Abstract
Following the classification of hepatocellular nodules by the International Working Party in 1995 and further elaboration by the International Consensus Group for Hepatocellular Neoplasia in 2009, entities under the spectrum of hepatocellular nodules have been better characterized. Research work hence has been done to answer questions such as distinguishing high-grade dysplastic nodules from early hepatocellular carcinoma (HCC), delineating the tumor cell origin of HCC, identifying its prognostic markers, and subtyping hepatocellular adenomas. As a result, a copious amount of data at immunohistochemical and molecular levels has emerged. A panel of immunohistochemical markers including glypican-3, heat shock protein 70 and glutamine synthetase has been found to be of use in the diagnosis of small, well differentiated hepatocellular tumors and particularly of HCC. The use of liver fatty acid binding protein (L-FABP), β-catenin, glutamine synthetase, serum amyloid protein and C-reactive protein is found to be helpful in the subtyping of hepatocellular adenomas. The role of tissue biomarkers for prognostication in HCC and the use of biomarkers in subclassifying HCC based on tumor cell origin are also discussed.
Key words: Hepatocellular tumors, Immunohistochemical, Classification
INTRODUCTION
In 1995, the International Working Party proposed a classification of hepatocellular nodules[1]. Such classification eases communication, as well as facilitates better characterization of each entity under the umbrella of hepatocellular nodular lesions. Under this scheme, hepatocellular nodules were divided into regenerative lesions including focal nodular hyperplasia (FNH), and dysplastic or neoplastic lesions, which comprise hepato- cellular adenoma (HCA), dysplastic focus, dysplastic nodule (DN) of low or high grade, and hepatocellular carcinoma (HCC). For lesions showing dysplasia, dysplastic foci by definition measure less than 1 mm; while anything larger than 1 mm belong to DNs[2]. Given this classification, there are major diagnostic issues including differentiating benign nodular lesions (HCA, FNH) from malignant ones; and differentiating DNs, especially high-grade DNs (HGDNs), from early well- differentiated HCC[3].
In 2009, the International Consensus Group for Hepatocellular Neoplasia provided additional pathological criteria to distinguish HGDN from early HCC[4]. Low-grade DNs (LGDNs) are vaguely nodular lesions with peripheral fibrous scar.There is a mild increase in cellularity, yet no cytological atypia, pseudoglands, or thickened trabeculae areobserved[4]. HGDNs show architectural and/or cytological atypia featuring increased cell density. Small cell change is the most frequent form of cytological atypia. Nodule-in- nodule appearance is occasionally seen[4].
Early HCC, small well-differentiated HCC of vaguely nodular type, shows increased cell density (>2 times than that of surrounding tissue), increased N/C ratio and irregular thin-trabecular pattern. The nodules consist of varying numbers of portal tracts and unpaired arteries. Pseudoglandular pattern and diffuse fatty change are also histological features. One distinguishing feature of HGDN from HCC is the presence of tumor cell invasion into the intratumoral portal tracts in HCC[4].
Given such detailed histological criteria, distinction of dysplastic from malignant lesions is still sometimes difficult. With the advances in immunohistochemical markers and molecular techniques, this diagnostic problem can be better addressed and attended. Besides, the immunohistochemical and molecular characteristics of hepatocellular nodules have been more explicitly explored. In this review, a brief summary of some recent works of these markers will be illustrated.
HCAs
HCA and FNH are benign hepatic nodules. Diagnosis of these nodules has all along been based on morphological features, which may not always be straightforward. Diagnostic problems include differenti- ating HCA and FNH (the latter is the most common benign hepatic nodule and carries a lowerrisk of tumor rupture resulting in hemoperitoneum), as well as differentiating these lesions from HCC.
Besides, various histological features of HCA have aroused researchers’ interest to explore further on this benign hepatocellular neoplasm. In recent years, a genotype classification on HCA has been proposed[5-9].
According to Bioulac-Sage, et al., such classification of HCA is based on the reasons[8] to: 1) dissect the pathogenesis of HCA, 2) aid diagnosis by radiological means, 3) stratify the risk of progression to HCC, and 4) facilitate genetic screening in familial cases.
The classification of HCA based on genotype consists mainly of three groups: 1) HCA with HNF-1α inactivating gene mutation (H-HCA), 2) HCA with mutation of the β-catenin gene (b-HCA), and 3) inflammatory HCA (I-HCA).
Each group of HCA is characterized by the expression of specific genes of interest by quantitative reverse transcription polymerase chain reaction (qRT-PCR) method[9]. FABP1 and UGT2B7, encoding liver fatty acid binding protein (L-FABP) and regulated by HNF-1α, are expressed in normal liver tissues. A low expression of these genes is found in H-HCA cases as compared with other non-mutated subtypes. The expression of the transcripts of GLUL (encoding glutamine synthetase [GS]) and GPR49, two genes regulated by β-catenin, correlates with β-catenin mutation in the b-HCA subgroup. Up-regulation of SAA2 (encoding serum amyloid A2) and CRP (encoding C-reactive protein) characterizes the I-HCA. Besides, the transcript expression levels by qRT-PCR of the specific genes were found to correlate with the protein expression levels. Immunohistochemical stains thus are useful in classifying HCA based on the immunoprofile[9]. In summary, H-HCA is characterized by a lack of L-FABP staining; b-HCA shows GS (specificity 89%; sensitivity 100%) and β-catenin staining (specificity 100%; sensitivity 85%); while I-HCA expresses CRP and SAA (specificity 94%; sensitivity 94%), with or without β-catenin[5, 9]. Given the above, 5%-10% of HCAs are still unclassifiable[5].
With reference to the above classification, the clinicopathological features of each group are summarized in Table 1.
