Abstract
Objective
Most recurrent intrahepatic cholangiocarcinoma (RICC) lost the opportunity of radical resection while most nonsurgical management failed to prolong patients’ survival. The efficacy and safety of radiofrequency ablation (RFA) as a local treatment for recurrent hepatocellular carcinoma have been confirmed by many clinical studies. The purpose of this study was to evaluate the efficacy, long-term survival and complications of RFA for RICC.
Methods
A total of 12 patients with 19 RICCs after radical resection were included in this study. The tumors were 1.9-6.8 cm at the maximum diameter (median, 3.2±1.6 cm). All patients were treated with ultrasound guided RFA. There were two RFA approaches including percutaneous and open.
Results
A total of 18 RFA treatment sessions were performed. Ablation was successful (evaluated by 1-month CT after the initial RFA procedure) in 18 (94.7%) of 19 tumors. By a median follow-up period of 29.9 months after RFA, 5 patients received repeated RFA because of intrahepatic lesion recurrence. The median local recurrence-free survival period and median event-free survival period after RFA were 21.0 months and 13.0 months, respectively. The median overall survival was 30 months, and the 1- and 3-year survival rates were 87.5% and 37.5%, respectively. The complication rate was 5.6% (1/18 sessions). The only one major complication was pleural effusion requiring thoracentesis.
Conclusion
This study showed RFA may effectively and safely manage RICC with 3-year survival of 37.5%. It provides a treatment option for these RICC patients who lost chance for surgery.
Key words: Intrahepatic cholangiocarcinoma, Hepatectomy, Recurrence, Radiofrequency ablation, Survival
INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer, arising from the biliary epithelium of the second branch (segmental branch) or the proximal branch of bile duct[1, 2]. Although ICC only accounts for 5%-10% of primary hepatic cancers, the therapeutic outcomes for patients with ICC are poor due to the highly malignant pathologic nature of the cancer[3]. Surgical resection has been regarded as the gold standard treatment for ICC, however, recurrence rate was as high as 50%-80% after radical surgery[3-6].
The majority of recurrences confined to the liver[3, 7]. No specific therapy has been recommended for recurrent intrahepatic cholangiocarcinoma (RICC). The resectability of RICC is influenced by several factors including the stage of the disease, anatomic conditions and patient’s medical co-morbidities. Also, the efficacy of repeated resection for RICC remains unclear, despite of several studies in small number of selected patients[6, 8]. The prognosis of patients with unresectable ICC is devastating, at less than 1 year[3]. Therefore, establishing and maintaining control of the intrahepatic disease remains the biggest problem for all RICC patients.
Recently, radiofrequency ablation (RFA) has been increasingly used for treating recurrent tumors involving the liver after hepatectomy[9-11]. RFA has low morbidity and mortality rates, and can effectively destroy tumor and preserve the maximal normal liver parenchyma as a local treatment for hepatic tumors, which has been confirmed by many clinical studies[9-11]. These features are particularly important for patients with limited liver remanent after liver resection[10,11]. To the best of our knowledge, few studies have focused on RFA treatment for RICC. The purpose of this study was to evaluate the long-term survival results and safety of RFA for RICC after radical hepatic resection.
MATERIALS AND METHODS
Patients
All the patients met the following criteria for RFA treatment: (1) nodular ICC ≤7 cm in maximum diameter, multi-nodule (up to three in number) ICC ≤3 cm in maximum diameter each; (2) tumors visible on ultrasound; (3) the absence of portal venous thrombosis; (4) Child-Pugh A or B grade; (5) platelet count greater than 50×109/L; and (6) extrahepatic metastases that had been surgically resected or locally controlled.
This study was approved by the institutional ethics committees, and written informed consent was obtained from all patients.
