hY3, was synthesized by Thermo Scientific (Chicago, IL) and also resuspended at 2.5 mg/ml for experimentation.
For alkaline phosphatase treatment, 10 µg in vitro-transcribed RNA was treated with 30 U calf intestine alkaline phosphatase (Stratagene, La Jolla, CA) for 3 h at 37°C in a buffer containing 50 mM Tris-HCl, pH 9.5, 0.1 mM EDTA in the presence of 10 U RNase inhibitor (RNAguard, Amer-sham-Biosciences, Scituate, MA). After calf intestine alkaline phosphatase treatment, the RNA was reisolated using the previously described method.
For RNase experiments, hY3 was treated with 8 µg/ml RNase A (Ab-gene, AB-0548) for 3 h at −37°C minus and plus RNase Inhibitor (N808-0119 [700 U]).
Macrophage transfection with ssRNA
ssRNA preparations used for transfection of macrophages were carried out following the specific manufacturer’s instructions using a commercial kit (DOTAP Liposomal Transfection Reagent, Roche, Germany). Briefly, 2.5 µg ssRNA are mixed with 15 µl DOTAP reagent to a final volume of 75 µl reaction buffer, and incubated at 22°C for 15 min. The mixture is then added to IFN-γ–primed macrophages for an overnight transfection at 37°C.
Preparation of SSA/Ro60, ssRNA, and IgG complexes
Native Ro60 was purchased from GenWay Biotech (San Diego, CA). Endotoxin was removed as described previously. Equimolar amounts of Ro60 (4.7 µg) and varied ssRNA (2.5 µg) were mixed and incubated for 1 h at 22°C on rotation. Then, 150 µg of either CHB IgG or normal IgG (nIgG), 30 µg of either AP60 or anti–60-ScFv (from Ref. 7) were added, and the mixture further incubated for 1 h under the same conditions. The reaction complexes were then added to cultured, INF-γ–primed macrophages (see below).
Isolation and preparation of macrophages
Human macrophages derived from PBMCs are isolated from WBC concentrate (Leukopak; New York Blood Center, New York, NY) by centrifugation on Ficoll-Hypaque gradients and purified by positive selection using CD14 microbeads (Miltenyi Biotech Cat. 130-050-201, Miltenyi Biotech, Auburn, CA) and LS columns (Miltenyi Biotech Cat. 130-042-401). The resulting monocytes are then cultured in Teflon beakers (RPMI 1640/10% FCS plus 10 ng/ml GM-CSF; PHC2014, Invitrogen, San Diego, CA) for 7 d. Monocyte-derived macrophages (5 × 105 per milliliter) were plated on growth medium containing 10% serum and incubated at 37°C. After 48 h, attached macrophages were incubated with serum-free fresh medium containing INF-γ (0.05 ng/ml) for 6 h. After a double wash with HBSS buffer, macrophages were DOTAP-transfected or incubated with the Ro60-containing ICs.
For flow cytometry experiments evaluating the expression of TLR7 and TLR8, macrophages (primed using IFN-γ) were stained with PE-conjugated anti-human CD14 Ab (12-0149, eBioscience, San Diego, CA). Cells were double labeled with anti-TLR7 Ab (IMG-665A, Imgenex, San Diego, CA) or FITC-conjugated anti-TLR8 Ab (IMG-321C, Imgenex) and followed by staining with FITC-labeled goat anti-rabbit IgG (F0382, Sigma-Aldrich, St. Louis, MO).
For the evaluation of mRNA, total RNA was isolated from macrophages (primed with IFN-γ) using standard conditions. cDNA synthesis and PCR amplification were performed using specific primers for TLR7 and TLR8 (16).
In vitro coculturing experiments and assessments
Macrophages were stimulated with ssRNA (DOTAP transfection) and Ro60 ICs in the absence and presence of endotoxin free-ODN, IRS661 (gift from Dynavax Technologies, Berkeley, CA), chloroquine (Sigma-Aldrich), or with anti-human anti-FcγRIIIa (CD16a) (IM0813; 20 µg/ml, Beckman Coulter, Fullerton, CA) as described in the figure legends. For each condition, macrophages were plated as monolayers. Supernatants were retrieved for analysis as described (17). The release of TNF-α was determined using the human TNF-α ELISA kit (Cell Sciences, Canton, MA).
Isolation and preparation of cardiac fibroblasts
Fibroblasts are isolated from the hearts of abortuses aged 16–24 wk, as previously described (18). Fibroblasts at passages three to five are used. The expression of SMAc (see below) has been used as our assessment of myofibroblasts. In brief, fibroblasts are plated on glass coverslips (1.2 × 104/cm2) and macrophage supernatants added (as generated previously and indicated in figure legends). Cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% triton ×100. For indirect immunofluorescence: mAb α-SMAc 1:200 dilution (Sigma-Aldrich) or isotype control was used at a dilution of 1:200 (Sigma-Aldrich). After addition of anti-mouse IgG Cy3 (1:200, Sigma-Aldrich) the samples were analyzed by indirect immunofluorescence and images captured by digital acquisition. Evaluation of SMAc staining was performed in the absence of knowledge of the experimental condition for generation of the supernatants.
Collagen assay
The concentration of soluble collagen in supernatants collected from cultures of fibroblasts treated with each of the supernatants generated from the macrophages incubated with the various reagents as previously described was measured by the Sircol soluble collagen assay (Biocolor, Belfast, Ireland). Briefly, 100 µl supernatant from the fibroblasts were incubated with 1 ml Sircol Dye reagent for 30 min at 22°C on rotation. The mixture was centrifuged at > 10,000×g for 10 min. After discharging the supernatant, the pellet was washed with cold pure ethanol and centrifuged. The pellet was then resuspended in 1 ml Alkali reagent and incubated for 10 min at 22°C on rotation. The 200 µl were transferred to a multiwell plate reader and absorbance determined at 540 nm. The standard curve was obtained by running parallel 5-, 10-, 25-, and 50-µg collagen standards.
Tissue sections from fetal hearts
Formalin-fixed paraffin sections were obtained from the heart of a fetus with fatal CHB [clinical description and gross anatomy previously published (6)], and a normal human fetus electively terminated at 23 wk of gestation. For immunostaining, sections of the fetal heart are prepared as described (6). Briefly, anti-TLR7 (Biocarta, IMG 581A), rabbit IgG (0111-01, isotype control for anti-TLR7, Southern Biotechnology Associates, Birmingham, AL), anti-CD45 (1076, Immunotech, Westbrook, ME), or mouse IgG (isotype control for anti-CD45, Accurate Chemical and Scientific, Westbury, NY) were used as primary Ab. Stains were visualized using anti-rabbit IgG alkaline phosphatase (brown) or anti-mouse IgG peroxidase (red). Sections were counterstained before photography.
Statistical analysis
The Wilcoxon matched pairs test and the paired t test were used as appropriate to compare TNF-α released by macrophages and collagen release by fibroblasts between the different groups. Values of p < 0.05 were considered significant.