Table 1. Immunohistochemical and histological characteristics of three types of HCA.
| H-HCA | b-HCA | I-HCA | |
|---|---|---|---|
| Immunohistochemical | L-FABP- | β-catenin+ | CRP+ |
| characteristics | β-catenin- | GS+ | SAA+ |
| β-catenin+/- | |||
| Histological characteristics | Marked steatosis; | Occasional cytological | Inflammatory infiltrates; |
| No cytological abnormalities; | Abnormality; | Sinusoidal congestion and dilatation; | |
| No inflammation | Rosette formation | Thick-walled arteries; Ductular reaction |
L-FABP, liver fatty acid binding protein; CRP, C-reactive protein; GS, glutamine synthetase; SAA, serum amyloid A.
H-HCA constitutes about 35%-40% of HCA[5]. The mean age of presentation is 39 years and oral contraceptive intake was noted in 92% of cases[7]. It involves bi-allelic inactivating mutations of the HNF-1α gene. Histologically, H-HCA shows marked steatosis, no inflammatory infiltrates and no cytological abnormalities. Immunohistochemically, as mentioned above, there is lack of L-FABP expression among tumor cells in contrast with adjacent liver tissue[5].
b-HCA constitutes around 10%-15%[5]. Oral contraceptive intake is noted in 100% of the cases. Morphologically, occasional cytological abnormalities and rosette formation are observed[7]. Immunohisto- chemically, aberrant nuclear and cytoplasmic expression is characteristic. Besides, GS, encoded by GLUL, is also expressed in this group of HCA[5]. Recognition of b-HCA is important as it is associated with a higher risk of HCC[10].
I-HCA accounts for more than 50% of HCA[5]. The mean age of presentation is 41 years, and oral contraceptive intake was observed in 90% cases[7]. Clinically, association with high body mass index and alcohol consumption in patients is observed. Signs of inflammatory syndromes, e.g. raised CRP levels, are noted. Histologically, features of I-HCA include inflammatory infiltrates, sinusoidal dilatation or congestion, presence of thick-wall arteries (some being dystrophic), and ductular reaction. Steatosis may be present but not as extensive[5,7]. In addition, there is increased expression of SAA and CRP at mRNA and protein levels, and can be detected by immunohisto- chemical methods[5].
FNH
FNH is not subjected to classification at this stage. However, there has been increasing knowledge on the molecular and immunohistochemical characteristics, which aid diagnosis of this entity. Recent developments in understanding the molecular characteristics of FNH have shown polyclonality, with an increase of ANGPT1/ ANGPT2 mRNA ratio, and features of activation of β-catenin pathway without β-catenin mutation[11]. Immunohistochemical study shows overexpression of GS in a map-like heterogeneous pattern[7,9]. Staining is often surrounding hepatic veins[8,9]. No staining was observed for SAA or β-catenin (nuclear or cytoplasmic) in a typical FNH case and L-FABP staining is preserved[9] (Table 2).
Table 2. Comparison of immunohistochemical characteristics between FNH and HCA.
| FNH | HCA | |
|---|---|---|
| GS | Map-like heterogeneous pattern | Homogenous pattern (b-HCA) |
| β-Catenin | Negative | Positive (b-HCA) |
| L-FABP | Preserved | Negative (H-HCA) |
| SAA | Negative | Positive (I-HCA) |
GS, glutamine synthetases; L-FABP, liver fatty acid binding protein; SAA, serum amyloid A.
HCC-New DiagnosticImmunohistochemical Markers
Apart from morphology, efforts have been made to diagnose early HCC from its possible precursors using molecular and immunohistochemical methods. Markers like Hep-Par, pCEA or CD10 stain up the hepatocytes in benign, dysplastic and malignant conditions; hence they are not very contributory in solving diagnostic problems. While AFP has a low sensitivity of about 50%[12], the identification of Glypican-3 (GPC-3) as a marker for HCC has become an important breakthrough.
GPC-3
GPC-3 is a member of the heparan sulfate proteoglycan family. It is expressed in the embryo and silenced in adult tissues[13,14]. It is linked to the outer surface of the cell membrane through aglycosylphos- phatidylinositol anchor[15]. GPC-3 is involved in cell growth, differentiation and migration[16]. Overexpression of GPC-3 modulates cell proliferation by inhibiting fibroblast growth factor 2 and bone morphogenetic protein 7 signaling via the Smadpathway[17]. The marker first came to attention based on the fact that the mRNA expression of GPC-3 in HCC tissue among serum AFP- negative patients could be as high as 90%[18,19]. GPC-3 mRNA was found to be expressed in HCC tissues, in contrast to cirrhotic and non-cirrhotic adult liver tissues[20]. Increased protein expression at immunohisto- chemical level was later confirmed[20].
A number of studies has been performed to investigate the staining pattern, sensitivity and specificity of this antibody to HCC. For resection specimens, the sensitivity ranges from 75.7% to 94.8% in the cohorts and specificity from 96% to 97%[20-25]. In studies using tissue microarrays (TMAs) for analysis, the sensitivity is 63.6%[26] and 90%, respectively[27].
GPC-3 staining has also been found to be specific for the HCC component in combined hepatocellular- cholangiocarcinoma (in 8 out of 11 cases)[25]. FNA is commonly performed on liver neoplasms. On FNA specimens of the liver, GPC-3 shows an immuno- reactivity in 56.8%-90.0% of cases, as compared with a negative staining in benign hepatocytic lesions or metastatic lesions[28, 29].
Upon clinicopathological correlation, the staining pattern does not seem to correlate with tumor differentiation or growth pattern[20, 22, 23, 30]. However, in one study, membranous staining has been observed in poorly differentiated cases as well as metastatic lesions[31]. Poorly differentiated tumors demonstrate a higher positive staining rate than the well-differentiated ones, with 93.3% versus 66.7% in one study[20], although the difference is not statistically significant[20, 25,].
Generally, normal liver tissues or cirrhotic liver tissues show negative staining[25,32] or only a small proportion of cases shows focal positive staining in the cirrhotic liver adjacent to HCC[22]. However, GPC-3 expression has recently been found in 84% of chronic hepatitis C samples with high activity[33]. So this has to be taken into account when interpreting the stain.
