Abstract
Objective
Toevaluate the role of class III β-tubulin (TUBB3), thymidylate synthase (TS), thymidine phosphorylase (TP), and excision repair cross-complementing group 1 (ERCC1) in clinical outcome of advanced gastric cancer patients receiving capecitabine plus paclitaxel or cisplatin.
Methods
The clinical data and tumor specimens from 57 advanced gastric cancer patients receiving first-line capecitabine plus paclitaxel (cohort 1, n=36) and capecitabine plus cisplatin (cohort 2, n=21) were retrospectively collected, and TUBB3, TS, TP, and ERCC1 expressions were detected by real-time quantitative PCR. The associations between expressions of biomarkers and response or survival were analyzed statistically.
Results
The median age of 57 patients was 57 years (range: 27–75 years) with 38 males and 19 females. Of all patients, the response rates of patients with high TP, low TP and high TS, low TS expressions were 57.1%, 27.6% (P=0.024), and 55.2%, 28.6% (P=0.042), respectively. Among cohort 1, the response rates and median overall survivals of patients with low and high TUBB3 expressions were 61.1% vs. 33.3% (P=0.095) and 13.8 months vs. 6.6 months (P=0.019), respectively; the response rate (87.5%) of patients with low TUBB3 and high TP expressions was higher than that (14.3%) of patients with high TUBB3 and low TP expressions (P=0.01). Among cohort 2, the response rates of patients with low ERCC1 and high ERCC1 expressions were 45.5% and 20.0% respectively (P=0.361).
Conclusion
TUBB3, TS and TP expressions could predict the response of advanced gastric cancer patients receiving capecitabine-based and paclitaxel-based chemotherapy. These results will be further confirmed in future large samples.
Key words: Advanced gastric cancer, TS/TP/TUBB3/ERCC1, Capecitabine, Paclitaxel, Cisplatin
INTRODUCTION
Capecitabine combined with cisplatin or paclitaxel has been an effective combination regimen used in patients with advanced gastric cancer. These regimens have increased response rate to over 40%, but have not prolonged the median overall survival time. Early studies showed that more than half of gastric cancer patients do not benefit from capecitabine-based chemotherapy[1-4]. Therefore, a great of effort has been made to identify patients who are most likely to benefit from these regimens.
A growing body of evidence suggests that the intratumor gene expression of drug-metabolizing enzymes, DNA repair enzymes, or angiogenic enzymes may have important implications for anticancer drug efficacy[5-7]. Both thymidylate synthase (TS) andthymidine phosphorylase (TP) are two key enzymes involved in metabolic pathway offluoropyrimdines. As the target of taxanes, class III β-tubulin (TUBB3) over- expression may have clinical relevance in the efficacy of taxanes therapy. The product of excisionrepair cross-complementing group 1 (ERCC1) gene is an essential member of the nucleotide excision repair (NER) pathway which is involved in the repair of DNA double-strand breaks. ERCC1gene expression may have associations with clinical outcome to platinum-basedchemotherapy. Therefore, these biomarkers may be potential candidates for predicting chemotherapy efficacy in gastric cancer patients treated with capepiciabine andpaclitaxel or cisplatin.
So far, no standard chemotherapy regimen has been established for advanced gastric cancer treatment. Thus, it is critical to identify biomarker(s) associated with clinical outcomes that may improve treatment success and tailor the chemotherapy regimen. This study was designed to identify potential predictors that may influence response to chemotherapy and survival in patients with advanced gastric cancer.
MATERIALS AND METHODS
Patients Eligibility Criteria
All patients in this study had histologically confirmed metastatic adenocarcinoma of stomach and at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST). These patients were treated by capecitabine/paclitaxel or capecitabine/cisplatin chemotherapy in the Department of Gastrointestinal Oncology, BeijingCancerHospital and had completed at least two cycles of study treatment. No patients with prior chemotherapy except for neoadjuvant or adjuvant chemotherapy treatment completed 12 months before the study were enrolled. All patients underwent endoscopic biopsy from primary stomach or vacuum-associated core needle biopsy from metastatic lesion before chemotherapy. The tissue samples were collected retrospectively from patients who met these criteria. Chemotherapeutic response was clinically evaluated by measuring the change in tumor size according to the standard RECISE as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). CR and PR were defined as responders. Stable disease and progressive disease were defined as nonresponders. The time to progression (TTP) and overall survival (OS) were calculated from the date therapy was started, to the date of disease progression and death, respectively. Written informed consent was obtained before treatment and evaluation of tumor samples. The use of all patient material was approved by our institutional review board.