Between January 2000 and July 2011, a total of 12 patients with ICC who had previously undergone radical hepatectomy as a first-line treatment were included in the study. At the time of RFA, 10 patients had liver function of Child-Pugh A, and the other two who had hepatitis B infection had Child-Pugh B grade. Majority of patients (83.3%, 10 of 12 patients) had a single tumor, while 2 (16.7%) had multiple tumors. There were 5 well-differentiated RICC, 2 moderately-differentiated RICC, and 5 poorly- differentiated RICC based on the Edmondson-Steiner grading system[12]. The median tumor size was 3.2±1.6 cm (range, 2.1-6.8 cm) at maximum diameter. Of the 12 patients with 19 tumors, 5 had tumors with diameters ≤3.0 cm, 3 had tumors with diameters of 3.1-5.0 cm, and the rest 4 had tumors with diameters >5.0 cm. One RICC patient was with lymph node metastasis. Radiation was performed to locally control the metastasis. Pre-RFA laboratory tests included glutamate-pyruvate transaminase (ALT), oxalacetic transaminase (AST), bilirubin, albumin, complete blood count, prothrombin time, electrolyte and carbohydrate antigen (CA19-9).
The curative resection procedures for the RICC patients before RFA included: extended right lobectomy in 2 patients, right lobectomy in 5, and left lobectomy in 5. The surgical margins of the liver resection specimens in all the patients were pathologically negative for tumor cells. RICC was found from 2 to 72 months after hepatectomy (mean 9.0±21.0 months) by CT/MRI or ultrasound. Four of the 12 patients were found having RICC within 1 year from the date of hepatectomy, while RICC occurred beyond 1 year in the remaining 8 patients. All patients underwent RFA within 1-2 months after diagnosis of recurrence. Before RFA, 5 patients with RICC had received preventive transcatheter arterial chemoembolization (TACE) treatments.
Approaches of RFA
In our center, RFA can be performed through percutaneous and open approaches. The choice of approach was made according to the location of the tumor and patients’ systemic conditions. In this group, 10 patients underwent RFA by percutaneous approach while the other 2 received RFA by open approach due to unfavorable locations (close to inferior vena cava).
RFA Procedure and Anesthesia
All RFA was performed under real-time sonographic guidance by one experienced radiologist. For tumors with clear boundary, the ablative volume enveloped the entire tumor, as well as a 0.5-1.0 cm margin of surrounding normal tissue regardless of the RF devices. For tumors with irregular shape or with obscure boundary, the ablative volume enveloped the entire tumor with a margin of 1.0 cm or more. Multiple overlapping ablations were used for tumors >3.5 cm[13]. Individualized treatment strategies and adjunctive measures for tumors located at problematic locations were used whenever possible[14].
All patients through percutaneous approach underwent moderate sedation anesthesia. Open RFA was performed in the patient under general anesthesia in the operating room.
Equipment
Two kinds of RFA system were used in this study. From 2000 to 2008, the RFA system used in this study was a 460-kHz generator (Model 1500; Rita Medical Systems, Mountain View, CA).
From 2009 to now, a multipolar RFA system (CelonLab POWER; Celon Medical Instruments, Teltow, Germany) was used[15].
An Aloka SSD-5000 or α 10 ultrasonography system (Aloka Co, Ltd, Tokyo, Japan) with a 3.5-5.0 MHz probe was used to guide percutaneous RFA. Multi-frequency (5-10 MHz) “T” style finger-grip transducer was used to guide intraoperative RFA.
Evaluation of Clinical Response and Follow-up
For irregular tumors larger than 5.0 cm, enhanced CT within 24 h after the treatment was used to detect any residual viable tissue that would require the second RFA and to observe early possible complications. One month after RFA, enhanced CT, a repeated blood test and tumor marker (CA19-9) were conducted. Follow-up imaging and laboratory were conducted every 3 months during the first year, and every 4-6 months during the following years. Information concerning the tolerance and complications was provided by all patients during follow-up for evaluating the safety and efficacy of RFA. All periods of follow-up ranged from 6 to 91 months (median, 29.9 months) after RFA. At the time of writing, 6 of the 12 patients had died, and six patients were still alive.
Primary technique effectiveness rate was identified if the ablation area showed no enhancement and had well-defined margins. Residual tumors were defined as irregular peripheral enhancing foci in the ablation zone on 1-month follow-up CT. Additional RFA was needed for residual tumors if possible.
