Abstract
Objective
To identify clinical and pathologic factors that were associated with the survival of stage IB upper lobe non-small cell lung cancer (NSCLC) patients.
Methods
A retrospective study of 147 subjects who had undergone curative resection for stage IB upper lobe NSCLC was performed. Patients who had received any adjuvant or neo-adjuvant chemotherapy were excluded. Survival function curves were estimated using the Kaplan-Meier procedure. Crude and adjusted hazard ratios (HRs) of potential prognostic factors were estimated using Cox proportional hazards models.
Results
Five factors, including age, tumor size, histologic grade of differentiation, number of removed superior mediastinal lymph node stations and presence of visceral pleura invasion, were significantly and independently associated with mortality risk. Adjusted HRs were 2.6 [95% confidence interval (95% CI): 1.1-6.5] and 4.6 (95% CI: 1.9-11) for those aged 58−68 years and those >68 years, respectively, relative to those aged <58 years. HRs for those with poorly and moderately differentiated tumors were 6.4 (95% CI: 2.3-18) and 1.4 (95% CI: 0.7-2.8), respectively. HRs for those with tumor size 3.1−5 cm and >5 cm (vs≤3.0 cm) were 2.3 (95% CI: 1.1-4.9) and 4.3 (95% CI: 1.9-10), respectively. The presence of visceral pleura invasion also increased the risk of mortality (HR=4.0, 95% CI: 1.3-12).
Conclusion
Advanced age, larger tumor size, poorly differentiated histology, smaller number of removed superior mediastinal lymph node stations, and presence of visceral pleura invasion were associated with poor survival of surgically treated stage IB upper lobe NSCLC patients.
Key words: Non-small cell lung cancer, Stage IB, Prognosis, Lymphadenectomy
INTRODUCTION
Among all patients with non-small cell lung cancer (NSCLC), those with stage IB accounts for about 20%-30%. Currently, surgery is still the preferred treatment for stage IB NSCLC and is considered to be the only procedure that could potentially cure the condition. However, current long-term survival for patients of stage IB NSCLC is still not optimistic. Despite surgical resection,its 5-year survival rate is only 60%-70%, and nearly half of the patients have died from local recurrence or distant metastasis within 5 years[1, 2].
Lymph node metastatic is a main pathway for spreading lung cancer and is one of the most important factors affecting the prognosis. In general, lymph node metastasis of lung cancer follows the pathway of lymph drainage: as the metastasis sequence of pulmonary lymph nodes→ hilarlymph nodes→ mediastinal lymph nodes. Upper lobeNSCLC, compared to the middle or lower lobe NSCLC, tends to have a higher probability of occurring mediastinal lymph node metastasis, and its mediastinal lymph node metastasis is often constricted to regional lymph nodes in superior mediastinum (for right upper lobe NSCLC metastasis more likely to occur at station 4R and for left upper lobe metastasis more likely to occur at station 5)[3,4].
Therefore, it is critical to identify factors that carry an increased risk of mortality in patients with stage IB NSCLC. Based on lymph node metastasis characteristics of upper lobe NSCLC, we analyzed the data from 147 stage IB upper lobe NSCLC patients undergoing complete resection from May 2001 to December 2004 at the ShanghaiChestHospital retrospectively. We aimed to investigate the clinical and pathological factors related to the prognosis of stage IB upper lobe NSCLC patients.
Materials and Methods
Case Selection and Inclusion Criteria
One hundred and forty-sevenconsecutive stage IB upper lobe NSCLC patients undergoing complete resection from May, 2001 to December, 2004 at the Shanghai Chest Hospital were included in this study. The inclusion criteria were as follows: 1) participants were Shanghai local residents; 2) their primary lesions were in the left/right upper lobe; 3) all patients had been evaluated by physical examination, chest and brain computed tomographic (CT) scan, ultrasound of the abdomen, bone scan and fiberoptic bronchoscopy prior operation; 4) a complete resection surgery was performed according to the Internation Association for the Study of Lung Cancer (IASLC) 2005 criteria[5];and 5) the diagnoses were confirmed as non-small cell lung cancer by postoperative pathology as the pathological stage of T2N0M0 (stage IB). Deaths due to non-cancer causes and cases who had received any adjuvant or neo-adjuvant chemotherapy were excluded.