Treatment Protocols
The following first-line chemotherapy regimens were administered to the patients in our study: capecitabine plus paclitaxel or capecitabine plus cisplatin; capecitabine was orally administered at a dosage of 1,000 mg/m2 twice daily from the evening of day 1 until the morning of day 15 within each 3 weeks cycle. Paclitaxel was given at a dosage of 80 mg/m2 as a 180-min iv infusion on days 1 and 8 of each cycle. Cisplatin was given at a dosage of 80 mg/m2 as a 120-minute iv infusion on day 1 of each cycle. Treatment was continued until disease progression, unacceptable toxicity, or patient/physician decision.
RNA Isolation and cDNA Synthesis
Total RNA from tumor specimens was extracted from formalin-fixed, paraffin-embedded tissues using 10-μm sections. For histologic diagnosis, representative sections were stained with haematoxylin and eosin by standard methods. Macroscopic dissection was used to ensure the extraction of RNA from defined areas with at least 60% tumor cells. Total RNA was extracted using HighPure RNA Paraffin Kit (Roche Diagnostics GmbH, Mannheim, Germany) in accordance withthe supplied protocol. Total RNA were reversetranscribed for single-strandcDNA using Exscript™ RT reagent Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacture's protocol (Applied Biosystems). Reverse transcription reaction was performed at 25°C for 10 min, 37°C for 120 min, followed by heating at 85°C for 5 min.
Reverse Transcription-PCR Quantification of mRNA
The relative quantitation of cDNA for TP, TS, TUBB3, ERCC1 and an internal reference gene (β-actin) was done using a fluorescence-based, real-time detection method (Rothe Sequence Detection System; SYBR GREEN I; Applied Biosystems), according to the supplier’s instructions. The primer sequences are shown in Table 1.
Table 1. Sequences of primers.
| Forward | Reverse | ||
|---|---|---|---|
| TP | 5’-TGGCTGCAAGGTGCCAATG-3’ | 5’-AGCACTTGCATCTGCTCTGG-3’ | |
| TS | 5’-GCCTCGGTGTGCCTTTCA-3’ | 5’-CCCGTGATGTGCGCAAT-3’ | |
| TUBB3 | 5’-GGCCAAGTTCTGGGAAGTCAT-3’ | 5’-GAGGCCTCGTTGTAGTAGACG-3’ | |
| ERCC1 | 5’-GGGAATTTGGCGACTGTAATTC-3’ | 5’-GCGGAGGCTGAGGAACAG-3’ | |
| β-actin | 5’-TGAGCGCGGCTACAGCTT-3’ | 5’-TCCTTAATGTCACGCACGATTT-3’ |
The PCR reaction mixture consisted of 600 nmol/L each primer, 2.5 U AmpliTaq Gold polymerase, 200 nmol/L each dATP, dCTP, dGTP and 400 μmol/L dUTP, 5.5 mmol/L MgCl2, and 1 μlTaqman Buffer A containing a reference dye, to a final volume of 25 μl (all reagents were purchased from Applied Biosystems). Cycling conditions were 50°C for 2 min, 95°C for 10 min, followed by 46 cycles at 95°C for 15 s and 60°C for 1 min.
Relative gene expression quantification was calculated according to the comparative Ct method by using β-actin as an endogenous control. Final results were determined by the formula 2-∆∆Ct and analyzed with the statistic analysis software.