It remains a consistent finding that all benign nodular lesions like HCA, FNH, and large regenerative nodule (LRN) are negative for GPC-3[20]. It is however remarkable that a negative staining of GPC-3 does not exclude the diagnosis of HCC[20] and this is especially so in biopsy cases, as staining may be heterogeneous. For dysplastic lesions, only around 10.6% of DNs expresses GPC-3, as shown from the results summarized by Wang, et al. from a number of studies[20], while Coston, et al. has come up with a sensitivity of 7.0% (for LGDN) and 23.0% (for HGDN)[22]. In this review article, we summarize the findings of additional studies (Table 3, Figure 1)
Table 3. A summary for sensitivity of GPC-3 in various liver nodules.
| FNH | HCA | Cirrhosis | LRN | NTL | LGDN | HGDN | HCC | |
|---|---|---|---|---|---|---|---|---|
| Baumhoer 2008@[26] | 11/95 | 0/8 | 0/16 | 0/7 | 6/31 | 140/220 | ||
| Di Tommaso 2009[34] | 1/13 | 0/21 | 4/50 | 65/92 | ||||
| Yamauchi 2005[35] | 1/3 | 0/7 | 0/43 | 0/25 | 2/8 | 6/8 | 47/56 | |
| Wang 2008[20] | 0/30 | 0/48 | 0/32 | 84/111 | ||||
| Wang 2010 #[30] | 0/9 | 0/5 | 0/7 | 45/54 | ||||
| Libbrecht 2006[23] | 0/11 | 0/14 | 2/29 | 36/47 | ||||
| Cappuro 2003*[21] | 0/4 | 0/7 | 0/22 | 0/7 | 1/5 | 20/26 | ||
| Wang 2006 @*[27] | 0/14 | 16/94 | 0/42 | 2/23 | 2/9 | 50/74 | ||
| Shafizadeh 2008[18] | 0/8 | 4/35 | 0/10 | 3/7 | 58/79 | |||
| Coston 2008[22] | 0/16 | 0/19 | 4/78 | 95/107 | ||||
| Total | 1/73 | 0/103 | 35/372 | 1/105 | 0/48 | 4/80 | 24/139 | 640/866 |
| (positivity rates in pooled series) | (1.4%) | (0%) | (10.4%) | (1.0%) | (0%) | (5.0%) | (17.3%) | (73.9%) |
@, tissue microarray; #, biopsy cases; *HCC including fibrolamellar HCC but excluding combined type; NTL, normal liver tissue.
Figure 1.
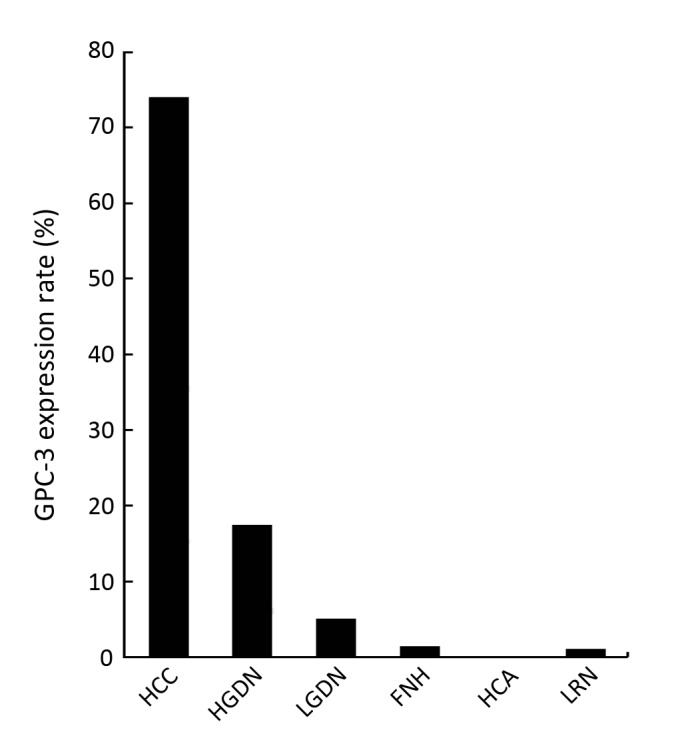
GPC-3 expression rate in various types of hepatocellular nodules (in the pooled series of 10 studies).
However, a caution of note in interpreting the stain is that expression of GPC-3 is not limited to hepatocytic lesions. Some cases of cholangiocarcinoma[26], melanoma, yolk sac tumors[16], testicular germ cell tumors, neuroendocrine tumors and ovarian tumors[22] etc. were also found to express GPC-3.
Correlation between GPC-3 immunohistochemical expression and mRNA expression has been confirmed[36]. As mentioned above, it was reported that mRNA expression of GPC-3 is higher in HCCs than in cirrhotic livers or DNs[23, 29, 37, 38]. Expression of GPC-3 was also confirmed in HCC cell lines by Western blot[21, 31].
After discussing the diagnostic utilization of GPC-3 on immunohistochemical and molecular levels, its serum level in HCC patients is worth the investigation. Since GPC-3 is released from the cell surface to regulate several signaling pathways, it is a serum marker for HCC[21,39-44].