Follow-up and Measurements
All the 147 stage IB cases were followed up until December 30, 2009. The endpoint mortality data during the follow-up period were obtained from the ShanghaiCenter for Disease Control and Prevention. The postoperative survival time was calculated as the difference between the surgery date and the date of death. Those who survived beyond December 30, 2009 were censored on that day and their survival time was determined as the difference between December 30, 2009 and the surgery date.
For all enrolled subjects, we collected the following data: hospital admission number, date of surgery, age, gender, tumor size, tumor location, histological type, grade of differentiation, visceral pleural invasion, number of removed lymph nodes, number of removed mediastinal lymph nodes and number of removed superior mediastinal lymph node stations.
Statistical Analysis
Survival time curves were estimated using the Kaplan-Meier product method. We calculated the crude hazard ratios (HRs) of potential prognostic factors using the Cox proportional hazards model. To assess the independent predictive values of potential predictors, we further calculated adjusted HRs after adjusting for other variables using multivariate Cox proportional hazards models. A backward stepwise approach was used to determine the final model which included all significant variables. A P-value of less than 0.05 was considered significant. All statistical analyses were performed using SPSSversion 15.0 (SPSS Inc., Chicago, IL).
RESULTS
Characteristics of the Study Participants
A total of 147 eligible patients met the inclusion criteria. Their clinical and pathological characteristics are shown in Table 1. Among these eligible patients, 76 cases were in the group of stage IB right upper lobe NSCLC and 71 cases in the group of stage IB left upper lobe NSCLC. The differences between the two groups in age, gender, histological type, grade of differentiation, visceral pleural invasion, and tumor size did not reach a statistical significance. A total of 925 lymph nodes were removed during the surgery in all enrolled patients with an average of 6.29 lymph nodes, and a total of 491 mediastinal lymph nodes (266 and 225 for superior and inferior mediastinal lymph nodes respectively) were removed; the number of cases whose number of removed superior mediastinal lymph node stations was less than 2 amounted to 55, and the number of cases whose number of removed superior mediastinal lymph nodes was not less than 2 amounted to 92.The differences between the two groups in the numbers of removed lymph nodes, and removed mediastinal lymph nodes did not reach a statistical significance. However, the number of removed superior mediastinal lymph node stations was significantly higher in the right upper lobe group than in the left upper lobe group.
Table 1. Characteristics of 147 patients with stage IB upper lobe NSCLC.
| Number(%) | Right upper lobe |
Left upper lobe |
P | |
|---|---|---|---|---|
| Number(%) | Number(%) | |||
| Age, years | 0.30 | |||
| <58 | 51(34.7) | 30(39.5) | 21(29.6) | |
| 58−68 | 56(38.1) | 29(38.2) | 27(38.0) | |
| >68 | 40(27.2) | 17(22.4) | 23(32.4) | |
| Sex | 0.09 | |||
| Female | 64(43.5) | 28(36.8) | 36(50.7) | |
| Male | 83(56.5) | 48(63.2) | 35(49.3) | |
| Histological type | 0.77 | |||
| Adenocarcinoma | 96(65.3) | 50(65.8) | 46(64.8) | |
| Squamous | 37(25.2) | 20(26.3) | 17(23.9) | |
| Adenosquamous | 14(9.5) | 6(7.9) | 8(11.3) | |
| Grade of differentiation | 0.82 | |||
| Well | 74(50.3) | 41(53.9) | 33(46.5) | |
| Moderate | 53(36.1) | 26(34.2) | 27(38.0) | |
| Poor | 13(8.8) | 6(7.9) | 7(9.9) | |
| Unknown | 7(4.8) | 3(3.9) | 4(5.6) | |
| Visceral pleura invasion | 0.73 | |||
| Present | 129(87.8) | 66(86.8) | 63(88.7) | |
| Absent | 18(12.2) | 10(13.2) | 8(11.3) | |
| Tumor size, cm | 0.09 | |||
| ≤3.0 | 60(40.8) | 37(48.7) | 23(32.4) | |
| 3.1−5.0 | 63(42.9) | 30(39.5) | 33(46.5) | |
| >5.0 | 24(16.3) | 9(11.8) | 15(21.1) | |
| Number of removed LN | 0.22 | |||
| <7 | 112(76.2) | 52(68.4) | 60(84.5) | |
| ≥7 | 35(23.8) | 24(31.6) | 11(15.5) | |
| Number of removed MLN | 0.06 | |||
| <4 | 106(72.1) | 47(61.8) | 59(83.1) | |
| ≥4 | 41(27.9) | 29(38.2) | 12(16.9) | |
| Number of removed SMLNS | 0.004 | |||
| <2 | 55(37.4) | 20(26.3) | 35(49.3) | |
| ≥2 | 92(62.6) | 56(73.7) | 36(50.7) |
LN=lymph nodes; MLN=mediastinal lymph nodes; SMLNS=superior mediastinal lymph node stations
Overall survival status
During a median follow-up period of 6.2 years, among 147 patients with stage IB upper lobe NSCLC, 42 patients of them died. The 3-year and 5-year survival rates were 79% and 72%, respectively. Although the group of stage IB right upper lobe NSCLC patients had slightly better survival than the left upper lobe group, the difference between two groups did not reach statistical significance, P=0.25 (Figure 1).