Results
Transfection of ssRNA (hY3 and m-pre5S) induces TLR-dependent TNF-α release by macrophages
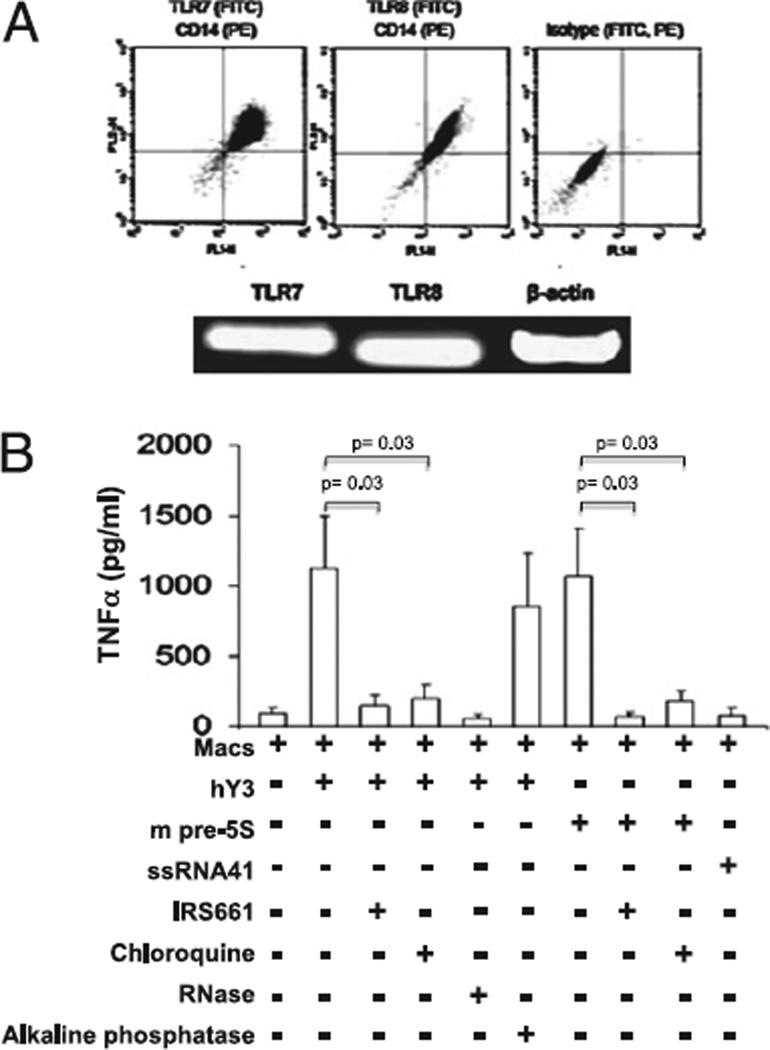
To initially evaluate the expression of TLR7/8, INF-γ–primed macrophages were permeabilized with digitonin, stained with Abs reactive with CD14 (PE) and TLR7 (FITC) or TLR8 (FITC), and assessed by FACS. CD14+ cells expressed both TLR7 and TLR8 (Fig. 1A, n = 3). RT-PCR confirmed the protein data (Fig. 1A, n = 3). Having established expression of TLR7/8 in the cultured macrophages, the capacity of ssRNAs to serve as agonists was evaluated. Treatment of macrophages with hY3 (DOTAP transfection) significantly stimulated TNF-α release compared with primed macrophages alone (1121 ± 373 pg/ml versus 92 ± 40 pg/ml, respectively, p = 0.0001, n = 14). m-pre5S RNA also significantly stimulated macrophages to secrete TNF-α (1072 ± 338 pg/ml, p = 0.0001 versus macrophages alone), an effect not observed with transfected ssRNA41 (control RNA, 78 ± 42, p = NS versus macrophages alone, Fig. 1B). The dependence on TLR signaling was then addressed. Both the TLR7 antagonist IRS661 (32 ng/µl) and chloroquine (10 µM) significantly decreased TNF-α release induced by either hY3 or m-pre5S RNA (IRS661: 159 ± 77 pg/ml, p = 0.03, n = 9 for hY3, and 71 ± 29 pg/ml, p = 0.03, n = 9 for pre-5S; chloroquine: 202 ± 89 pg/ml, p = 0.03, n = 9 for hY3, and 180 ± 70 pg/ml, p = 0.03, n = 9 for pre-5S, Fig. 1B). Coincubation of hY3 with RNase displayed a strong trend to reduce TNF-α release (53 ± 31 pg/ml, p = 0.06, n = 5).
FIGURE 1.
Stimulation of macrophages by hY3 and mpre-5S RNAs is TLR-dependent. A, IFN-γ–primed macrophages express TLR7 and TLR8 (FACS, RT-PCR). B, TNF-α was measured in the supernatants generated from human primed macrophages transfected with hY3, mpre-5S RNA or SSRNA41 under varied conditions. Treatments include coincubations in the presence or absence of IRS661, chloroquine, RNase as well as alkaline phosphatase. Bars represent means ± SEM.
To evaluate the capacity of in vitro-derived RNA to stimulate macrophages via a RIG1-dependent pathway, hY3 RNA was treated with alkaline phosphatase [which cleaves a 5′-triphosphate (19)]. Treatment of hY3 with alkaline phosphatase did not reduce the TNF-α release (778 ± 334 [macrophages and hY3] versus 849 ± 386 [macrophages and hY3] with pretreatment using alkaline phosphatase, n = 4, Fig. 1B).
The complex of human Ro60-hY3 and Ab to Ro60 generate FcγR-dependent TNF-α secretion by macrophages
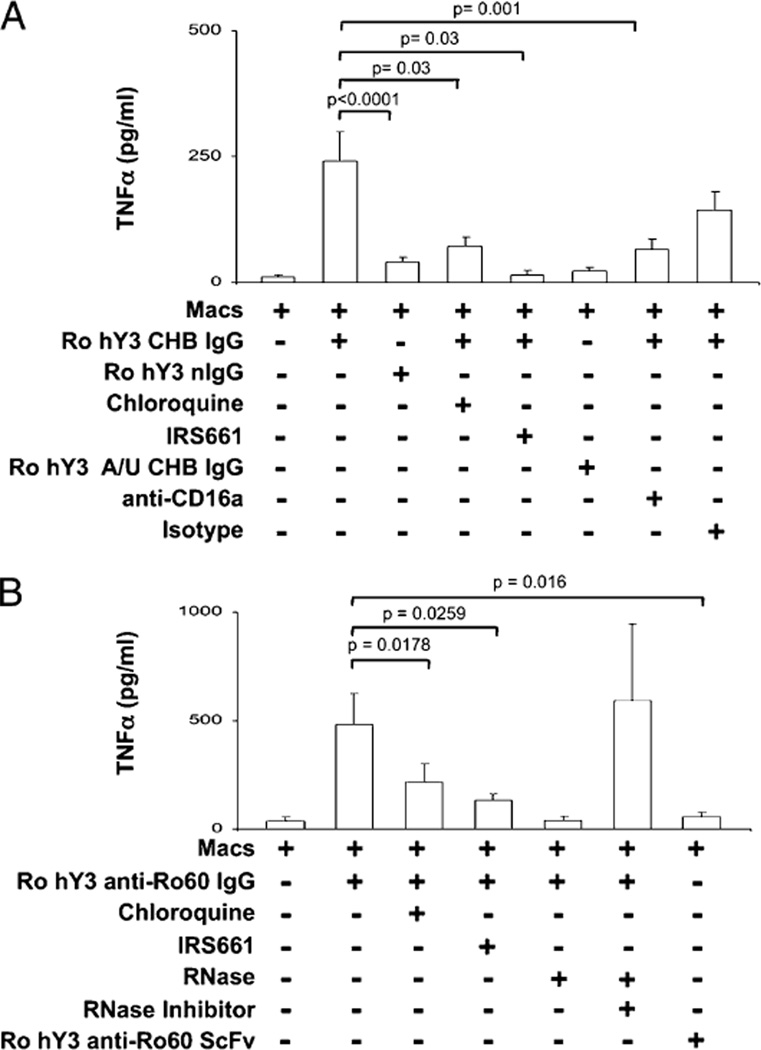
Having demonstrated TNF-α secretion by Ro60-associated ssRNA, ICs containing Ro60-associated ssRNA were generated as a proxy for opsonized apoptotic cardiocytes. ICs composed of an IgG fraction from a CHB mother (serum contains anti-Ro60) and native Ro60-hY3 (Ro-hY3–CHB IgG) significantly increased TNF-α secretion compared with nIgG (healthy donor absent anti-Ro) with Ro60-hY3 (Ro-hY3–nIgG), (241 ± 59 pg/ml versus 40 ± 10 pg/ml, respectively, p < 0.0001, n = 15, Fig. 2A). The specificity of the ssRNA was demonstrated by the absence of TNF-α release after treatment with Ro-hY3 A/U-CHB IgG. Similar to the transfection experiments, dependence on TLR signaling was then addressed. Both IRS661 (32 ng/µl) and chloroquine (10 µM) significantly decreased TNF-α release induced by Ro-hY3–CHB IgG (IRS661: 23 ± 7 pg/ml, p = 0.03, n = 6; chloroquine: 72 ± 18 pg/ml, p = 0.03, n = 6, Fig. 2A).