Multi-Marker Panel
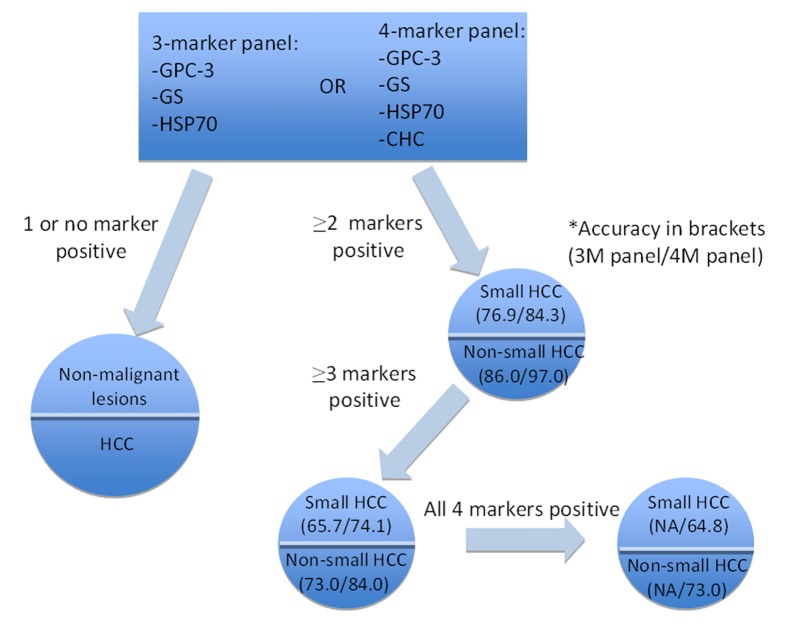
Three-marker panel (HSP70, GPC-3 and GS)
To enhance the diagnostic accuracy, a panel of markers was tested on HCC samples. Heat shock protein 70 (HSP70), GPC-3, and GS were shown to offer a sensitivity of 70% and a specificity of 100% (for 2 markers)[45]. With this 3-marker panel, 11 out of 50 HGDNs stained up with one marker only; while none stained up with any two markers[34]. Even for very well differentiated and grade 1 HCC, the accuracy was 57% (3 markers) and 72.9% (2 markers) and a 100% specificity[34,45].An all-negative phenotype was observed in 100% LRNs and LGDNs; 72.7% of HGDNs and 3.1% of early HCC/well differentiated HCC[45].
Four-marker panel (HSP70, GPC-3, GS and clathrin heavy chain)
Recently, Di Tommaso’s group put forward a 4-marker panel with the introduction of clathrin heavy chain (CHC)[46]. With the 4-maker panel, staining by at least 2 markers shows a diagnostic accuracy of 97% and 84.3% in non-small HCCs and small HCCs, respectively. CHC was also reported as the most single sensitive marker for small grade 1 HCC, giving a sensitivity of 58.8%, versus GS (41.2%), HSP70 (17.6%) and GPC3 (11.8%)[46] (Figure 2).
Figure 2.
Algorithm for applying the 3-marker and 4-marker panels in the diagnosis of premalignant hepatic nodules.
Future perspectives on this topic will be the use of GPC-3 as a prognostic marker for HCC. Multivariate analysis identified GPC-3 expression as an independent prognostic factor for overall survival[36,47]. The possible candidate(s) in a multiple marker panel need also to be investigated.
Immunohistochemical markers on tumor cell origin of HCC
Edmondson and Steiner classified primary carcinoma of the liver in 1954 into 4 groups: liver-cell carcinoma, bile duct carcinoma, multiple or combined primary cancers, and squamous cell carcinoma[48]. Since then, HCC and cholangiocarcinoma (CC) have long been taken as the two largest groups of primary liver carcinoma, presumably representing hepatocytic and cholangiocytic origin respectively, based onmorphologicalresemblanceto the normal counterparts.
Yet, combined/mixed hepatocellular-cholangio- carcinoma (HCC-CC) has raised researchers’ interest, especially since the 1980s, given the observation of an ‘intermediate’ or ‘transition’ morphology between the two well-established HCC and CC groups. Besides, the entity primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype was also introduced by some and this entity morphologically displayed strands or trabeculae of small, uniform, round- to-oval cells with scanty cytoplasm and hyperchromatic nuclei among a desmoplasticstroma[49]. This observation was supported by immunohistochemical findings, in which tumor cells in this particular group express both hepatocytic and cholangiocytic markers. In this regard, new ideas on subtyping of HCC or primary liver carcinoma with reference to the tumor cell origin emerged. It was postulated that HCC might actually consist of two subtypes, one derived from progenitor cells that are able to show hepatocytic and biliary differentiation and the other derived from differentiated hepatocytes[50]. To investigate this problem, studies on immunohistochemical characteristics of tumor cells were performed. In these studies, immunohistochemical markers for hepatocytes (Hep-Par) and for cholangiocytes (AE1/3 and CK19) were implemented. Durnez, et al. attempted to delineate the subgroups in a cohort of 109 HCC using two immunohistochemical markers only, CK7 and CK19. They showed that 28% contained cells expressing CK7 and/or CK19 (taken as biliary/progenitor cell markers) and concluded that these HCCs were possibly derived from hepatic progenitor cells. The immunostains were considered positive if >5% of tumor cells stained up[51]. Based on these findings, possible histogenesis of HCC was further dissected. Further subtyping of cells of origin of HCC included hepatocyte (CK7-CK19-), intermediate hepatocyte (CK7+CK19-), and hepatic progenitor cell (CK7+ CK19+), the latter two groups of which gave rise to the morphological category of combined/mixed HCC-CC[51]. Morphologically, tumor cells immuno-reacting with CK7 and/or CK19 corresponded to the small or intermediate-size cells[51]. The small cells displayed small nuclei and a narrow rim of cytoplasm, resembling the non-neoplastic hepatic progenitor cells[52].
Investigations involving specific immunohisto- chemical markers for progenitor cells have also been carried out. CK14, a progenitor cell marker, is frequently expressed in those HCCs showing stainingfor CK19, AE1/3 and HepPar1 by immunohistochemical analysis, thus supporting a progenitor cell origin of this subset of HCC[49]. C-kit was found in 10 of 13 cases in liver carcinoma of intermediate cell type in another study[49]. The hypothesis that a proportion of HCC originates from hepatic progenitor cells correlates with the identification of the activated hepatic progenitor cells in chronic liver diseases (chronic viral hepatitis, alcoholic and non-alcoholic hepatitis), which are known examples of carcinogenic processes[51].
The investigations on identifying HCC subgroups also uncovered immunohistochemical markers conferring prognostic significance (also see paragraph below). HCC with biliary differentiation was reported to show features related to aggressive behaviour, such as poorer tumor cell differentiation and a higher proliferative index[50]. CK19+ HCC was found to correlate with poorer prognosis, in terms of a higher recurrence rate after transplantation[51]. In addition, CK19 expression was associated with AFP expression in tumor and AFP serum level, and thus conferring a poorer prognosis[51].