Local recurrence was defined as enhancement in the periphery of RFA-treated area, and remote intrahepatic recurrence was defined as a new liver lesion other than the RFA-treated area. Follow-up CT/MRI imaging studies were reviewed by two radiologists with more than 10 years experiences in reading liver scans. When recurrences were confirmed, the tumors were usually treated with additional RFA if possible. If an additional RFA was not feasible, TACE or other palliative treatments were performed. Patients who had a CT contrast allergy underwent magnetic resonance (MR) or contrast enhanced ultrasound (CEUS) for follow-up.
Complications were categorized using the definitions recommended by the Society of Interventional Radiology reporting standards[16]. Major complications were those that if untreated, it might lead to death, substantial morbidity and disability, or a lengthened hospital stay. All other complications were called minor.
Statistic Analysis
All quantitative data were expressed as median or range, unless otherwise indicated. The overall survival time was defined as the interval between the first RFA and the death or the last visit to the outpatient clinic. The local recurrence-free survival was defined as the time interval from the initial RFA to local tumor progression or death. The event-free survival was defined as the time interval from the initial RFA to local tumor progression, remote intrahepatic recurrence, exhepatic metastasis or death. Cumulative survival rate was calculated using the Kaplan-Meier method. The level of significance was set at 0.05 for all tests. All data were analyzed with SPSS 13.0 software (SPSS Inc., Chicago, USA).
RESULTS
Clinical Response
There were a total of 19 RICCs among 12 patients, for which 18 RFA treatment sessions were performed. Most of the ablation procedures were carried out through percutaneous (n=10, 83.3% patients) approach, whereas 2 patients underwent open RFA.
Primary technique effectiveness rate was 94.7% (18 of 19 tumors). One patient with residual unablated tumor was ablated successfully by the second session through percutaneous approach (Figure 1). During the follow-up period, local recurrence developed in another 2 tumors (10.5%) in 2 patients (16.7%). Remote intrahepatic recurrence was observed in 5 (41.7%) of 12 patients. Three of the 5 patients with remote intrahepatic recurrence received repeated RFA. The rest 2 patient with multiple distant recurrences (>3 in number) did not meet our inclusion criteria for RFA, and TACE was performed instead. Four patients had extrahepatic metastases (pulmonary metastases in 1 case, lymph node metastasis in 2 cases, and adrenal metastases in 1 case). Radiotherapy and/or chemotherapy were performed to control tumor progression in these patients.
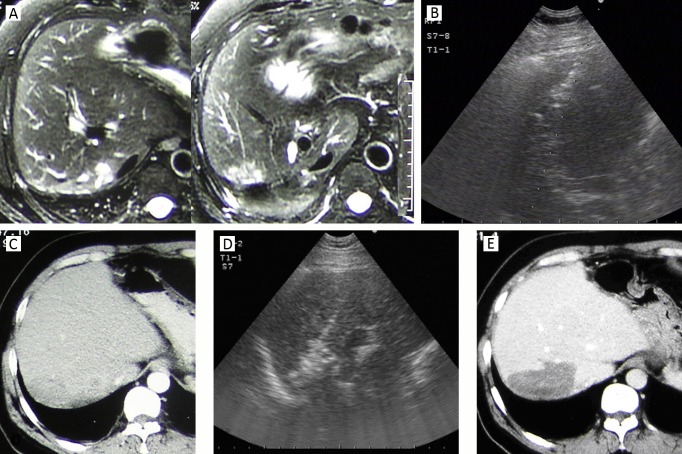
Figure 1.
A 64-year-old man in whom ICC recurred 9 months after partial resection of right hepatic lobe. A. MRI showed an irregular tumor located at the edge of resection on the T2-weighted image; B. Intercostal sonograms showed a 4.2 cm tumor and it was treated by sonography-guided RFA; C. On 1-month CT, the margin of the ablation zone was obscure and coarse, without sufficient safety margin; D. A repeated RFA was performed for the unablated tumor area. E. CT scan 3 months after repeated RFA showed that ablated zone had clear margin and no enhancement. This patient survived for 30 months after the initial RFA treatment and died of tumor spread and metastasis.
The median local recurrence-free survival period after RFA in this group was 21.0±4.1 months and the median event-free survival period after RFA was 13.0±3.4 months. The median overall survival was 30±6.2 months, and 1- and 3-year survival rates was 87.5%, and 37.5%, respectively. (Figure 2, 3)
Figure 2.