FIGURE 2.
Stimulation of macrophages by ICs composed of Ro60-associated ssRNA is TLR- and FcγRIIIa-dependent. In A, TNF-α was measured in the supernatants generated from human macrophages incubated with native Ro60 in complex with hY3 or hY3 A/U, and CHB IgG or nIgG. Treatments included coincubations in the presence or absence of chloroquine or IRS661 or anti-CD16a or an isotype Ab (control). B, TNF-α was measured in supernatants generated from human macrophages incubated with native Ro60 in complex with hY3 and affinity purified anti-Ro60 (anti-Ro60 IgG) or monoclonal anti-Ro (anti-Ro60 ScFv). Treatments included coincubations with chloroquine, IRS661, RNase, or RNase plus RNase inhibitor. Bars represent means ± SEM.
The potential importance of FcγR-mediated delivery of ssRNA was evaluated with a preparation of Ro60 hY3 ICs (Ro-hY3–CHB IgG). The addition of anti-CD16a to Ro-hY3–CHB IgG-stimulated macrophages significantly reduced TNF-α secretion (66 ± 20 pg/ml, p = 0.001 versus Ro-hY3–CHB IgG, n = 11, Fig. 2A).
To assure specificity of the IC, affinity purified anti-Ro60 (anti-Ro60 IgG) and anti-Ro60 ScFv (absent Fc domain) were evaluated in parallel experiments. Equivalent to the results obtained with Ro-hY3–CHB IgG, complexes of Ro-hY3–anti-Ro60 IgG stimulated macrophages to release TNF-α (481 ± 144 pg/ml versus 35 ± 19 pg/ml [macrophages alone], p < 0.0001, n = 7, Fig. 2B). In contrast, macrophages challenged with complexes of Ro-hY3–anti-Ro60 ScFv released TNF-α at levels comparable to macrophages alone supporting the dependency on FcγR engagement. Coincubation of Ro-hY3–anti-Ro60 IgG with chloroquine and IRS661 significantly reduced TNF-α secretion (IRS661: 133 ± 30 pg/ml p = 0.026, n = 7; chloroquine: 216 ± 87 pg/ml p = 0.0178, n = 7). Furthermore, treatment of the IC Ro-hY3–anti-Ro60 IgG with RNase resulted in TNF-α secretion equivalent to that obtained from the macrophages alone (49 ± 19 pg/ml, n = 8). In contrast, cotreatment of the ICs with RNase, and an inhibitor of RNase, restored TNF-α secretion by stimulated macrophages to that seen with the ICs alone (Fig. 2B).
TLR stimulation is linked to fibrosis in the human fetal cardiac fibroblast
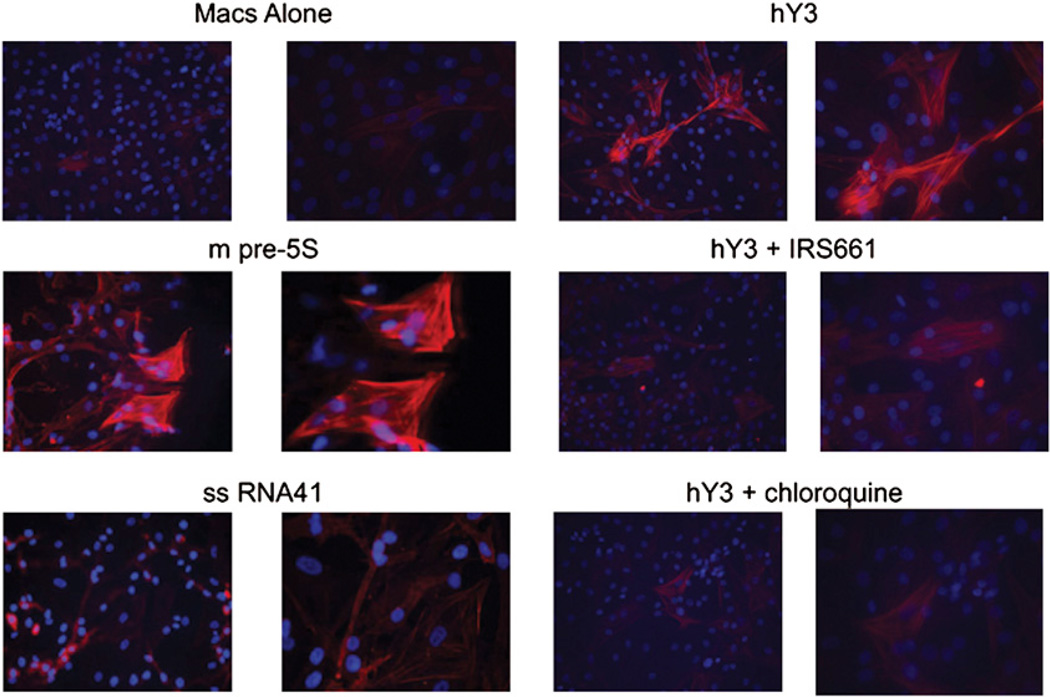
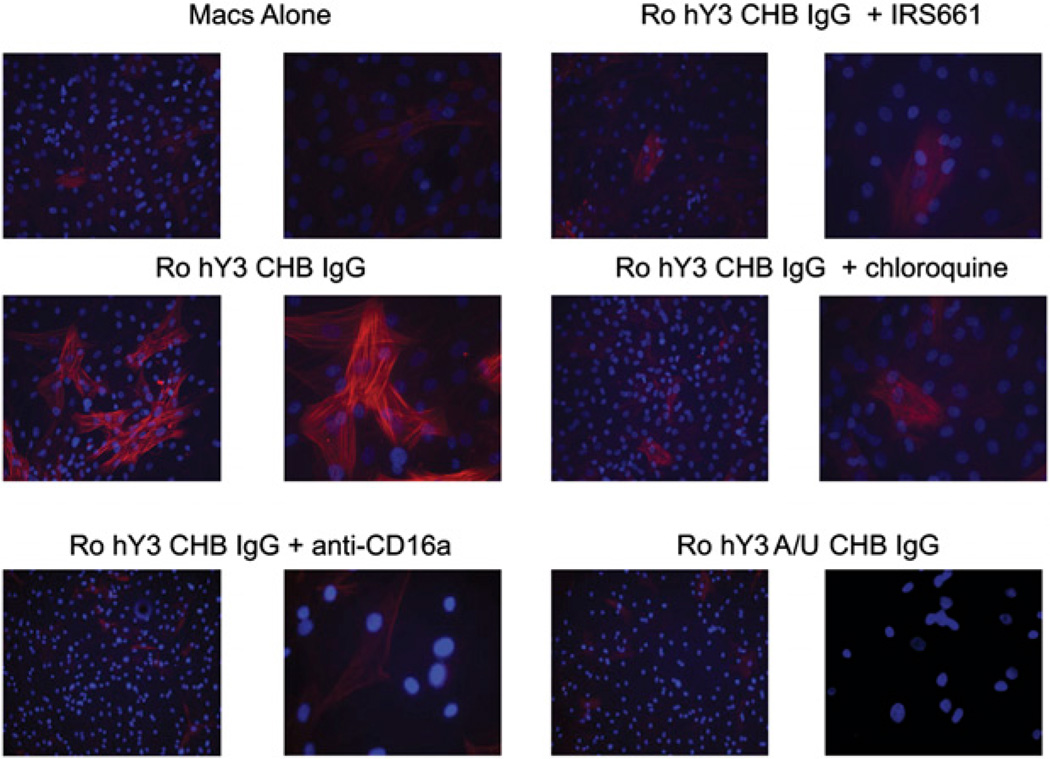
The next set of experiments was designed to address whether TLR activation generated by macrophage stimulation with ssRNA via transfection or ICs might link inflammation to fibrosis. The approach exploited two readouts including human fetal cardiac fibroblast protein expression of SMAc and the release of collagen by stimulated fibroblasts. Transdifferentiation of fibroblasts (SMAc staining) was markedly increased by incubation with supernatants generated from macrophages transfected with hY3 or m-pre5s RNA, but not ssRNA41 (Fig. 3, representative of six experiments). The increased expression of SMAc was not observed when fibroblasts were exposed to supernatants of macrophages transfected with hY3 in the presence of IRS661 or chloroquine (Fig. 3, representative of six