Studies at the molecular level have also provided supportive results on the different genetic characteristics and molecular pathways in the heterogeneous group of HCC, as well as shedding light on the prognostication role of these markers. With reference to the two proposed molecular pathways of HCC carcinogenesis (β-catenin mutation versus p53 and axin1 mutations respectively) [53], it was shown that HCC expressing CK7 and/or CK19 was less frequently involved in the β-catenin carcino- genic pathway[51].
Immunohistochemical Markers for Prognostication of HCC
Prognosis of HCC is traditionally based on staging, which includes pathological parameters on tumor morphology. As there is increasing understanding on the cellular mechanisms and molecular pathways implicated in the HCC, some molecular markers have been identified for diagnostic utility. The next question will be whether these markers are also useful in stratification and prognostication in HCC, supplementing the existing prognosticators commonly in use. A number of studies have been published to answer this question. However, up to date, these markers have not yet been prevalently incorporated into prognostication systems.
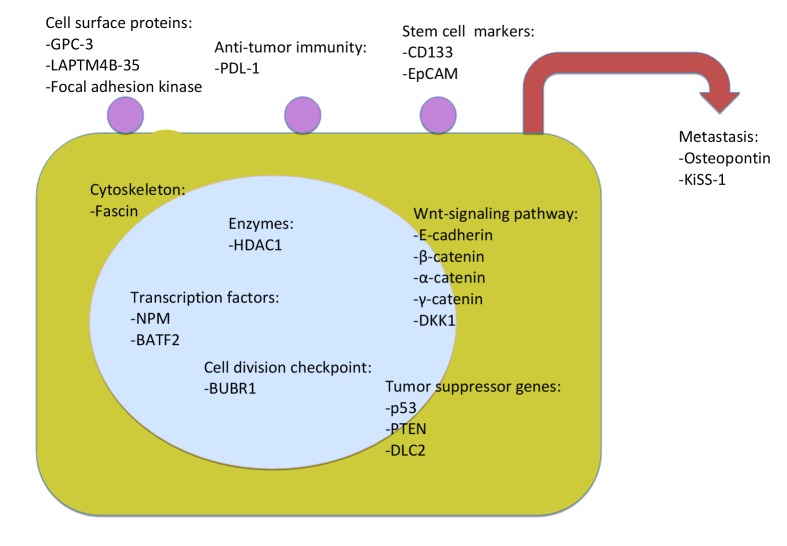
The following discussion will focus on some recent works on markers of which the immunohistochemical expression was assessed. This is by no means and cannot be exhaustive. The markers will be categorized into groups with reference to the nature of the molecules (Figure 3).
Figure 3.
Some immunohistochemical prognostic markers according to subcellular localization of the molecules.
Cell surface proteins
Circumferential membranous staining with GPC-3 was found to be associated with worse prognosis. For those with hepatitis C virus (HCV) infection, high membranous staining is an independent prognostic factor in disease-free survival[36,47]. Lysosomal protein transmembrane 4 beta-35 (LAPTM4B-35) is a tetra- transmembrane glycoprotein. It promotes cell proliferation and tumorigenesis through regulation of cell cycle and signaling pathways. The expression of LAPTM4B-35 at immunohistochemical level is associated with poorer overall survival and disease-free survival[54]. Expression of focal adhesion kinase is also found to correlate with poorer survival and may be implicated in tumor progression[55].
Cytoskeleton
Fascin, a cross-linking protein, is involved in cell motility. Its expression is associated with pathological parameters such as tumor size (larger) and cell differentiation (poorer), as well as clinical parameters such as serum AFP level. It is also an independent unfavorable prognostic factor for disease-free survival[56].
Enzymes
Histone deacetylases (HDACs) are involved in chromatin remodeling, gene repression and regulating cell cycle progression and differentiation. A high expression of HDAC1 is believed to play a role in tumor aggressiveness and cell dedifferentiation. They are independent prognostic factors on multivariate analysis[57].
Stem cell markers
Expression of stem cell marker CD133 is associated with higher tumor grade, advanced disease stage, and elevated AFP levels. High CD133 expression is associated with shorter overall survival and higher recurrence rates[58]. Epithelial cell adhesion molecule (EpCAM) is another marker for progenitor cell under investigation[59].
Transcription
Nucleophosmin (NPM) correlates with clinical parameters such as serum AFP, tumor grading and liver cirrhosis[60].BATF2, a leucine zipper protein, is a regulator of gene expression. Negative expression of BATF2 is associated with shorter survival. Its decreased expression correlates with parameters like age, tumor size and tumor differentiation[61].
Tumor suppressor genes
p53 tumor protein, encoded by the gene TP53, is involved in regulating the cell cycle and maintaining the genome stability. There has been evidence suggesting that p53 expression resulting from mutation of the gene, on its own or in conjunction with other biomarkers, is an adverse prognostic marker for HCC[62,63]. PTEN, located on chromosome 10q23, is another tumor suppressor gene involved in various malignancies. A reduced PTEN expression is correlated with tumor progression, and is associated with p53 expression[64]. A significant correlation between a low expression level of deleted in liver cancer gene 2 (DLC2) and cellular differentiation of HCC has also been reported, and its underexpression is associated with overexpression of RhoA and a poorer prognosis[65].
Cell division checkpoint
BUBR1, a major player in mitotic checkpoint, is overexpressed in about half of HCCs. It is associated with a poor prognosis[66].
Immunity
Programmed cell death 1 ligands 1 and 2 (PDL-1 and -2) weaken anti-tumor immunity. Expression of PDL-1 is associated with poorer survival and postoperative recurrence[67].
Metastasis
Osteopontin is associated with metastasis in many types of cancers. Its overexpression is associated with capsular infiltration, venous invasion, lymph node metastasis, and worse prognosis[68]. Whereas expression of KiSS-1, a metastasis suppressor gene, correlates with a shorter disease-free and overall survival and serves as an independent prognostic factor[69].