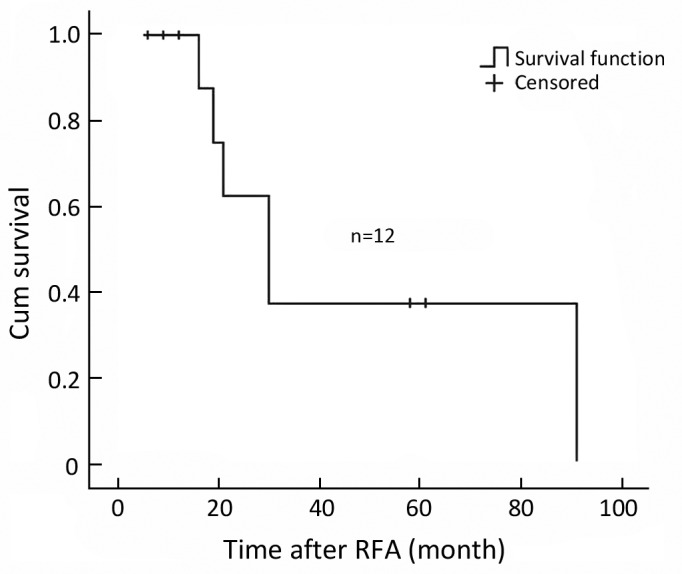
Overall survival curve of 12 RICC patients after RFA. The median overall survival was 30±6.2 months and the 1- and 3-year survival rates were 87.5% and 37.5%, respectively.
Figure 3.
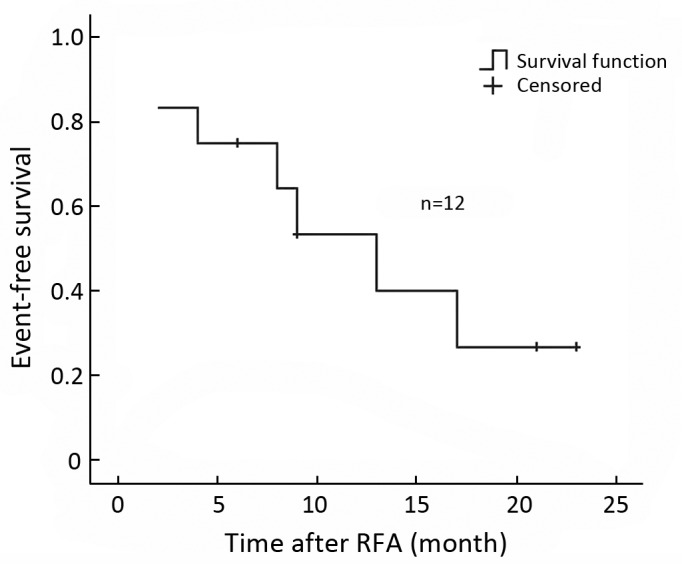
Event-free survival curve of 12 RICC patients after RFA.
Complications
All patients tolerated the RFA procedure well. No procedure-related deaths occurred. There was only one major complication (5.6%, 1/18 sessions) identified during follow-up. This patient, who had a recurrent tumor 3 cm in diameter just under the diaphragm, received two ablations through open approach. Three days after RFA, she developed pleural effusion and had symptoms of dyspnea. The patient received two times of thoracentesis and 1,200 ml fluid was aspirated. Finally her symptom was relieved within two weeks. Minor complications were also observed, including asymptom pleural effusion in 2 patients, mild bile duct dilation with/without jaundice in 3 patients, and acute cholecystitis in 1 patient. All patients with minor complications relieved after conservative treatments.
DISCUSSION
Among the methods used to treat ICC, surgery is unquestionably the optimum, and yields a 5-year survival rate of 17%-46%[4-7]. However, only a small subset of patients can benefit from surgery and the recurrent rate is as high as 80%[6, 7]. At the time of recurrence, the tumor is mostly multifocal or the patient has poor general condition or limited remnant liver. So management of patients with RICC following surgical resection is challenging. Reports of repeated hepatectomies due to RICC are rare[6, 8]. At the same time, some researchers considered RICC as contraindication to curative surgical management[17,18]. The prognosis for patients with unresectable ICC is extremely poor, with survival of 5-8 months[5-8].