experiments). In addition, exposure of fibroblasts to supernatants from macrophages incubated with the IC, Ro-hY3–CHB IgG, resulted in increased expression of SMAc (Fig. 4, representative of three experiments) that was not observed with supernatants generated from macrophages treated with the IC and Abs to CD16a. Supernatants of macrophages coincubated with Ro-hY3–CHB IgG in the presence of chloroquine or IRS661 strongly reduced SMAc expression (Fig. 4, representative of three experiments). In contrast, fibroblasts treated with supernatants from macrophages incubated with Ro-hY3–nIgG or supernatants from macrophages incubated with Ro-hY3 A/U-CHB IgG, did not express SMAc (not shown).
FIGURE 3.
Transdifferentiation of human fetal cardiac fibroblasts exposed to supernatants from macrophages transfected with hY3 ssRNA is TLR-dependent. Human fetal cardiac fibroblasts were prepared as monolayers. Cells were incubated with supernatants of macrophages diluted 1:1 with fibroblast medium. The supernatants were generated from human macrophages transfected with hY3, m-pre5S RNA or ssRNA41, or hY3 in the presence of IRS661 or chloroquine (shown in Fig. 1). Fibroblasts were then stained with Hoechst and anti–SMAc-Cy3, and analyzed by fluorescence microscopy (original magnifications ×10 and ×40). Results are representative of three experiments.
FIGURE 4.
Transdifferentiation of human fetal cardiac fibroblasts exposed to supernatants from macrophages incubated with ICs composed of Ro60-associated ssRNA is TLR- and FcγRIIIa-dependent. Human fetal cardiac fibroblasts were prepared as monolayers. Cells were incubated with supernatants of macrophages diluted 1:1 with fibroblast media. The supernatants were generated from human macrophages incubated with a complex of native Ro60 plus hY3 or hY3 A/U and CHB IgG, and Ro hY3 CHB IgG with or without IRS661, chloroquine and anti-CD16a (as shown in Fig. 2). Fibroblasts were then stained with Hoechst and anti–SMAc-Cy3, and analyzed by fluorescence microscopy (original magnification ×10 and ×40). Results are representative of three experiments.
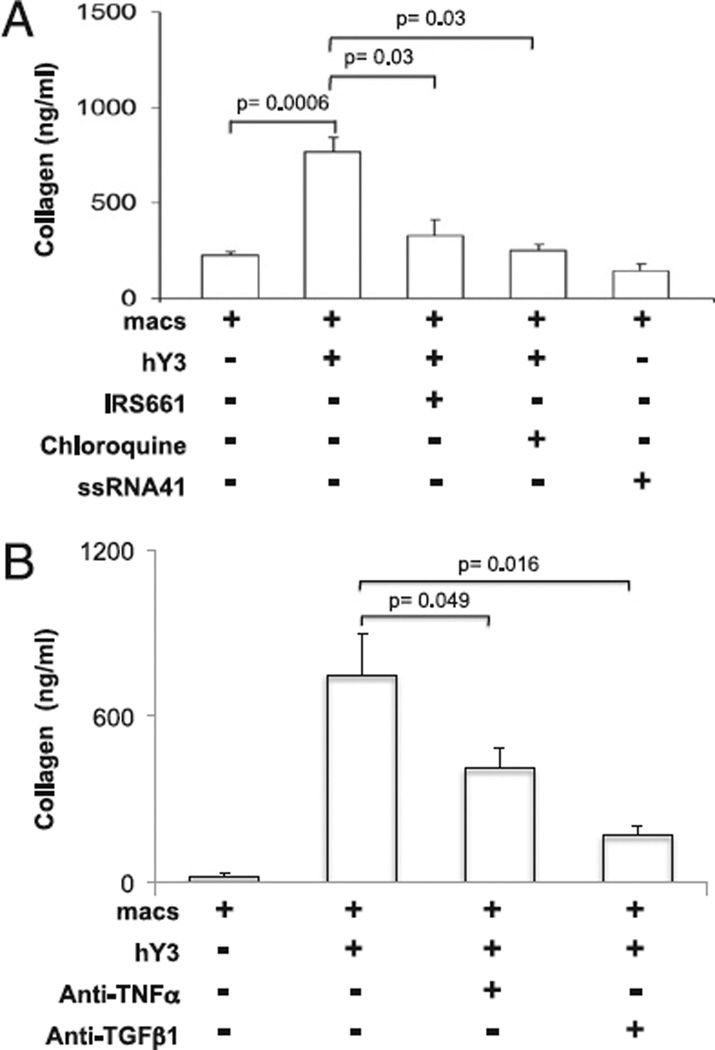
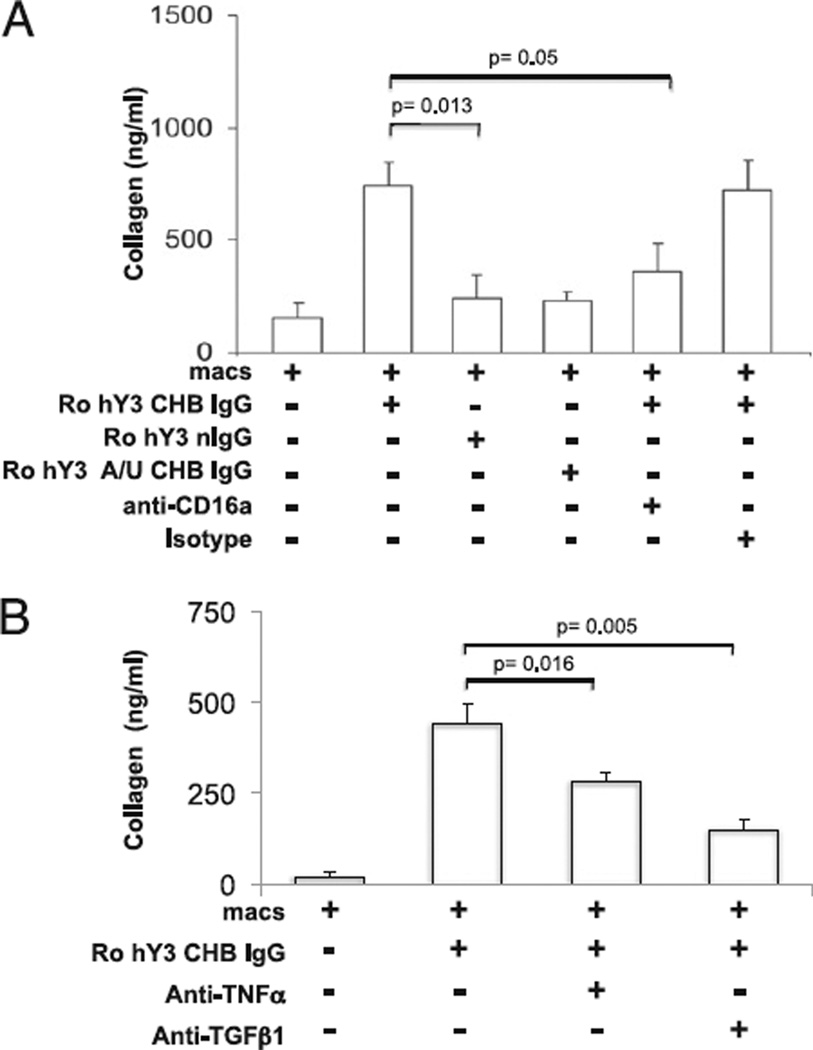
Collagen secretion by the cultured fetal cardiac fibroblasts paralleled transdifferentiation. Collagen secretion was increased after the addition of supernatants from macrophages transfected with hY3 compared with supernatants from macrophages alone (766 ± 82 ng/ml versus 224 ± 23.4 ng/ml, respectively, n = 7, p = 0.0006, Fig. 5A). Co-treatment with IRS661 or chloroquine significantly reduced the hY3-induced collagen synthesis (for IRS661, 331 ± 78 ng/ml, p = 0.03, n = 9; for chloroquine, 254 ± 33 ng/ml, p = 0.03, n = 9, Fig. 5A). Exposure of fibroblasts to supernatants of macrophages transfected with ssRNA41 did not stimulate collagen release (150 ± 30 ng/ml, p = NS versus macrophages alone). In addition, exposure of fibroblasts to supernatants generated from macrophages incubated with Ro-hY3–CHB IgG significantly increased the levels of collagen compared