Statistical Analysis
Based on TS, TP, TUBB3 and ERCC1 mRNA expression levels, we used the median value as a cutoff point to divide patients into two groups (high vs. low) for each gene. Thus, all subgroups would have a similar number of subjects. The relationship between TS, TP, TUBB3, ERCC1 mRNA expression levels and response to capecitabine/paclitaxel or capecitabine/cisplatin was analyzed by χ2 test and Fisher’s exact test. The association of each marker with OS was examined using Kaplan-Meier plots, the log-rank test, and its associated 95% confidence interval (95% CI) was calculated. Logistic regression analysis was used to assess the association between the tumor response and the biomarker parameters. All significant factors by univariate analysis were included in the Cox’s proportional hazards model in multivariate analysis to identify independent factors influencing survival. A value of P<0.05 was considered statistically significant. Statistical analyses were done using SPSS 13.0 (SPSS Inc, Chicago, Illinois, USA).
RESULTS
Patient Characteristics
Fifty-seven patients were recruited into this study between July 2003 and July 2008. Median age of the patients was 57 years (range 27-75years). Thirty-six patients were treated by paclitaxel and capecitabine chemotherapy (cohort 1). Twenty-one patients were treated with cisplatin and capecitabine chemotherapy (cohort 2). The characteristics of the 57 studied patients are presented in Table 2. The majority of patients showed Karnofsky performance score (KPS) ranging from 90 to 100, and metastatic disease to the liver and lymph nodes.
Table 2. Basic characteristics of the patients.
| Characteristic | Cohort 1 (n=36) |
Cohort 2 (n=21) |
Total (n=57) |
|||
|---|---|---|---|---|---|---|
| No. of patients % | No. of patients % | No. of patients % | ||||
| Sex | ||||||
| Male | 21 | 58.3 | 17 | 81 | 38 | 66.7 |
| Female | 15 | 41.7 | 4 | 19 | 19 | 33.3 |
| Age, years | ||||||
| Median | 57 | 57 | 57 | |||
| Range | 27-74 | 42-75 | 27-75 | |||
| KPS | ||||||
| 90−100 | 25 | 69.4 | 15 | 71.4 | 40 | 70.2 |
| 70−80 | 11 | 30.6 | 6 | 28.6 | 17 | 29.8 |
| Metastatic disease | ||||||
| Liver | 13 | 36.1 | 6 | 28.6 | 19 | 33.3 |
| Lung | 2 | 5.6 | 4 | 19.0 | 6 | 10.5 |
| Lymph nodes | 35 | 97.2 | 20 | 95.2 | 55 | 96.5 |
| Peritoneum | 7 | 19.4 | 4 | 19.0 | 11 | 19.3 |
| Other | 11 | 30.6 | 5 | 23.8 | 16 | 28.1 |
| Tumor marker elevated histotype | 22 | 61.1 | 10 | 47.6 | 32 | 56.1 |
| Poor | 26 | 72.2 | 14 | 66.7 | 40 | 70.2 |
| Good | 10 | 27.8 | 7 | 33.3 | 17 | 29.8 |
Response to Treatment
Thirty-five patients had died at the time of analysis. The overall response rate in the 57 patients who were evaluated was 42.1%, with 24 patients with PR (42.1%), 17 patients with SD (29.8%), and 16 patients with PD (28.1%). The median TTP of the entire cohort was 4.1 months, and the median OS was 9.2 months. Treatment response and survival estimates were similar for patients in cohorts 1 and 2 (Table 3). The objective response rate was 41.7% (pls check the number) in cohort 1, and 33.3% in cohort 2. The median number of treatment cycles was 4 (2–8) in both cohorts.