Figure 1.
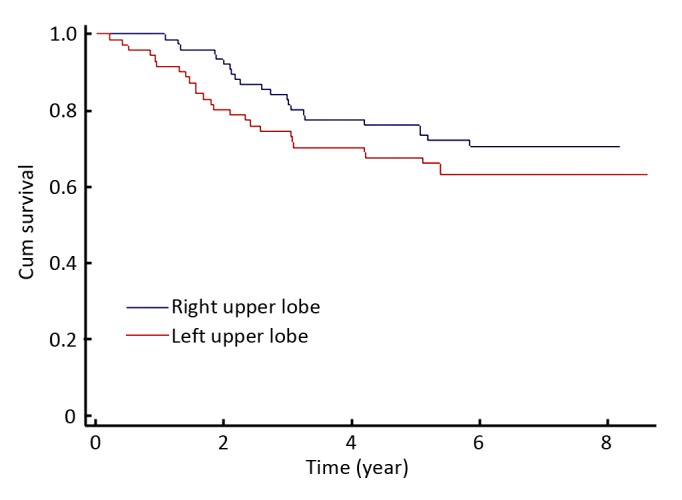
Kaplan-Meier survival probabilities according to pulmonary location. right upper lobe group vs left upper lobegroup.
Crude HRs
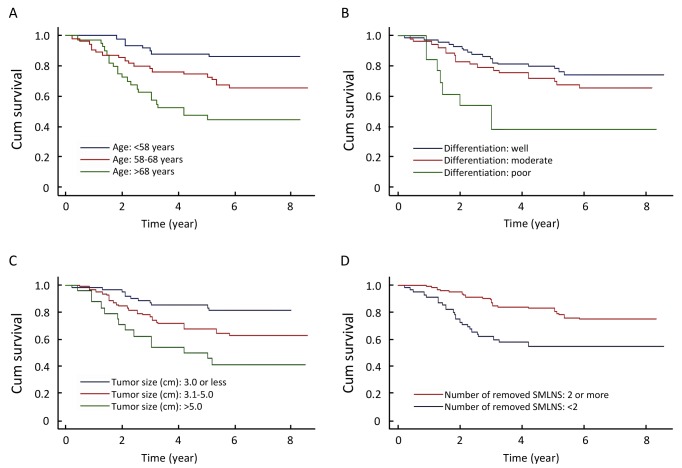
As shown in Figure 2, we found that four variables were significantly associated with the survival of the patients. Those variables were age, tumor size, histologic differentiation grade, and number of removed superior mediastinal lymph node stations. As shown in Table 2, the crude HRs were 2.8 and 5.4 for patients aged 57-68 and >68 years, respectively, compared with those aged <57 years. Tumor size was positively and significantly associated with mortality. HRs were 2.3 and 4.3 for those with tumor size of 3.1 to 5 cm and >5 cm, respectively, relative to the patients with tumors <3.0 cm. The prognosis of the group with a larger number of removed superior mediastinal lymph node stations (≥2) was better than that of the group with the number <2. The mortality risk for the former group was reduced by 60% compared to the latter group (HR=0.4). Patients who had poorly differentiated tumors had an increased mortality risk than those with well differentiated histological grade. The mortality risk also varied with the levels of other variables but those differences did not reach statistical significance (Table 2).
Figure 2.
Kaplan-Meier cumulative survival. A: According to age. B: According to histologic grade of differentiation. C: According to tumor size. D: According to the number of removed superior mediastinal lymph node stations (SMLNS).