with Ro-hY3–nIgG (743 ± 103 ng/ml versus 242 ± 101 ng/ml, respectively, p = 0.013, n = 4, Fig. 6A). In fibroblasts treated with macrophage supernatants generated after incubation with Ro-hY3–CHB IgG and anti-CD16a, the levels of collagen were significantly reduced (360 ± 123 ng/ml, p = 0.05 versus supernatants of macrophages and Ro-hY3–CHB IgG, n = 4, Fig. 6A). Supernatants from macrophages incubated with Ro-hY3 A/U-CHB IgG did not induce collagen secretion.
FIGURE 5.
Collagen secretion by human fetal cardiac fibroblasts exposed to supernatants from macrophages transfected with hY3 ssRNA is TLR-dependent. Human fetal cardiac fibroblasts were prepared as monolayers and treated using conditions described in Fig. 3. Cells were plated into four-chamber slides (24 h) and serum starved (24 h). A, Conditions match those in Fig. 3. B, To examine the effect of neutralizing Abs on transdifferentiation of cardiac fibroblasts, cells were incubated with supernatants in the absence or presence of a TNF-α–neutralizing Ab (1 µg/ml) or a TGF-β–neutralizing Ab (1 µg/ml) for 24 h. After the 24-h incubation, supernatants of fibroblast cultures were retrieved and total collagen was detected by the Sircol assay. Results are representative of three experiments. Bars represent means ± SEM.
FIGURE 6.
Collagen secretion by human fetal cardiac fibroblasts exposed to supernatants from macrophages incubated with ICs composed of Ro60-associated ssRNA is FcγRIIIa-dependent. A, Human fetal cardiac fibroblasts were prepared as monolayers and treated using conditions described in Fig. 4. Cells were plated into four-chamber slides (24 h) and serum starved (24 h). B, Cells were incubated with supernatants in the absence or presence of a TNF-α–neutralizing Ab (1 µg/ml) or a TGF-β–neutralizing Ab (1 µg/ml) for 24 h. After the 24-h incubation, supernatants of fibroblast cultures were retrieved and total collagen was detected by the Sircol assay. Results are representative of three experiments. Bars represent means ± SEM.
To examine the potential role of TNF-α and TGF-β1 in the transdifferentiation of cardiac fibroblasts by supernatants from macrophages transfected with hY3 or macrophages incubated with Ro-hY3–CHB IgG, the effects of their respective neutralizing Abs were evaluated. TNF-α–neutralizing Ab partially attenuated the collagen release by conditions using hY3 (746 ± 152 versus 410 ± 46, absence and presence of anti–TNF-α Ab, respectively, p = 0.049, n = 5 Fig. 5B) and Ro-hY3–CHB IgG (439 ± 56 versus 280 ± 26, absence and presence of anti–TNF-α Ab, respectively, p = 0.016, n = 5, Fig. 6B). However, incubation of the same macrophage supernatants with a neutralizing anti–TGF-β1 Ab resulted in nearly complete attenuation of the elicited collagen synthesis by conditions with hY3 and Ro-hY3–CHB IgG (for hY3, 746 ± 152 versus 168 ± 34, absence and presence of anti–TGF-β Ab, respectively, p = 0.016, n = 5, Fig. 5B and for ICs, 439 ± 56 versus 148 ± 25, absence and presence of anti–TGF-β Ab, respectively, p = 0.005, n = 5, Fig. 6B).