Wnt-signaling pathway
The molecular players in the Wnt-signaling pathway have been studied. High tumor Wnt-1 expression correlates with nuclear β-catenin accumulation, reduced membranous E-cadherin expression, and increased rate of tumor recurrence after resection[70]. Reduced expression of E-cadherin correlates with intrahepatic metastasis and capsular invasion. Expression of catenins (α-, β- &γ-catenin) is associated with tumor size, while expression of the E-cadherin/catenin complex is associated with patient’s survival[71]. Dickkopf-1 (DKK1) expression correlates with β-catenin accumulation. It is an independent factor for overall survival and disease-free survival[72].
Summary and Perspectives
Given the extensive amount of work from different groups, the identification of markers with the greatest prognostic power is yet to be determined[73,74]. With the immense amount of work done in characterizing molecular and cell signaling pathway alterations in HCC, researchers in HCC are actively investigating into their use in prognostication of HCC. A recent piece of work from Barcelona-Clinic Liver Cancer Group has shed some insight on the prognostic implication of genomic profiling[75]. In addition, the study of the roles of microRNA is likely to provide exciting and encouraging results on the functions and interplay of specific genes implicated in the progression of HCC[76,77]. Hopefully, there will soon be prognostication models similar to the breast cancer prognostication molecular markers e.g. Oncotype® and MammaPrint®, in which a substantial amount of markers are evaluated simultaneously to generate a prognostic score.
REFERENCES
- 1.Wanless IR. Terminology of nodular hepatocellular lesions. Hepatology 1995;22:983-93 [DOI] [PubMed] [Google Scholar]
- 2.Kojiro M, Roskams T.Early hepatocellular carcinoma and dysplastic nodules. Semin Liver Dis 2005;25:133-42 [DOI] [PubMed] [Google Scholar]
- 3.Roskams T, Kojiro M.Pathology of early hepatocellular carcinoma: conventional and molecular diagnosis. Semin Liver Dis 2010;30:17-25 [DOI] [PubMed] [Google Scholar]
- 4.Kojiro M, Wanless IR, Alves V, et al. Pathologic diagnosis of early hepatocellular carcinoma: A report of the international consensus group for hepatocellular neoplasia. Hepatology 2009;49:658-64 [DOI] [PubMed] [Google Scholar]
- 5.Bioulac-Sage P, Balabaud C, Zucman-Rossi J.Subtype classifi- cation of hepatocellular adenoma. Dig Surg 2010;27:39-45 [DOI] [PubMed] [Google Scholar]
- 6.Bioulac-Sage P, Blanc LF, Rebouissou S, et al. Genotype phenotype classification of hepatocellular adenoma. World J Gastroenterol 2007;13:2649-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bioulac-Sage P, Cubel G, Balabaud C, et al. Revisiting the pathology of resected benign hepatocellular nodules using new immunohistochemical markers. Semin Liver Dis 2011;31:91-103 [DOI] [PubMed] [Google Scholar]
- 8.Bioulac-Sage P, Laumonier H, Rullier A, et al. Over-expression of glutamine synthetase in focal nodular hyperplasia: a novel easy diagnostic tool in surgical pathology. Liver Int 2009;29:459-65 [DOI] [PubMed] [Google Scholar]
- 9.Bioulac-Sage P, Rebouissou S, Thomas C, et al. Hepatocellular adenoma subtype classification using molecular markers and lmmunohistochemistry. Hepatology 2007;46:740-8 [DOI] [PubMed] [Google Scholar]
- 10.Micchelli STL, Vivekanandan P, Boitnott JK, et al. Malignant transformation of hepatic adenomas. Mod Pathol 2008;21:491-7 [DOI] [PubMed] [Google Scholar]
- 11.Rebouissou S, Couchy G, Libbrecht L, et al. The beta-catenin pathway is activated in focal nodular hyperplasia but not in cirrhotic FNH-like nodules. J Hepatol 2008;49:61-71 [DOI] [PubMed] [Google Scholar]
- 12.Wee A.Diagnostic utility of immunohistochemistry in hepatocellular carcinoma, its variants and their mimics. Appl Immunohistochem Mol Morphol 2006;14:266-72 [DOI] [PubMed] [Google Scholar]
- 13.Jakubovic BD, Jothy S. Glypican-3: From the mutations of Simpson-Golabi-Behmel genetic syndrome to a tumor marker for hepatocellular carcinoma. Exp Mol Pathol 2007;82:184-9 [DOI] [PubMed] [Google Scholar]
- 14.Li M, Choo B, Wong ZM, et al. Expression of OCI-5 glypican 3 during intestinal morphogenesis: Regulation by cell shape in intestinal epithelial cells. Exp Cell Res 1997;235:3-12 [DOI] [PubMed] [Google Scholar]
- 15.Filmus J.The contribution of in vivo manipulation of gene expression to the understanding of the function of glypicans. Glycoconj J 2002;19:319-23 [DOI] [PubMed] [Google Scholar]
- 16.Filmus J, Selleck SB. Glypicans: proteoglycans with a surprise. J Clin Invest 2001;108:497-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Midorikawa Y, Ishikawa S, Iwanari H, et al. Glypican-3, overexpressed in hepatocellular carcinoma, modulates FGF2 and BMP-7 signaling. Int J Cancer 2003;103:455-65 [DOI] [PubMed] [Google Scholar]
- 18.Shafizadeh N, Ferrell LD, Kakar S. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Mod Pathol 2008;21:1011-8 [DOI] [PubMed] [Google Scholar]
- 19.Zhou XP, Wang HY, Yang GS, et al. Cloning and expression of MXR7 gene in human HCC tissue. World J Gastroenterol 2000;6:57-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HL, Anatelli F, Zhai QH, et al. Glypican-3 as a useful giagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions. Arch Pathol Lab Med 2008;132:1723-8 [DOI] [PubMed] [Google Scholar]
- 21.Capurro M, Wanless IR, Sherman M, et al. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003;125:89-97 [DOI] [PubMed] [Google Scholar]