Table 3. Analysis of response and survival of patients with gastric cancer receiving capecitabine plus paclitaxel (cohort 1) or cisplatin (cohort 2).
| Outcome | Cohort 1 (n=36) |
Cohort 2 (n=21) |
Total (n=57) |
||||
|---|---|---|---|---|---|---|---|
| No. of patients % | No. of patients % | No. of patients % | |||||
| Response | |||||||
| Partial response | 17 | 47.2 | 7 | 33.3 | 24 | 42.1 | |
| Stable disease | 10 | 27.8 | 7 | 33.3 | 17 | 29.8 | |
| Progression disease | 9 | 25.0 | 7 | 33.3 | 16 | 28.1 | |
| Overall response rate | 15 | 47.2 | 7 | 33.3 | 22 | 42.1 | |
| Median PFS, months | 4.5 | 3.9 | 4.1 | ||||
| 95% CI | 1.5-7.5 | 1.9-6.0 | 2.2-6.0 | ||||
| Median OS, months | 8.5 | 9.2 | 9.2 | ||||
| 95% CI | 6.3-10.7 | 4.2-14.2 | 7.1-11.3 | ||||
Correlation of TP and TS mRNA Expressions to Clinical Outcome of Capecitabine Therapy
The mRNA expressions of TS and TP were detectable in all 57 samples. The median TS expression, relative to the housekeeping gene β-actin, was 0.57 (minimum expression, 0.00; maximum expression, 6.5). The median TP gene expression was 1.24 (minimum, 0.00; maximum, 39.7). The association between the mRNA expression of TP, TS and response to capecitabine-based chemotherapy is listed in Table 4. Response rate was 55.2% among patients with low TS expression compared with 28.6% anong patients with high TS expression (P=0.042). Response rate was 57.1% for the high TP expression group compared with 27.6% for the low TP expression group (P=0.024). Additional progression-free survival (PFS) and OS were analyzed, and a similar trend was observed. However, this was not statistically significant.
Table 4. Analysis of response and survival of patients with gastric cancer accepted capecitabine therapy: correlation with TP and TS mRNA expression (univariate analysis).
| Biomarker | RR% | P | PFS (month) | P | OS (month) | P |
|---|---|---|---|---|---|---|
| TS≤5.7 | 16/29 (55.2%) | 5.9 | 9.7 | |||
| TS>5.7 | 8/28 (28.6%) | 0.042 | 3.2 | 0.617 | 7.3 | 0.257 |
| TP≤1.24 | 8/28 (27.6%) | 3.5 | 6.6 | |||
| TP>1.24 | 16/28 (57.1%) | 0.024 | 5.9 | 0.469 | 11.6 | 0.204 |
| TS≤5.7 | 11/19 (57.9%) | 5.9 | 13.8 | |||
| TP>1.24 | ||||||
| TS≤5.7 | 5/10 (50%) | 0.714 | 3.9 | 0.290 | 9.7 | 0.245 |
| TP≤1.24 | ||||||
| TS>5.7 | 3/19 (15.8%) | 0.007 | 3.0 | 0.498 | 6.6 | 0.054 |
| TP≤1.24 | ||||||
| TS>5.7 | 5/9 (55.6%) | 1.0 | 4.0 | 0.482 | 9.4 | 0.535 |
| TP>1.24 |
When TP and TS gene expressions were combination-analyzed, the response rate was 57.9% for the low TS and high TP subgroup and 15.8% for the high TS and low TPsubgroup (P=0.007) (Table 4). Median survival time was 13.8 months (95% CI, 4.4 to 23.3 months) for thelow TSand high TPsubgroup and 6.6 months (95% CI, 2.8 to 10.3 months) for the high TS and low TPsubgroup (P=0.054) (Figure 1).
Figure 1.
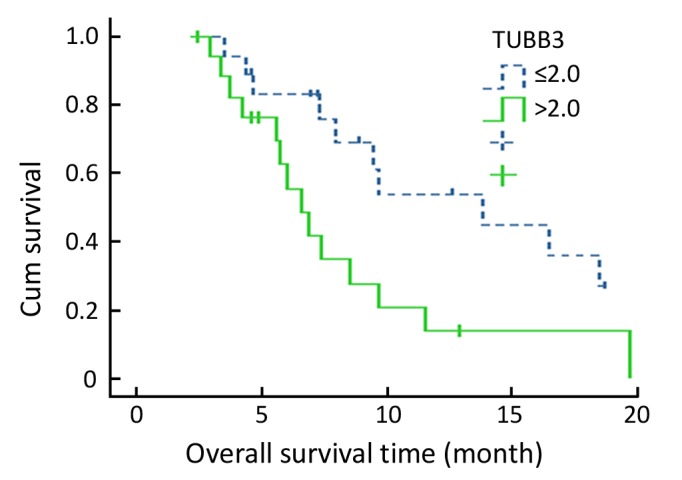
Kaplan-Meier survival curve for low TS and high TP expressors and high TS and low TP expressors.