The expression of TLR7 in the conduction system of a fetus dying with CHB
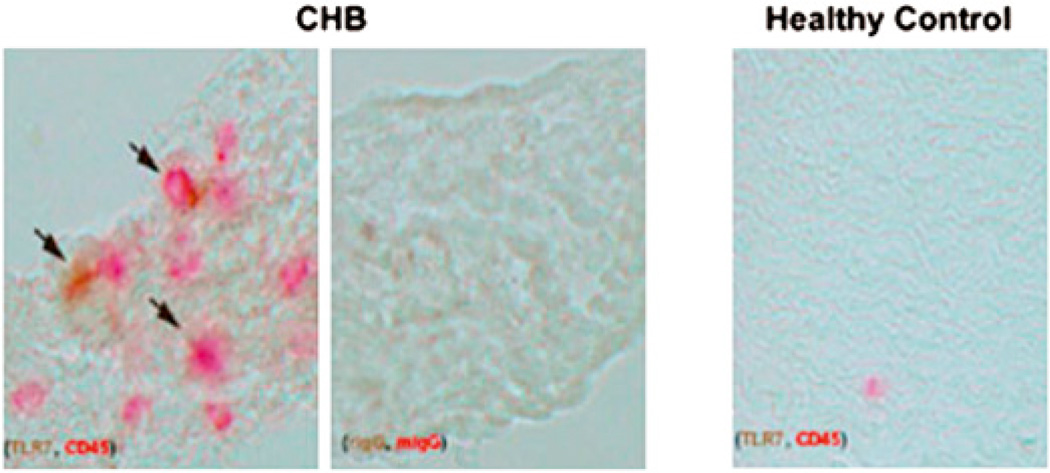
In vitro evidence supporting the potential participation of TLR in the pathogenesis of tissue injury in CHB was sought by evaluation of autopsy tissue from a fetus diagnosed with CHB at 19 wk and dying at 22 wk [previously described (6)]. Immunostain of slides from the affected heart revealed small clusters of CD45 and TLR7 double positive cells in areas of scar tissue (Fig. 7). Isotype controls stained appropriately, and CD45 positive cells were extremely rare in healthy fetal hearts.
FIGURE 7.
TLR7 infiltrating mononuclear cells in heart tissue obtained from a fetus dying with CHB. Sections from the septal region of a 22-wk fetus with CHB and an age-matched control electively terminated were stained with anti-TLR7, rabbit IgG (isotype control for anti-TLR7), anti-CD45, or mouse IgG (isotype control for anti-CD45) as primary Ab. Stains are visualized using anti-rabbit IgG alkaline phosphatase (brown) or anti-mouse IgG peroxidase (red; original magnification ×40).
Discussion
Pathogenic mechanisms linking anti–SSA/Ro-SSB/La Abs to cardiac injury in the developing fetus have been approached in several laboratories with most focusing attention on the protein target of the maternal immune response (20–22). Given recent clues from the evolving study of gene expression in SLE, the potential importance of the ssRNA associated with the Ag was addressed. The hypothesis driving the current study is that opsonization of apoptotic cardiocytes by maternal anti–SSA/Ro-SSB/La Abs induces macrophage activation via a TLR signaling pathway after uptake by an FcγR-dependent pathway with release of inflammatory mediators and profibrotic factors that set in motion the final step of irreversible scar (replacement of AV node and myocardium/endocardium by fibrosis). ICs composed of Ro60, Ro60-associated ssRNA, and IgG fractions containing anti-Ro60 reactivity were used in this study as a proxy for the opsonized apoptotic cardiocytes. Several in vitro lines of experimental evidence herein support the participation of TLR ligation and the dependence of ssRNA in this process. These data are consistent with the notion that unchecked TLR signaling is causally related to a substantial inflammatory response (23, 24), and advance the novel premise that TLR7 ligation may contribute to subsequent fibrosis. Precedent for this premise is the observation that TLR7 activation in the course of hepatitis-C viral infection is likened to a double-edged sword. Although activation to clear virus in the setting of acute infection is warranted, it may be ultimately responsible for liver scarring in the setting of chronic disease (25). In the case of CHB, autoantibody is inadvertently delivering the endogenous ssRNA (not viral) from an apoptotic cardiocyte during a period of vulnerability in fetal development.
The evaluation of TLR7 ligation was approached by exploiting the recently described oligonucleotide inhibitory immunoregulatory DNA sequence IRS661 identified by Barrat and coworkers (26). IRS661 specifically blocks TLR7 in a model of R848 stimulated dendritic cells (26). Pawar et al. have demonstrated that IRS661 dose dependently blocked the R848 induced production of TNF-α by splenic monocytes isolated from MRLlpr/lpr mice (27). In the current study, IRS661 reproducibly and significantly inhibited macrophage secretion of TNF-α induced by transfection with ssRNAs. Moreover, supernatants from TLR-dependent stimulation of the macrophages promoted a profibrosing phenotype of cocultured human fetal cardiac fibroblasts. The expression of TLR7 in the conduction system of a fetus dying with CHB is consistent with the speculation that TLR7 pathways contribute to fibrosis in this disease.
Given the precedent for the induction of TLR7-dependent secretion of inflammatory cytokines by ssRNA molecules of nonviral origin (28), the contribution of the RNA moiety in the anti-Ro60 complex to the pathogenesis of injury was considered highly relevant. Ro60 contains an α-helical HEAT repeat that forms a ring with a central hole that provides an extensive RNA binding surface to a large collection of possible RNAs, including Y RNAs, variant 5S rRNAs, and misfolded U2 small nuclear RNA (29). Although the current study focused solely on Ro60 as the proxy Ag, it is fully acknowledged that Abs to both Ro52 and La48 are associated with the development of CHB in an offspring (30). Although Ro52 is not reported to interact with RNA, its potential association with Ro60 may provide a means of introducing RNA in association with a molecular complex (31). La48 Ag binds to numerous RNA molecules such as newly synthesized RNA polymerase III products, and it has been shown to play a pivotal role in RNA polymerase III transcription and maturation of the transcripts during the cell cycle (32). Small hY-RNAs binding Ro60 are also associated with La48 at a distinct site (33). Albeit in a different experimental system, it is highly relevant that Vollmer et al. have reported that two synthetic oligoribonucleotides derived from the hY5 and hY3RNA, both containing G/U-rich sequences, stimulated IFN-α production from human PBMCs (34). In the current study, the dependence on U-rich sequences was supported by the absence of macrophage activation using an ssRNA whose sequences were identical to oligonucelotides hY3 but in which all the U nucleotides were replaced by A nucleotides.
In consideration of TLR activation as a pivotal lynch pin from Ab to fibrosis, the clinical association of CHB with Abs to SSA/Ro and SSB/La, but never to date with anti-Sm/RNP reactivities was the justification for focusing the current study solely on ssRNA associated with Ro60. However, it is fully acknowledged that there is strong in vitro data on the endogenous adjuvant activity of the RNA components of the Sm/RNP autoantigens (10, 34, 35). Thus, molecularly accounting for this clinical discrepancy is challenging. Although further work is needed to reconcile this consideration, several points may be applicable. Previous studies have demonstrated that IgG fractions isolated from patients with anti-RNP Abs absent anti–SSA/Ro-SSB/La do not bind the surface of either live or apoptotic cells (7, 36). In many of the published studies using noncardiocyte cell preparations, apoptotic cell debris or cells rendered necrotic with freeze/thawing were used as the source of RNP or Sm ribonucleoparticles (34, 37). Therefore, if the RNP protein moiety is not accessible on the apoptotic surface, the critical Ab link between the ssRNA and uptake into the macrophage would not be present eliminating Sm/RNP ssRNAs from consideration in this model of CHB. Therefore, the transplacental passage of maternal anti-RNP would not be predicted to chaperone the U series of small RNAs to the TLR. Finally, the focus in this CHB model on the macrophage and not the dendritic cells per se and TNF-α, not IFN-α, may be further contributory.