- 22.Coston WMP, Loera S, Lau SK, et al. Distinction of hepatocellular carcinoma from benign hepatic mimickers using glypican-3 and CD34 immunohistochemistry. Am J Surg Pathol 2008;32:433-44 [DOI] [PubMed] [Google Scholar]
- 23.Libbrecht L, Severi T, Cassiman D, et al. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. Am J Surg Pathol 2006;30:1405-11 [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Li P, Zhai Y, et al. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J Gastroenterol 2010;16:4410-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirakawa H, Kuronuma T, Nishimura Y, et al. Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer. Int J Oncol 2009;34:649-56 [DOI] [PubMed] [Google Scholar]
- 26.Baumhoer D, Tornillo L, Stadlmann S, et al. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: A tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol 2008;129:899-906 [DOI] [PubMed] [Google Scholar]
- 27.Wang XY, Degos F, Dubois S, et al. Glypican-3 expression in hepatocellular tumors: diagnostic value for preneoplastic lesions and hepatocellular carcinomas. Hum Pathol 2006;37:1435-41 [DOI] [PubMed] [Google Scholar]
- 28.Kandil DH, Cooper K. Glypican-3 A novel diagnostic marker for hepatocellular carcinoma and more. Adv Anat Pathol 2009;16:125-9 [DOI] [PubMed] [Google Scholar]
- 29.Nassar A, Cohen C, Siddiqui MT. Utility of glypican-3 and survivin in differentiating hepatocellular carcinoma from benign and preneoplastic hepatic lesions and metastatic carcinomas in liver fine-needle aspiration biopsies. Diagn Cytopathol 2009;37:629-35 [DOI] [PubMed] [Google Scholar]
- 30.Wang FH, Yip YC, Zhang M, et al. Diagnostic utility of glypican-3 for hepatocellular carcinoma on liver needle biopsy. J Clin Pathol 2010;63:599-603 [DOI] [PubMed] [Google Scholar]
- 31.Suzuki M, Sugimoto K, Tanaka J, et al. Up-regulation of glypican-3 in human hepatocellular carcinoma. Anticancer Res 2010;30:5055-61 [PubMed] [Google Scholar]
- 32.Anatelli F, Chuang ST, Yang XJ, et al. Value of glypican 3 immunostaining in the diagnosis of hepatocellular carcinoma on needle biopsy. Am J Clin Pathol 2008;130:219-23 [DOI] [PubMed] [Google Scholar]
- 33.Abdul-Al HM, Makhlouf HR, Wang GH, et al. Glypican-3 expression in benign liver tissue with active hepatitis C: implications for the diagnosis of hepatocellular carcinoma. Hum Pathol 2008;39:209-12 [DOI] [PubMed] [Google Scholar]
- 34.Di Tommaso L, Destro A, Seok JY, et al. The application of markers (HSP70 GPC3 and GS) in liver biopsies is useful for detection of hepatocellular carcinoma. J Hepatol 2009;50:746-54 [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi N, Watanabe A, Hishinuma M, et al. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol 2005;18:1591-8 [DOI] [PubMed] [Google Scholar]
- 36.Yorita K, Takahashi N, Takai H, et al. Prognostic significance of circumferential cell surface immunoreactivity of glypican-3 in hepatocellular carcinoma. Liver Int 2011;31:120-31 [DOI] [PubMed] [Google Scholar]
- 37.Sung YK, Hwang SY, Park MK, et al. Glypican-3 is overexpressed in human hepatocellular carcinoma. Cancer Sci 2003;94:259-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu ZW, Friess H, Wang L, et al. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut 2001;48:558-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malaguarnera G, Giordano M, Paladina I, et al. Serum markers of hepatocellular carcinoma. Dig Dis Sci 2010;55:2744-55 [DOI] [PubMed] [Google Scholar]
- 40.Hippo Y, Watanabe K, Watanabe A, et al. Identification of soluble NH2-terminal fragment of glypican-3 as a serological marker for early-stage hepatocellular carcinoma. Cancer Res 2004;64:2418-23 [DOI] [PubMed] [Google Scholar]
- 41.Jia HL, Ye QH, Qin LX, et al. Gene expression profiling reveals potential biomarkers of human hepatocellular carcinoma. Clin Cancer Res 2007;13:1133-9 [DOI] [PubMed] [Google Scholar]
- 42.Nakatsura T, Yoshitake Y, Senju S, et al. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun 2003;306:16-25 [DOI] [PubMed] [Google Scholar]
- 43.Spangenberg HC, Thimme R, Blum HE. Serum markers of hepatocellular carcinoma. Semin Liver Dis 2006;26:385-90 [DOI] [PubMed] [Google Scholar]
- 44.Yan D, He Q, Chen YP, et al. Detection of alpha-fetoprotein and glypican-3 mRNAs in the peripheral blood of hepatocellular carcinoma patients by using multiple FQ-RT-PCR. J Clin Lab Anal 2011;25:113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Tommaso L, Franchi G, Park YN, et al. Diagnostic value of HSP70, glypican 3, and glutamine synthetase in hepatocellular nodules in cirrhosis. Hepatology 2007;45:725-34 [DOI] [PubMed] [Google Scholar]
- 46.Di Tommaso L, Destro A, Fabbris V, et al. Diagnostic Accuracy of Clathrin Heavy Chain Staining in a Marker Panel for the Diagnosis of Small Hepatocellular Carcinoma. Hepatology 2011;53:1549-57 [DOI] [PubMed] [Google Scholar]
- 47.Shirakawa H, Suzuki H, Shimomura M, et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci 2009;100:1403-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7:462-503 [DOI] [PubMed] [Google Scholar]
- 49.Kim H, Park C, Han KH, et al. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol 2004;40:298-304 [DOI] [PubMed] [Google Scholar]
- 50.Wu PC, Fang JW, Lau VKT, et al. Classification of hepatocellular carcinoma according to hepatocellular and biliary differentiation markers - Clinical and biological implications. Am J Pathol 1996;149:1167-75 [PMC free article] [PubMed] [Google Scholar]