Because surgical therapy is not indicated in majority of RICC cases, other treatment modalities should be considered. Unfortunately, the nonsurgical management failed to prolong patient’s survival or had only slightly increased survival. External radiotherapy (RT) with or without intraoperative radiotherapy and intraluminal radiotherapy (brachytherapy) has been explored in the adjuvant setting but showed no significant benefits after R0 resections[19-21]. The unsuccessful results maybe related to this fact that ICC is mainly classified as adenocarcinoma, which is not sensitive to radiotherapy[1, 2].
Systemic chemotherapy for cholangiocarcinoma didn’t show significant survival benefits neither[22,23]. Although chemotherapy has been reported to be more beneficial than the best supportive care[24], systemic chemotherapy with a combination regimen (5-fluorouracil, doxorubicin, cisplatin, and mitomycin-C) is not entirely satisfactory in terms of survival outcomes. The range of median survival was only 6.5 to 11.5 months for patients treated with systemic chemotherapy[25,26]. There is no randomized, prospective trial data in this disease, and standard chemotherapy regimen has not been established yet.
Compared with systemic chemotherapy, TACE or transcatheter arterial chemoinfusion (TACI) has the advantages of increasing the local concentration of chemotherapeutic agents to kill cancer cells without damaging healthy liver tissue and of reducing systemic side effects. It was reported the median survival of TACE for RICC was ranged from 9.0-17.3 months[27-29]. Tumor vascularity is closely associated with treatment response[28,29]. ICC with rich arterial supply more likely has tumor response to TACE than ICC with a decreased arterial supply. However, the problem is that most ICCs are of hypovascular[1,2]. So the efficacy of TACE for treating inoperable cholangiocarcinoma in the majority of patients remains questionable.
Recent years, RFA which can produce localized tumor destruction by heating tumor tissue has shown some benefits in selected groups of RICC patients by several case series[17, 30, 31]. Kim, et al. firstly reported the role of percutaneous RFA in RICC and evaluated survival results in 20 patients with 29 RICC in 2010. In 20 patients with 29 RICCs, the technical effectiveness rate of RFA was 97% (28/29), median overall survival after RFA was 27.4 months, and the cumulative overall 1, 2, and 4 year survival rates were 70%, 60%, and 21%, respectively[32]. Our result is comparable to their study, the median overall survival was 30 months and the 1- and 3-year survival rates were 87.5% and 37.5%, respectively. One patient survived for 91 months and received RFA treatments four times. The advantage of RFA is that it can be repeated many times easily to treat residual tumor or intrahepatic recurrence. The studies above showed RFA was superior to other palliative therapies in prolonging patients’ survival in selected patients.
Chiou, et al. had reported a series of 10 ICC patients underwent RFA. They analyzed the correlation between RFA efficacy and tumor size. Based on 1 month CT, complete necrosis was seen in all of the five tumors (100%) with diameters of 3.0 cm or less, two of three tumors with diameters of 3.1-5.0 cm, and one of two tumors with diameters of more than 5.0 cm[33]. The results demonstrated that tumor size was a main risk factor for local tumor progression. However, in our study, with the individualized treatment strategies and adjunctive measures used[13, 14], the ablation success rate can be up to 94.7% for the tumors with the median size of 3.2 cm (range, 1.9-6.8 cm). In fact, as the improvement of RFA equipments, overlapping techniques and different approaches of RFA were developed, it has been reported RFA can effectively ablate intrahepatic tumors both metastatic and primary tumor with size up to 12 cm in diameter[17, 34].
In addition, open RFA was used in our study for 2 patients. It permits the ablation of liver tumors close to surrounding organs, such as bowel, kidney, gallbladder and diaphragm. The risk of injury will be high if these tumors are treated by percutaneous RFA. Also, percutaneous RFA is not fit for patients with serious intra-abdominal adhesions because adjuvant therapy such as artificial ascites usually failed. In fact, many researchers had proved open RFA can achieve a higher complete ablation rate and a lower local recurrence rate than percutaneous approach[34, 35].