Based on our identification of TNF-α in previous coculturing experiments (17) and identification of mRNA for TNF-α (5) in autopsy tissue, this cytokine was chosen as the readout for macrophage activation. Justification as a potential proxy for fibrosis is provided by the observations that TNF-α stimulates type I collagen, induces tissue inhibitor of metalloproteinase-1 expression and reduces matrix metalloproteinase-2 activity and collagen degradation in intestinal myofibroblasts (38) but does not increase SMAc (18). A role for TGF-β as a potential mediator of collagen secretion by the stimulated macrophage supernatants was suggested by the Ab neutralizing experiments. However, it remains unclear whether this cytokine is generated as a direct consequence of TLR ligation (because TGF-β was not detectable by commercial ELISA) or indirectly secreted by the fibroblasts. Therefore, it is fully acknowledged that the specific macrophage cytokine (or combination of cytokines) responsible for promoting the fibrotic replacement in the fetal heart has not as yet been identified.
All four TLRs that bind nucleic acid substrates are intracellular and contained in the endoplasmic reticulum or endosomal/lysosomal compartments (39), which raises the speculation that this location might be an attempt to thwart reactivity against self. Experimental data in our proposed model suggests that the ssRNA in the anti-Ro60 ICs co-ops an FcγR-dependent pathway to gain access to the TLR. Although it is appreciated that blockade of FcγR does not necessarily imply TLR involvement, the experimental design demonstrated causality. The association between readouts obtained with blockade of TLR and FcγR provides a reasonable assumption that they work cooperatively. Precedent for these findings are the reports demonstrating that FcγRII promotes the interaction between DNA and TLR9 in patients with anti-dsDNA Abs (40) and anti–RNP-Ab complexes and TLR7/8 in plasmacytoid dendritic cells (34). The current focus on FcγRIIIa was based on the observation that Abs to Ro60 are skewed toward IgG1 and IgG3 subclasses rather than IgG2 (41), the former subclasses being strongly linked to macrophage signaling by FcγRIIIa.
Because endosomal TLR binds ligand at low pH, pharmacologic approaches to attenuate TLR-dependent readouts have included chloroquine and baflomyocin, which interfere with acidification (35, 37). From a clinical perspective, it is notable that hydroxychloroquine is often recommended for continued use in pregnancies of mothers with SLE and Sjogren’s Syndrome (42, 43), which might have encouraging implications with regard to CHB-prevention. Consideration of this class of medications in the setting of anti-SSA/Ro Ab exposed pregnancies is timely given reports that hydroxychloroquine use may forestall the development of SLE or favorably affect survival in patients with established disease (44, 45). A retrospective review of several large databases suggests that hydroxychloroquine use during pregnancy may decrease the risk of CHB (46).
In summary, the data support an injury model whereby anti-SSA/Ro/SSB/La Abs may promote a “binary” insult in generating organ disease in one step because they bind to a complex containing an endogenous ligand (ssRNA) capable of ligating TLR. The consideration that TLR engagement may promote fibrosis extends the TLR “paradigm” from the afferent loop of autoimmune induction to an efferent loop in which downstream effectors eventuate in tissue damage. Specificity of the SSA/Ro-SSB/La associated ssRNA may relate to surface accessibility of the protein target. These observations provide further justification for consideration of preventative therapies aimed at antagonism of TLR signaling not only in SLE, but to forestall the rapid scarring observed in the passively acquired model of cardiac injury in neonatal lupus.
Acknowledgments
Normal heart tissue samples were obtained through Dr. Brad Poulos at the Human Fetal Tissue Repository of Albert Einstein College of Medicine, Bronx, NY. We thank the New York University genomic facility.
This work was supported by National Institutes of Health Grants AR-42455 and N01-AR-4-2271 and a grant from the Mary Kirkland Center for Lupus Research.
Abbreviations used in this paper
- AV
atrioventricular
- CHB
congenital heart block
- IC
immune complex
- m-pre5S
misfolded pre-5S
- nIgG
normal IgG
- SMAc
smooth muscle actin
- SLE
systemic lupus erythematosus
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Brucato A, Frassi M, Franceschini F, Cimaz R, Faden D, Pisoni MP, Muscarà M, Vignati G, Stramba-Badiale M, Catelli L, et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum. 2001;44:1832–1835. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 2.Buyon JP, Clancy RM. Neonatal lupus. In: Wallace DJ, Hahn BH, editors. Dubois’ Lupus Erythematosus. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 1058–1080. [Google Scholar]
- 3.Buyon JP, Clancy RM. From antibody insult to fibrosis in neonatal lupus - the heart of the matter. Arthritis Res. Ther. 2003;5:266–270. doi: 10.1186/ar763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nield LE, Silverman ED, Smallhorn JF, Taylor GP, Mullen JB, Benson LN, Hornberger LK. Endocardial fibroelastosis associated with maternal anti-Ro and anti-La antibodies in the absence of atrioventricular block. J. Am. Coll. Cardiol. 2002;40:796–802. doi: 10.1016/s0735-1097(02)02004-1. [DOI] [PubMed] [Google Scholar]
- 5.Clancy RM, Backer CB, Yin X, Kapur RP, Molad Y, Buyon JP. Cytokine polymorphisms and histologic expression in autopsy studies: contribution of TNF-alpha and TGF-beta 1 to the pathogenesis of autoimmune-associated congenital heart block. J. Immunol. 2003;171:3253–3261. doi: 10.4049/jimmunol.171.6.3253. [DOI] [PubMed] [Google Scholar]
- 6.Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–182. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 7.Clancy RM, Neufing PJ, Zheng P, O’Mahony M, Nimmerjahn F, Gordon TP, Buyon JP. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J. Clin. Invest. 2006;116:2413–2422. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahman AH, Eisenberg RA. The role of toll-like receptors in systemic lupus erythematosus. Springer Semin. Immunopathol. 2006;28:131–143. doi: 10.1007/s00281-006-0034-3. [DOI] [PubMed] [Google Scholar]
- 9.Kirou KA, Lee C, George S, Louca K, Papagiannis IG, Peterson MG, Ly N, Woodward RN, Fry KE, Lau AY, et al. Coordinate over-expression of interferon-alpha-induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50:3958–3967. doi: 10.1002/art.20798. [DOI] [PubMed] [Google Scholar]
- 10.Lövgren T, Eloranta ML, Kastner B, Wahren-Herlenius M, Alm GV, Rönnblom L. Induction of interferon-alpha by immune complexes or liposomes containing systemic lupus erythematosus autoantigen- and Sjögren’s syndrome autoantigen-associated RNA. Arthritis Rheum. 2006;54:1917–1927. doi: 10.1002/art.21893. [DOI] [PubMed] [Google Scholar]