Table 2. Crude HRs [95% confidence intervals (95% CI)].
| HR | 95%CI |
P | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age, years | <0.001 | |||
| <58 | 1 | |||
| 58−68 | 2.8 | 1.2 | 6.7 | |
| >68 | 5.4 | 2.3 | 13 | |
| Sex | 0.27 | |||
| Female | 1 | |||
| Male | 1.4 | 0.8 | 2.5 | |
| Tumor location | 0.25 | |||
| Right upper lobe | 1 | |||
| Left upper lobe | 1.4 | 0.8 | 2.5 | |
| Histological type | 0.13 | |||
| Adenocarcinoma | 1 | |||
| Squamous | 1.7 | 0.9 | 3.2 | |
| Adenosquamous | 2.0 | 0.8 | 5.0 | |
| Grade of differentiation | 0.02 | |||
| Well | 1 | |||
| Moderate | 1.4 | 0.7 | 2.7 | |
| Poor | 3.7 | 1.6 | 8.5 | |
| Visceral pleura invasion | 0.64 | |||
| Absent | 1 | |||
| Present | 1.3 | 0.5 | 3.2 | |
| Tumor size, cm | 0.002 | |||
| ≤3 | 1 | |||
| 3.1−5 | 2.3 | 1.1 | 4.6 | |
| >5 | 4.3 | 2.0 | 9.6 | |
| Number of removed LN | 0.16 | |||
| <7 | 1 | |||
| ≥7 | 0.6 | 0.3 | 1.2 | |
| Number of removed MLN | 0.20 | |||
| <4 | 1 | |||
| ≥4 | 0.6 | 0.3 | 1.3 | |
| Number of removed SMLNS | 0.004 | |||
| <2 | 1 | |||
| ≥2 | 0.4 | 0.3 | 0.8 | |
LN=lymph nodes; MLN=mediastinal lymph nodes; SMLNS=superior mediastinal lymph node stations
Multivariate Survival Analysis: Adjusted HRs
After adjustment for all factors included in this study using the multivariate Cox proportional hazards model, the associations of age, tumor size, pathologic grade of differentiation, number of removed superior mediastinal lymph node stations and the presence of visceral pleura invasion were independently and significantly associated with mortality risk. The final model including five factors which reached statistical significance were shown in Table 3. The four factors identified in the previous crude HR estimates remained significant after adjusting for other variables. In addition, in the context of controlling for other four variables,the presence of visceral pleura invasion became a significant predictor in multivariate analysis. Older age groups experienced substantially increased mortality risk. Adjusted HRs were 2.6 (95% CI: 1.1-6.5) and 4.6 (95% CI: 1.9-11) for those aged <58 years and 58 to 68 years, respectively. The patients with tumor size >5 cm had a 4.3 fold mortality risk (HR=4.3, 95% CI: 1.9-10) as those with tumor size ≤3.0 cm. Those with the number of removed superior mediastinal lymph node stations ≥2 only had 40% mortality risk as those with the number <2. Patients with a poorly differentiated histologic grade had a 6.4 fold risk of mortality as those with a well differentiated grade (HR=6.4, 95% CI: 2.3-18).
Table 3. Adjusted HRs and 95% CI with all variables in the Cox proportional hazards model.
| HR | 95%CI |
P | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age, years | <0.001 | |||
| <58 | 1 | |||
| 58−68 | 2.6 | 1.1 | 6.5 | |
| >68 | 4.6 | 1.9 | 11 | |
| Tumor size, cm | 0.002 | |||
| ≤3 | 1 | |||
| 3.1−5 | 2.3 | 1.1 | 4.9 | |
| >5 | 4.3 | 1.9 | 10 | |
| Number of removed SMLNS | 0.005 | |||
| <2 | 1 | |||
| ≥2 | 0.4 | 0.2 | 0.8 | |
| Grade of differentiation | 0.008 | |||
| Well | 1 | |||
| Moderate | 1.4 | 0.7 | 2.8 | |
| Poor | 6.4 | 2.3 | 18 | |
| Visceral pleura invasion | 0.008 | |||
| Absent | 1 | |||
| Present | 4.0 | 1.3 | 12 | |
SMLNS=superior mediastinal lymph node stations
DISCUSSION
In this study, we found that older age, larger tumor size, poorly differentiated pathology, smaller number of removed superior mediastinal lymph node stations and the presence of visceral pleura invasion carried an increased risk of mortality in patients with stage IB upper lobe NSCLC.