One symptomatic pleural effusion was reported in our study. The patient was a female patient with a 3.0 cm tumor close to the diaphragm. Three days after open RFA, she developed pleural effusion and had symptoms of dyspnea. Two times of thoracentesis was performed and the patient relieved within two weeks. In fact, most pleural effusion after ablation was asymptomatic and usually resolved spontaneously. The incidence of symptomatic pleural effusion requiring thoracentesis was about 0.2% among hepatocellular carcinoma (HCC) patients[36]. It was common to find pleural effusion after ablation in patients with tumors located less than 2 cm from the diaphragm[37]. Only the patients with symptoms of dyspnea need further treatment, and in some patients, the pleural effusion was refractory, necessitating repeated aspiration[37].
As a result of the low incidence of ICC and the initial use of RFA as a possible treatment option for RICC, our patient population is unfortunately small. The facts that our study is not randomized and lacks of control group are also limitations. However, our population size of 12 patients is comparable to those of other reports of palliative treatments for RICC and demonstrates the potential application of RFA in the management of RICC.
In conclusion, this preliminary clinical study showed minimally invasive RFA can effectively and safely manage RICC with 3-year survival of 37.5%. It provides a treatment option for these RICC patients who lost chance for surgery. Although our preliminary results are encouraging, well- designed controlled trials with a large sample are needed to further confirm the role of RFA in the treatment of postoperative recurrences of ICC.
REFERNCES
- 1.Nakajima T, Kondo Y, Miyazaki M, et al. A histopathologic study of 102 cases of intrahepatic cholangiocarcinoma: histologic classification and modes of spreading. Hum Pathol 1988;19:1228-34 [DOI] [PubMed] [Google Scholar]
- 2.Lim JH, Park CK. Pathology of cholangiocarcinoma. Abdom Imaging 2004;29540-7 [DOI] [PubMed] [Google Scholar]
- 3.Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84-96 [DOI] [PubMed] [Google Scholar]
- 4.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangio- carcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007;245:755-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konstadoulakis MM, Roayaie S, Gomatos IP, et al. Fifteen-year, single-center experience with the surgical management of intrahepatic cholangiocarcinoma: operative results and long-term outcome. Surgery 2008;143: 366-74 [DOI] [PubMed] [Google Scholar]
- 6.Saiura A, Yamamoto J, Kokudo N, et al. Intrahepatic cholangio- carcinoma: analysis of 44 consecutive resected cases including 5 cases with repeat resections. Am J Surg 2011;201:203-8 [DOI] [PubMed] [Google Scholar]
- 7.Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic cholangio- carcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg 2001;193:384-91 [DOI] [PubMed] [Google Scholar]
- 8.Ohtsuka M, Kimura F, Shimizu H, et al. Significance of repeated resection for recurrent intrahepatic cholangiocarcinoma. Hepatogastroenterology 2009;56:1-5 [PubMed] [Google Scholar]
- 9.Casaril A, Abu Hilal M, Harb A, et al. The safety of radiofrequency thermal ablation in the treatment of liver malignancies. Eur J Surg Oncol 2008;34:668-72 [DOI] [PubMed] [Google Scholar]
- 10.Choi D, Lim HK, Kim MJ, et al. Recurrent hepatocellular carcinoma: percutaneous radiofrequency ablation after hepatectomy. Radiology 2004;230:135-41 [DOI] [PubMed] [Google Scholar]
- 11.Yang W, Chen MH, Yin SS, et al. Radiofrequency ablation of recurrent hepatocellular carcinoma after hepatectomy: therapeutic efficacy on early- and late-phase recurrence. AJR Am J Roentgenol 2006;186:S275-83 [DOI] [PubMed] [Google Scholar]
- 12.Edmondson HA, Steiner PE. Primary carcinoma of the liver. A study of 100 cases among 48,900 necropsies. Cancer 1954;7:462-503 [DOI] [PubMed] [Google Scholar]
- 13.Chen MH, Yang W, Yan K, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients. Radiology 2004;232:260-71 [DOI] [PubMed] [Google Scholar]