- 51.Durnez A, Verslype C, Nevens F, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology 2006;49:138-51 [DOI] [PubMed] [Google Scholar]
- 52.Roskams TA, Theise ND, Balabaud C, et al. Nomenclature of the finer branches of the biliary tree: Canals, ductules, and ductular reactions in human livers. Hepatology 2004;39:1739-45 [DOI] [PubMed] [Google Scholar]
- 53.Laurent-Puig P, Legoix P, Bluteau O, et al. Genetic alterations associated with hepatocellular carcinomas define distinct pathways of hepatocarcinogenesis. Gastroenterology 2001;120:1763-73 [DOI] [PubMed] [Google Scholar]
- 54.Yang H, Xiong FX, Lin M, et al. LAPTM4B-35 overexpression is a risk factor for tumor recurrence and poor prognosis in hepatocellular carcinoma. J Cancer Res Clin Oncol 2010;136:275-81 [DOI] [PubMed] [Google Scholar]
- 55.Itoh S, Maeda T, Shimada M, et al. Role of expression of focal adhesion kinase in progression of hepatocellular carcinoma. Clin Cancer Res 2004;10:2812-7 [DOI] [PubMed] [Google Scholar]
- 56.Iguchi T, Aishima S, Umeda K, et al. Fascin Expression in Progression and Prognosis of Hepatocellular Carcinoma. J Surg Oncol 2009;100:575-9 [DOI] [PubMed] [Google Scholar]
- 57.Rikimaru T, Taketomi A, Yamashita YI, et al. Clinical significance of histone deacetylase 1 expression in patients with hepatocellular carcinoma. Oncology 2007;72:69-74 [DOI] [PubMed] [Google Scholar]
- 58.Song W, Li H, Tao K, et al. Expression and clinical significance of the stem cell marker CD133 in hepatocellular carcinoma. Int J Clin Pract 2008;62:1212-8 [DOI] [PubMed] [Google Scholar]
- 59.Yamashita T, Forgues M, Wang W, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res 2008;68:1451-61 [DOI] [PubMed] [Google Scholar]
- 60.Yun JP, Miao J, Chen GG, et al. Increased expression of nucleo- phosmin/B23 in hepatocellular carcinoma and correlation with clinicopathological parameters. Br J Cancer 2007;96:477-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma H, Liang X, Chen Y, et al. Decreased expression of BATF2 is associated with a poor prognosis in hepatocellular carcinoma. Int J Cancer 2011;128:771-7 [DOI] [PubMed] [Google Scholar]
- 62.Qin LX, Tang ZY, Ma ZC, et al. P53 immunohistochemical scoring: an independent prognostic marker for patients after hepatocellular carcinoma resection. World J Gastroenterol 2002;8:459-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan RH, Jeng YM, Hu RH, et al. Role of p53 and beta-catenin mutations in conjunction with CK19 expression on early tumor recurrence and prognosis of hepatocellular carcinoma. J Gastrointest Surg 2011;15:321-9 [DOI] [PubMed] [Google Scholar]
- 64.Hu TH, Huang CC, Lin PR, et al. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer 2003;97:1929-40 [DOI] [PubMed] [Google Scholar]
- 65.Xiaorong L, Wu W, Qian LY, et al. Underexpression of Deleted in liver cancer 2 (DLC2) is associated with overexpression of RhoA and poor prognosis in hepatocellular carcinoma. BMC Cancer 2008;8:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu AW, Cai J, Zhao XL, et al. The clinicopathological significance of BUBR1 overexpression in hepatocellular carcinoma. J Clin Pathol 2009;62:1003-8 [DOI] [PubMed] [Google Scholar]
- 67.Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 Significantly Associates with Tumor Aggressiveness and Postoperative Recurrence in Human Hepatocellular Carcinoma. Clin Cancer Res 2009;15:971-9 [DOI] [PubMed] [Google Scholar]
- 68.Xie H, Song J, Du R, et al. Prognostic significance of osteopontin in hepatitis B virus-related hepatocellular carcinoma. Dig Liver Dis 2007;39:167-72 [DOI] [PubMed] [Google Scholar]
- 69.Schmid K, Wang XW, Haitel A, et al. KiSS-1 overexpression as an independent prognostic marker in hepatocellular carcinoma: an immunohistochemical study. Virchows Arch 2007;450:143-9 [DOI] [PubMed] [Google Scholar]
- 70.Lee HH, Uen YH, Tian YF, et al. Wnt-1 Protein as a Prognostic Biomarker for Hepatitis B-Related and Hepatitis C-Related Hepatocellular Carcinoma after Surgery. Cancer Epidemiol Biomarkers Prev 2009;18:1562-9 [DOI] [PubMed] [Google Scholar]
- 71.Zhai B, Yan HX, Liu SQ, et al. Reduced expression of E-cadherin/catenin complex in hepatocellular carcinomas. World J Gastroenterol 2008;14:5665-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu B, Yang XR, Xu Y, et al. Elevated expression of DKK1 is associated with cytoplasmic/nuclear beta-catenin accumulation and poor prognosis in hepatocellular carcinomas. J Hepatol 2009;50:948-57 [DOI] [PubMed] [Google Scholar]
- 73.Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. J Cancer Res Clin Oncol 2004;130:497-513 [DOI] [PubMed] [Google Scholar]
- 74.Mann CD, Neal CP, Garcea G, et al. Prognostic molecular markers in hepatocellular carcinoma: a systematic review. Eur J Cancer 2007;43:979-92 [DOI] [PubMed] [Google Scholar]
- 75.Villanueva A, Hoshida Y, Battiston C, et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology 2011;140:1501-12 e2. [DOI] [PMC free article] [PubMed]
- 76.Liang L, Wong CM, Ying Q, et al. MicroRNA-125b suppressesed human liver cancer cell proliferation and metastasis by directly targeting oncogene LIN28B2. Hepatology 2010;52:1731-40 [DOI] [PubMed] [Google Scholar]
- 77.Wong CC, Wong CM, Tung EK, et al. The microRNA miR-139 suppresses metastasis and progression of hepatocellular carcinoma by down-regulating Rho-kinase 2. Gastroenterology 2011;140:322-31 [DOI] [PubMed] [Google Scholar]