Predictive Value of TUBB3 mRNA Level for Paclitaxel Chemotherapy
Thirty-six patients received capecitabine combined with paclitaxel chemotherapy. The median TUBB3 gene expression was 2.0 (minimum, 0.00; maximum, 13.45). Using the median value as a cutoff point, we divided treated patients into two groups (high vs. low). Response rates in the high expression group and low expression group were 61.1% and 33.3%, respectively (P=0.095). Five clinicopathological variables (sex, age, KPS, grading and elevated tumor maker) and TP expression were also examined for their association with treatment response. Multi-variable analysis showed that only the levels of TUBB3 and TP expression were independent variables to chemotherapy response (Table 5).
Table 5. Univariable and multivariable analysis results of relationship between TP, TUBB3 expressions and tumor response to chemotherapy with capecitabline plus paclitaxel.
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| RR | P | OR | P | |
| TP mRNA expression | ||||
| ≤1.24 | 29.4% | 0.043 | 0.023 | |
| >1.24 | 63.2% | 0.135 | ||
| TUBB3 expression | ||||
| ≤2 | 61.1% | 0.095 | 0.166 | 0.042 |
| >2 | 33.3% | |||
When survival time was analyzed, median PFS for low and high TUBB3 groups were 7.9 months and 3.2 months (P =0.019), respectively. The OS for each group were 13.8 months and 6.6 months, respectively (P=0.019. Figure 2). When TP and TUBB3 expressions were analyzed in combination, the response rate for low TUBB3 and high TP subgroup was 87.5%, which was higher than any other subgroup. The lowest response rate was 14.3% found in patients with high TUBB3 and low TP expression level (Table 6).
Figure 2.
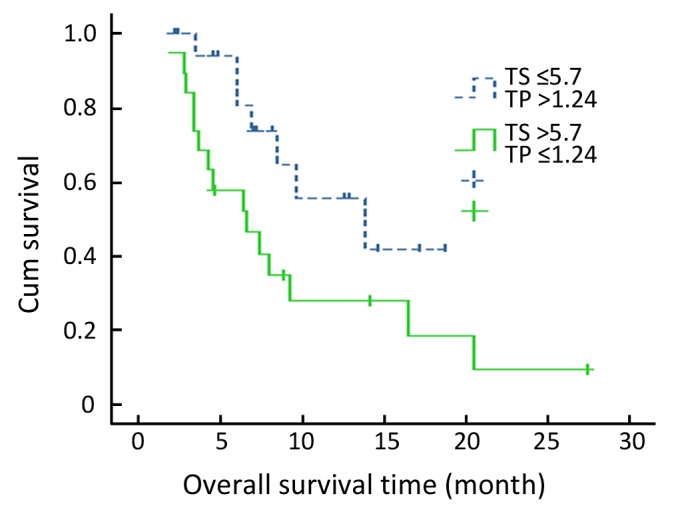
Kaplan-Meier survival curve for patients received paclitaxel chemotherapy with different TUBB3 expression levels.
Table 6. Tumor response to capecitabline plus pacilitaxel treatment depending on TP and TUBB3 mRNA expression.
| Tumor respond to chemotherapy |
||||
|---|---|---|---|---|
| PR | SD+PD | RR | P value | |
| TP and TUBB3 expression | ||||
| TP>1.24 | 7 | 1 | 87.5% | |
| TUBB3≤2 | ||||
| TP>1.24 | 5 | 6 | 45.5% | 0.147 |
| TUBB3>2 | ||||
| TP≤1.24 | 4 | 6 | 40.0% | 0.066 |
| TUBB3≤2 | ||||
| TP≤1.24 | 1 | 6 | 14.3% | 0.01 |
| TUBB3>2 | ||||
Among patents with low TUBB3 levels, paclitaxel treatment was more favorable than cisplatin, although this was not statistically significant (P=0.082) (Table 7).