- 14.Chen MH, Yang W, Yan K, et al. Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging 2008;33:428-36 [DOI] [PubMed] [Google Scholar]
- 15.Frericks BB, Ritz JP, Roggan A, et al. Multipolar radiofrequency ablation of hepatic tumors: initial experience. Radiology 2005;237:1056-62 [DOI] [PubMed] [Google Scholar]
- 16.Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009;20 (Suppl):S377-90 [DOI] [PubMed] [Google Scholar]
- 17.Zgodzinski W, Espat NJ. Radiofrequency ablation for incidentally identified primary intrahepatic cholangiocarcinoma. World J Gastroenterol 2005;11:5239-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou FF, Sheen-Chen SM, Chen YS, et al. Surgical treatment of cholangiocarcinoma. Hepatogastroenterology 1997;44:760-5 [PubMed] [Google Scholar]
- 19.Gerhards MF, van Gulik TM, González González D, et al. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg 2003;27:173-9 [DOI] [PubMed] [Google Scholar]
- 20.Serafini FM, Sachs D, Bloomston M, et al. Location, not staging, of cholangiocarcinoma determines the role for adjuvant chemoradiation therapy. Am Surg 2001;67:839-43 [PubMed] [Google Scholar]
- 21.Pitt HA, Nakeeb A, Abrams RA, et al. Perihilar cholangiocarcinoma. Postoperative radiotherapy does not improve survival. Ann Surg 1995;221:788-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yonemoto N, Furuse J, Okusaka T, et al. A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol 2007;37:843-51 [DOI] [PubMed] [Google Scholar]
- 23.Thongprasert S.The role of chemotherapy in cholangiocarcinoma. Ann Oncol 2005;16 (Suppl 2):ii93-6 [DOI] [PubMed] [Google Scholar]
- 24.Glimelius B, Hoffman K, Sjödén PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 1996;7:593-600 [DOI] [PubMed] [Google Scholar]
- 25.Okusaka T, Ishii H, Funakoshi A, et al. Phase II study of single-agent gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 2006;57:647-53 [DOI] [PubMed] [Google Scholar]
- 26.Kornek GV, Schuell B, Laengle F, et al. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomized phase II trial. Ann Oncol 2004;15:478-83 [DOI] [PubMed] [Google Scholar]
- 27.Herber S, Otto G, Schneider J, et al. Transarterial Chemoembolization (TACE) for Inoperable Intrahepatic Cholangiocarcinoma. Cardiovasc Intervent Radiol 2007;30:1156-65 [DOI] [PubMed] [Google Scholar]
- 28.Vogl TJ, Schwarz W, Eichler K, et al. Hepatic intraarterial chemotherapy with gemcitabine in patients with unresectable cholangiocarcinomas and liver metastases of pancreatic cancer: a clinical study on maximum tolerable dose and treatment efficacy. J Cancer Res Clin Oncol 2006;132:745-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burger I, Hong K, Schulick R, et al. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol 2005;16:353-61 [DOI] [PubMed] [Google Scholar]
- 30.Slakey DP. Radiofrequency ablation of recurrent cholangiocarcinoma. Am Surg 2002;68:395-7 [PubMed] [Google Scholar]
- 31.Oshima S, Takaishi K, Kurokawa E, et al. A case of successful management of recurrent intrahepatic cholangiocarcinoma by repeated radiofrequency ablations. Gan To Kagaku Ryoho (in Japanese)2009;36:2404-6 [PubMed] [Google Scholar]
- 32.Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur J Radiol 2011;80:e221-5 [DOI] [PubMed] [Google Scholar]
- 33.Chiou YY, Hwang JI, Chou YH, et al. Percutaneous ultrasound-guided radiofrequency ablation of intrahepatic cholangiocarcinoma. Kaohsiung J Med Sci 2005;21:304-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poon RT, Ng KK, Lam CM, et al. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg 2004;239:441-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choy PY, Koea J, McCall J, et al. The role of radiofrequency ablation in the treatment of primary and metastatic tumours of the liver: initial lessons learned. N Z Med J 2002;115:U128. [PubMed] [Google Scholar]
- 36.Mulier S, Mulier P, Ni Y, et al. Complications of radiofrequency coagulation of liver tumors. Br J Surg 2002;89:1206-22 [DOI] [PubMed] [Google Scholar]
- 37.Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology 2009;251:933-40 [DOI] [PubMed] [Google Scholar]