Currently, surgery is still the main treatment for stage IB NSCLC with pneumonectomy plus lymph node dissection. Although compared to middle or lower lobe NSCLC, the upper lobe NSCLC has a higher probability of occurring mediastinal lymph node metastasis, its mediastinal lymph node metastasis was often constricted to regional lymph nodes in superior mediastinum[3,6]. Our findings are critically important for clinicians to use routine clinical and pathologic characteristics to identify patients who are at an increased risk of mortality, so that such patients may be considered for and benefit from adjuvant treatments.
This study demonstrated that those patients older than 68 years had over 5 fold risk of mortality as those younger than 58 years. It has been controversial if age affects the treatment and prognosis of lung cancer, particularly for the early lung cancer. Mery, et al.[7] revealed that age was an important prognostic factor for survival of NSCLC patients of stage I-II after controlling for such factors as gender, histological type, clinical stage and type of surgery.Agarwal, et al.[8] also confirmed that the mortality rate increased sharply with age (one year increase of age brought nearly 6% increase in HR) in patients of stage I-II NSCLC. However, some researchers reported that age was not an important prognostic factor for the survival of early stage lung cancer[9], especially for elderly patients. In addition to facing the threat of cancer-related factors, elderly patients face impacts of complications, organ impairment and other non-cancer related factors. A study in Netherlands on the complication prevalence found that for cancer patients of over 65 years, their prevalence of one serious complication was 1.4 times of that of those younger than 64 years. The most common complication was cardiovascular disease. Mortality among those of over 70 years was much higher than that of among younger patients after the surgery of non-small cell lung cancer (11% vs. 2%, P<0.01); and cardiovascular and thrombotic events were also more common in older lung cancer patients[10].
In our study, tumor size was found to be a major factor associated with mortality risk. This is consistent with the findings in the literature. Okada,et al.[11] found that tumor size was an independent factor affecting prognosis in patients receiving complete resection of NSCLC. Christian, et al.[12] reported that 2 cm and 5 cmwere the two risk thresholds of tumor size and once tumor size reached the thresholds, risk of death would increase 58% and 118% respectively. In the 7th edition TNM staging of lung cancer published on World Lung Conference in 2009, T2 was subdivided into T2a (3cm<diameter ≤5cm) and T2b (5cm<diameter ≤7cm), and diameter >7cm in the original T2 was classified as T3, while T2bN0M0 and T3N0M0 were listed as IIA, IIB respectively[13]. Some researchers believed that the tumor size could not be simply regarded as an independent prognostic indicator of NSCLC but should be considered jointly with the pathological type. Lin, et al. [14], viewed that the threshold for early NSCLC should be set as 2.5cm for adenocarcinoma and the threshold could be relaxed to 4cm for squamous cell carcinoma.
It is still debatable whether systemic lymph node dissection should be performed on patients with stage IB NSCLC. Wu, et al.[15] reported in a prospective study that for NSCLC of stage I, systematic lymph node dissection could remove the tumor cells more thoroughly and reduce the postoperative risk of recurrence and distant metastasis. Izbicki, et al.[16] reported that systemic lymph node dissection did not improve the survival rate in patients of state N0. In a retrospective study, Ishiguro, et al.[17] reported that, there was no statistical significance in the 5-year survival rate between the way of intraoperative lobe-specific lymph node dissection and systematic lymph node dissection in patients with stage I NSLCL. Our study showed that patients with a higher number of removed lymph nodes and a higher number of removed mediastinal lymph nodes had a lower mortality risk but were not statistically significant. A higher number of removed superior mediastinal lymph node stations (≥2) was significantly associated with a 60% reduction in mortality risk. This result might be related to that upper lobe NSCLC tends to develop superior mediastinal lymph node metastasis and non-regional lymph node metastasis was rarely observed. Therefore, for early upper lobe NSCLC, the lobe-specific mediastinal lymph node dissection may be a more efficient way of lymph node dissection. It has been reported previously that poorly differentiated histologic grade is an independent prognostic factor for poor survival in patients with stage IB NSCLC[18]. In our study, those with poorly differentiated tumors had an over 6 fold mortality risk as those with well differentiated tumors. We also demonstrated that the presence of visceral pleura invasion was independently associated with mortality risk. Such an association was only observed in multivariate analysis, suggesting the presence of visceral pleura invasion is a useful prognostic factor in combination with other factors.