Table 7. Association between TUBB3 and response to different chemotherapy regimen.
| Therapy | RR% | P | OS (month) | P | PFS (month) | P | |
|---|---|---|---|---|---|---|---|
| TUBB3≤2 | Paclitaxel | 11/18(61.1%) | 7.9 | 13.8 | |||
| Ciplatin | 5/16(31.3%) | 0.196 | 2.9 | 0.196 | 9.2 | 0.324 | |
| TUBB3>2 | Paclitaxel | 6/18(33.3%) | 3.2 | 6.6 | |||
| Cisplatin | 2/5(40%) | 1.0 | 6.3 | 0.509 | 0.469 |
Predictive Value of ERCC1 mRNA level for Cisplatin Chemotherapy
Twenty-one of all the enrolled patients received capecitabine combined with cisplatin chemotherapy. The median ERCC1 expression was 3.25 (minimum, 0.35; maximum, 9.45) for these patients. Using the median value as the cutoff point, we divided patients into two groups (high and low). The response rate was 45.5% among low ERCC1 group and 20.0% among high ERCC1 group (P=0.361). Median PFS among low ERCC1 group was 5.9 months (95% CI, 1.4-10.4 months) compared with 2.1 months (95% CI, 1.1−4.6 months) among high ERCC1 group (P=0.516).
DISCUSSION
In this study, we analysed mRNA expression levels of TP and TS in primary tumors from 57 patients with advanced gastric cancer. Our objective was to determine whether these genes were closely related to treatment outcomes. We found that low TP expression and high TS expression in gastric cancer specimens were linked to poor outcome in patients receiving capecitabine-based regimen. In contrast, the subgroup of patients with low TS and high TP expression had the best treatment response. Patients with high TS and low TP expression had poorest response rate (15.8% vs. 57.9%) and overall survival (6.6 vs. 13.8 months) compared to the patients with low TS/high TP expression. These data show that the combination of TS and TP might add more predictive power than each gene expression alone.
We also studied the correlation between expression of TS and TP and the efficacy of capecitabine combined with different cytotoxic agents (paclitaxel or cisplatin). We found that the expression of TS and TP was clearly associated with a better tumor response. The results confirm the value of TS and TP as predictive markers when either used alone or in combination in patients with metastatic gastric cancer. Previous studies have shown the association between TS/TP expression and efficacy of chemotherapy with capecitabine in solid tumor. However, there is still no consensus. Some investigators reported a better outcome for patients with low TS expression or high TP expression treated with capecitabine[8-10]. In contrast, different investigations have not demonstrated a significant association between TP or TS expression and clinical outcome[11,12]. Besides the function of TP in 5-FU phosphorylation, TP (which is identical to platelet-derived endothelial cell growth factor) has been shown to play crucial roles in cancer angiogenesis, invasiveness, metastasis, and antiapoptoticeffects[13]. Some studies have reported that tumors with high TP expression levels were associated with poorer outcomes in gastric cancer patients who received 5-FU- based chemotherapy[14,15]. These data support that TP expression is a negative predictor of treatment with 5-FU in gastric cancer. So the TP expression might have different effects on capecitabine-treated and 5-FU treated patients. If our observations were validated, the evaluation of TP expression would be a prerequisite step before choosing either 5-FU-based or capecitabine-based regimen. Therefore, large scale studies are needed to examine the relationship between TP/TS mRNA expression levels and clinical outcomes.