- 11.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J. Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien CA, Wolin SL. A possible role for the 60-kD Ro autoantigen in a discard pathway for defective 5S rRNA precursors. Genes Dev. 1994;8:2891–2903. doi: 10.1101/gad.8.23.2891. [DOI] [PubMed] [Google Scholar]
- 15.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 16.Nagase H, Okugawa S, Ota Y, Yamaguchi M, Tomizawa H, Matsushima K, Ohta K, Yamamoto K, Hirai K. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J. Immunol. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 17.Miranda-Carús ME, Askanase AD, Clancy RM, Di Donato F, Chou TM, Libera MR, Chan EK, Buyon JP. Anti-SSA/Ro and anti-SSB/La autoantibodies bind the surface of apoptotic fetal cardiocytes and promote secretion of TNF-alpha by macrophages. J. Immunol. 2000;165:5345–5351. doi: 10.4049/jimmunol.165.9.5345. [DOI] [PubMed] [Google Scholar]
- 18.Clancy RM, Askanase AD, Kapur RP, Chiopelas E, Azar N, Miranda-Carus ME, Buyon JP. Transdifferentiation of cardiac fibroblasts, a fetal factor in anti-SSA/Ro-SSB/La antibody-mediated congenital heart block. J. Immunol. 2002;169:2156–2163. doi: 10.4049/jimmunol.169.4.2156. [DOI] [PubMed] [Google Scholar]
- 19.Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 20.Salomonsson S, Dörner T, Theander E, Bremme K, Larsson P, Wahren-Herlenius M. A serologic marker for fetal risk of congenital heart block. Arthritis Rheum. 2002;46:1233–1241. doi: 10.1002/art.10232. [DOI] [PubMed] [Google Scholar]
- 21.Boutjdir M, Chen L, Zhang ZH, Tseng CE, El-Sherif N, Buyon JP. Serum and immunoglobulin G from the mother of a child with congenital heart block induce conduction abnormalities and inhibit L-type calcium channels in a rat heart model. Pediatr. Res. 1998;44:11–19. doi: 10.1203/00006450-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Miranda-Carús ME, Boutjdir M, Tseng CE, DiDonato F, Chan EK, Buyon JP. Induction of antibodies reactive with SSA/Ro-SSB/La and development of congenital heart block in a murine model. J. Immunol. 1998;161:5886–5892. [PubMed] [Google Scholar]
- 23.Andreakos E, Foxwell B, Feldmann M. Is targeting Toll-like receptors and their signaling pathway a useful therapeutic approach to modulating cytokine-driven inflammation? Immunol. Rev. 2004;202:250–265. doi: 10.1111/j.0105-2896.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 24.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol. Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 25.Schott E, Witt H, Neumann K, Taube S, Oh DY, Schreier E, Vierich S, Puhl G, Bergk A, Halangk J, et al. A Toll-like receptor 7 single nucleotide polymorphism protects from advanced inflammation and fibrosis in male patients with chronic HCV-infection. J. Hepatol. 2007;47:203–211. doi: 10.1016/j.jhep.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, Duramad O, Coffman RL. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawar RD, Ramanjaneyulu A, Kulkarni OP, Lech M, Segerer S, Anders HJ. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J. Am. Soc. Nephrol. 2007;18:1721–1731. doi: 10.1681/ASN.2006101162. [DOI] [PubMed] [Google Scholar]
- 28.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs G, Stein AJ, Fu C, Reinisch KM, Wolin SL. Structural and biochemical basis for misfolded RNA recognition by the Ro autoantigen. Nat. Struct. Mol. Biol. 2006;13:1002–1009. doi: 10.1038/nsmb1156. [DOI] [PubMed] [Google Scholar]
- 30.Buyon JP, Ben-Chetrit E, Karp S, Roubey RA, Pompeo L, Reeves WH, Tan EM, Winchester R. Acquired congenital heart block. Pattern of maternal antibody response to biochemically defined antigens of the SSA/Ro-SSB/La system in neonatal lupus. J. Clin. Invest. 1989;84:627–634. doi: 10.1172/JCI114208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurien BT, Chambers TL, Thomas PY, Frank MB, Scofield RH. Autoantibody to the leucine zipper region of 52 kDa Ro/SSA binds native kDa Ro/SSA: identification of a tertiary epitope with components from 60 kDa Ro/SSA and 52 kDa Ro/SSA. Scand. J. Immunol. 2001;53:268–276. doi: 10.1046/j.1365-3083.2001.00870.x. [DOI] [PubMed] [Google Scholar]
- 32.Rosa MD, Gottlieb E, Lerner MR, Steitz JA. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol. Cell. Biol. 1981;1:785–796. doi: 10.1128/mcb.1.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Venrooij WJ, Slobbe RL, Pruijn GJ. Structure and function of La and Ro RNPs. Mol. Biol. Rep. 1993;18:113–119. doi: 10.1007/BF00986765. [DOI] [PubMed] [Google Scholar]
- 34.Vollmer J, Tluk S, Schmitz C, Hamm S, Jurk M, Forsbach A, Akira S, Kelly KM, Reeves WH, Bauer S, Krieg AM. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J. Exp. Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly KM, Zhuang H, Nacionales DC, Scumpia PO, Lyons R, Akaogi J, Lee P, Williams B, Yamamoto M, Akira S, et al. “Endogenous adjuvant” activity of the RNA components of lupus autoantigens Sm/RNP and Ro 60. Arthritis Rheum. 2006;54:1557–1567. doi: 10.1002/art.21819. [DOI] [PubMed] [Google Scholar]
- 36.Reed JH, Neufing PJ, Jackson MW, Clancy RM, Macardle PJ, Buyon JP, Gordon TP. Different temporal expression of immunodominant Ro60/60 kDa-SSA and La/SSB apotopes. Clin. Exp. Immunol. 2007;148:153–160. doi: 10.1111/j.1365-2249.2007.03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lövgren T, Eloranta ML, Båve U, Alm GV, Rönnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 38.Theiss AL, Simmons JG, Jobin C, Lund PK. Tumor necrosis factor (TNF) alpha increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J. Biol. Chem. 2005;280:36099–36109. doi: 10.1074/jbc.M505291200. [DOI] [PubMed] [Google Scholar]
- 39.Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 40.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng CE, Caldwell K, Feit S, Chan EK, Buyon JP. Subclass distribution of maternal and neonatal anti-Ro(SSA) and La(SSB) antibodies in congenital heart block. J. Rheumatol. 1996;23:925–932. [PubMed] [Google Scholar]
- 42.Clowse ME, Magder L, Witter F, Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum. 2006;54:3640–3647. doi: 10.1002/art.22159. [DOI] [PubMed] [Google Scholar]
- 43.Costedoat-Chalumeau N, Amoura Z, Huong DL, Lechat P, Piette JC. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases. Review of the literature. Autoimmun. Rev. 2005;4:111–115. doi: 10.1016/j.autrev.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 44.James JA, Kim-Howard XR, Bruner BF, Jonsson MK, McClain MT, Arbuckle MR, Walker C, Dennis GJ, Merrill JT, Harley JB. Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus. 2007;16:401–409. doi: 10.1177/0961203307078579. [DOI] [PubMed] [Google Scholar]
- 45.Alarcón GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alén J, Bastian HM, Vilá LM, Reveille JD LUMINA Study Group. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L) Ann. Rheum. Dis. 2007;66:1168–1172. doi: 10.1136/ard.2006.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Izmirly P, Kim M, Le P, Lianos C, Katholi M, Clancy R, Salmon JE, Buyon JP. Decreased risk of anti-Ro/La associated congental heart block in fetuses exposed to hydroxychloroquine. Arthritis Rheum. 2008;58:S810. [Google Scholar]