In conclusion, we identified that older age, larger tumor size, poorly differentiated histology, lower number of removed superior mediastinal lymph node stations, and the presence of visceral pleura invasion in patients with stage IB upper lobe NSCLC were associated with an increased mortality risk. Those five prognostic factors are useful for identifying high-risk patients who may benefit from adjuvant treatments.
REFERENCES
- 1.Pfannschmidt J, Muley T, Bülzebruck H, et al. Prognostic assessment after surgical resection for non-small cell lung cancer: Experiences in 2083 patients. Lung Cancer 2007;55:371-7 [DOI] [PubMed] [Google Scholar]
- 2.Goya T, Asamura H, Yoshimura H, et al. Prognosis of 6644 resected non-small cell lung cancers in Japan: A Japanese lung cancer registry study. Lung Cancer 2005;50:227-34 [DOI] [PubMed] [Google Scholar]
- 3.Kim AW. Lymph node drainage patterns and micrometastasis in lung cancer. Semin Thorac Cardiovasc Surg 2009;21:298-308 [DOI] [PubMed] [Google Scholar]
- 4.Kotoulas CS, Foroulis CN, Kostikas K, et al. Involvement of lymphatic metastatic spread in non-small cell lung cancer accordingly to the primary cancer location. Lung Cancer 2004;44:183-91 [DOI] [PubMed] [Google Scholar]
- 5.Rami-Porta R, Wittekind C, Goldstraw P.Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33 [DOI] [PubMed] [Google Scholar]
- 6.Kawano R, Hata E, Ikeda S, et al. Lobe-specific skip nodal metastasis in non-small cell lung cancer patients. Ann Thorac Cardiovasc Surg 2008;14:9-14 [PubMed] [Google Scholar]
- 7.Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest 2005;128:237-45 [DOI] [PubMed] [Google Scholar]
- 8.Agarwal M, Brahmanday G, Chmielewski GW, et al. Age, tumor size, type of surgery, and gender predict survival in early stage (stage I and II) non-small cell lung cancer after surgical resection. Lung Cancer 2010;68:398-402 [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto K, Padilla Alarcón J, Calvo Medina V, et al. Surgical results of stage I non-small cell lung cancer: comparison between elderly and younger patients. Eur J Cardiothorac Surg 2003;23:21-5 [DOI] [PubMed] [Google Scholar]
- 10.Janssen-Heijnen ML, Houterman S, Lemmens VE, et al. Prognostic impact of increasing age and co-morbidity in cancer patients: A population-based approach. Crit Rev Oncol Hematol 2005;55:231-40 [DOI] [PubMed] [Google Scholar]
- 11.Okada M, Nishio W, Sakamoto T, et al. Evaluation of surgical outcomes for non small cell lung cancer: time trends in 1465 consecutive patients undergoing complete resection. Ann ThoracSurg 2004;77:1926-30 [DOI] [PubMed] [Google Scholar]
- 12.Christian C, Erica S,MorandiU. The prognostic impact of tumor size in resected stage I non-small cell lung cancer: Evidence for a two thresholds tumor diameters classification. Lung Cancer 2006;54:185-91 [DOI] [PubMed] [Google Scholar]
- 13.Milroy R.Staging of Lung Cancer. Chest 2008;133:593-95 [DOI] [PubMed] [Google Scholar]
- 14.Lin PY, Chang YC, Chen HY, et al. Tumor size matters differently in pulmonary adenocarcinoma and squamous cell carcinoma. Lung Cancer 2010;67:296-300 [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6 [DOI] [PubMed] [Google Scholar]
- 16.Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small lung cancer. Ann Surg 1998;227:138-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishiguro F, Matsuo K, Fukui T, et al. Effect of selective lymph node dissection based on patters of lobe-specific lymph node metastases on patient outcome in patients with resectable non-small cell lung cancer: A large retrospective cohort study applying a propensity. J ThoracCardiovascSurg 2010;139:1001-6 [DOI] [PubMed] [Google Scholar]
- 18.Ou SH, Zell JA, Ziogas A, et al. Prognostic factors for survival of stage I non-small cell lung cancer patients: a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer 2007;110:1532-41 [DOI] [PubMed] [Google Scholar]