We also analyzed mRNA expression levels of TP, TS and TUBB3 in primary tumors from 36 patients who received the treatment of capecitabinecombinated with paclitaxel.Our data demonstrated a significant association between TUBB3 expression levels and survival or PFS in gastric cancer. Patients with low TUBB3 expression had a higher response rate and a longer survival time while receiving capecitabine plus paclitaxel therapy. Additional analysis on PFS and OS showed that the predictive value of TUBB3 mRNA expression levels was better than that of TP and TS. Our result suggests that TUBB3 was likely to be a predictive marker for resistance to antitubulin agents. Our finding is consistent with previous studies that assessed the prognostic or predictive value of TUBB3 expression in patients with advanced lung, ovarian, breast, and those with cancers of unknown primary sites[16-20]. A small cohort study of 20 patients with advanced gastric cancer who received preoperative docetaxel-based chemo- therapy also showed a correlation between expression of TUBB3 (as determined by immunohistochemistry) and response to chemotherapy[21]. These results showed that expression of TUBB3 is associated either with resistance to taxane agents, with poor prognosis, or both. But the investigation on TUBB3 expression and survival or TTP in gastric cancer is scant. Thus, to address the question of the predictive or prognostic value of TUBB3, further clinical trials to compare gastric patients treated with and without taxane are needed.
The capecitabine/cisplatin regimen is another effective option for advanced gastric cancer patients. Cisplatine/DNA adducts are repaired by the NER system. ERCC1 has been found to be essential for this repair process. ERCC1 and TS messenger RNA (mRNA) levels have been reported to predict the response rate and survival of gastric cancer patients on a combination of cisplatin and fluorouracil chemotherapy[22]. So in our study, we evaluated the values of TP, TS and ERCC1 in twenty-one gastric cancer patients given capecitabine and cisplatin regimens as first-line chemotherapy. ERCC1 levels remain important, as indicated by its association with survival and a trend toward a lower response rate in patients with high ERCC1 mRNA levels. This result was consistent with previous studies in patients with gastric cancer receiving platinum-based chemotherapy[23,24]. Although several retrospective studies have reported that the expression of ERCC1 is related to the outcomes of platinum-based chemotherapy, prospective study is needed to confirm predictave value of ERCC1.
In conclusion, the present study provides evidence that low TS and high TP gene expression levels in gastric cancer specimens are risk factors for poor outcomes in gastric cancer patients receiving capecitabine-based chemotherapy. In addition, TUBB3 expression level is a potential predictive marker for the efficacy of panclitaxel-based chemotherapy. However, our conclusions are drawn from a limited retrospective study with relatively small sample size. Further large-scale clinical studies are needed to precisely determine the association between candidate biomarkers and the efficacy of chemotherapy in patients with advanced gastric cancer.
REFERENCES
- 1.Kang HJ, Chang HM, Kim TW, et al. A phase II study of paclitaxel and capecitabine as a first-line combination chemotherapy for advanced gastric cancer. Br J Cancer 2008;98:316-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim TW, Kang YK, Ahn JH, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced gastric cancer. Ann Oncol 2002;13:1893-8 [DOI] [PubMed] [Google Scholar]
- 3.M Jin, L Shen, B Hu, et al.Mature data on capecitabine (X) + fractionated cisplatin (P) as first-line therapy in patients (pts) with advanced gastric carcinoma (AGC).J ClinOncol 24:2006 (June 20 suppl;abstract4075).
- 4.Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 2009;20:666-73 [DOI] [PubMed] [Google Scholar]
- 5.Marsh S, McLeod HL. Cancer pharmacogenetics. Br J Cancer 2004;90:8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park DJ, Stoehlmacher J, Lenz HJ. Tailoring chemotherapy in advanced colorectal cancer. Curr Opin Pharmacol 2003;3:378-85 [DOI] [PubMed] [Google Scholar]
- 7.Ulrich CM, Robien K, McLeod HL. Cancer pharmacogenetics: polymorphisms, pathways and beyond. Nat Rev Cancer 2003;3:912-20 [DOI] [PubMed] [Google Scholar]
- 8.Andreetta C, Puppin C, Minisini A, et al. Thymidine phosphorylase expression and benefit from capecitabine in patients with advanced breast cancer. Ann Oncol 2009;20:265-71 [DOI] [PubMed] [Google Scholar]
- 9.Koizumi W, Okayasu I, Hyodo, et al. Prediction of the effect of capecitabine in gastric cancer by immunohistochemical staining of thymidine phosphorylase and dihydropyrimidinedehydro- genase. Anticancer Drugs 2008;19:819-24 [DOI] [PubMed] [Google Scholar]
- 10.Puglisi F, Cardellino GG, Crivellari D, et al. Thymidine phosphorylase expression is associated with time to progression in patient receiving low dose, docetaxel-modulated capecitabine for metastatic breast cancer. Ann Oncol 2008;19:1541-6 [DOI] [PubMed] [Google Scholar]
- 11.Layman RM, Thomas DG, Griffith KA, et al. Neoadjuvantdocetaxel and capecitabine and the use of thymidine phosphorylase as a predictive biomarker in breast cancer. Clin Cancer Res 2007;13:4092-7 [DOI] [PubMed] [Google Scholar]
- 12.Vallböhmer D, Yang DY, Kuramochi H, et al. DPD is a molecular determinant of capecitabine efficacy in colorectal cancer. Int J Oncol 2007;31:413-8 [PubMed] [Google Scholar]
- 13.Toi M, Atiqur Rahman M, Bando H, et al. Thymidine phosphorylase (platelet-derived endothelial-cell growth factor) in cancer biology and treatment. Lancet Oncol 2005;6:158-66 [DOI] [PubMed] [Google Scholar]
- 14.Napieralski R, Ott K, Kremer M, et al. Combined GADD45A and thymidine phosphorylase expression levels predict response and survival of neoadjuvant treated gastric cancer patients. Clin Cancer Res 2005;11:3025-31 [DOI] [PubMed] [Google Scholar]
- 15.Ichikawa W, Takahashi T, Suto K, et al. Gene expressions for thymidylate synthase (TS), orotatephosphoribosyltransferase (OPRT), and thymidine phosphorylase (TP), not dihydro- pyrimidine dehydrogenase (DPD), influence outcome of patients (pts) treated with S-1 for gastric cancer (GC). J ClinOncol 22:2004 (July 15 suppl;abstract4050).
- 16.Rosell R, Scagliotti G, Danenberg KD, et al. Transcripts in pretreatment biopsies from a three-arm randomized trial in metastatic non-small-cell lung cancer. Oncogene 2003;22:3548-53 [DOI] [PubMed] [Google Scholar]
- 17.Dumontet C, Isaac S, Souquet PJ, et al. Expression of class III beta tubulin in non-small cell lung cancer is correlated with resistance to taxane chemotherapy. Bull Cancer 2005;92:E25-30 [PubMed] [Google Scholar]
- 18.Mozzetti S, Ferlini C, Concolino P, et al. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res 2005;11:298-305 [PubMed] [Google Scholar]
- 19.Ferrandina G, Zannoni GF, Martinelli E, et al. Class III beta-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin Cancer Res 2006;12:2774-9 [DOI] [PubMed] [Google Scholar]
- 20.Bernard-Marty C, Treilleux I, Dumontet C, et al. Microtubule- associated parameters as predictive markers of docetaxel activity in advanced breast cancer patients: results of a pilot study. Clin Breast Cancer 2002;3:341-5 [DOI] [PubMed] [Google Scholar]
- 21.Urano N, Fujiwara Y, Doki Y, et al. Clinical significance of class III beta-tubulin expression and its predictive value for resistance to docetaxel-based chemotherapy in gastric cancer. Int J Oncol 2006;28:375-81 [PubMed] [Google Scholar]
- 22.Metzger R, Leichman CG, Danenberg KD, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol 1998;16:309-16 [DOI] [PubMed] [Google Scholar]
- 23.Wei J, Zou Z, Qian X, et al. ERCC1 mRNA levels and survival of advanced gastric cancer patients treated with a modified FOLFOX regimen. Br J Cancer 2008;98:1398-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon HC, Roh MS, Oh SY, et al. Prognostic value of expression of ERCC1, thymidylate synth- ase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann Oncol 2007;18:504-9 [DOI] [PubMed] [Google Scholar]


