Abstract
Objective
To observe the efficacy of the inhalation of an aerosolized group A streptococcal (GAS) preparation in treating orthotopic lung cancer in mouse models and assess the feasibility, safety, and effectiveness of this administration mode for lung cancer.
Methods
Lewis lung carcinoma (LLC) cell strains were administered via intrathoracic injection to establish orthotopic lung cancer mouse models. After the tumor-bearing models were successfully established, as confirmed by computed tomography, the mice were administered by inhalation with an aerosolized GAS preparation (GAS group) or aerosolized normal saline (control group). The anti-tumor effect of the aerosolized GAS preparation was evaluated histologically; meanwhile, the survival and quality of life were compared between these two groups.
Results
The aerosolized GAS preparation showed remarkably anti-tumor effect, causing the necrosis of the orthotopic lung cancer cells in tumor-bearing mice. Furthermore, mice in the GAS group had significantly better quality of life and longer survival than those in control group.
Conclusions
The inhalation of aerosolized GAS preparation may be a feasible, safe and effective solution for lung cancer.
Key Words: Group A streptococcal (GAS), lung cancer, Lewis lung carcinoma cell
Introduction
In 2012, more than 1.6 million new cancer cases and close to 0.6 million deaths (about 35% of new cases) from cancer are projected to occur in the United States. Lung cancer represents the most common cause of cancer-related mortality in the United States and around the world. Despite medical advances, lung cancer still accounts for more than 150,000 deaths annually in the United States (1). For patients with advanced lung cancer, even after treatment with the third-generation platinum-based chemotherapy, the median survival time can only be prolonged from 4-6 months (2-4), and the prognosis is even worse for pulmonary metastases. Obviously, such prognosis cannot be satisfactory for both clinicians and patients, and new ideas and new ways for the treatment of lung cancer should be actively explored (5,6).
OK432 [a Group A streptococcal (GAS) preparation] is a bacteria-derived biological response modifier (BRM). During its preparation, human group A hemolytic streptococci were treated with penicillin in the Berubeimer basic culture at 37 °C for 20 minutes and subsequently heat-treated at 45 °C for 30 minutes to yield low-virulence products (7). It can regulate the immune system by enhancing the activities of multiple immunocytes including natural killer (NK) cells and CD4+/CD8+ T cells (8). OK432 has been applied in clinical practices for over three decades and shown certain therapeutic effectiveness in controlling malignant effusions via serosal cavity injection (9). However, due to its side effects, the administration modes of OK432 only include intramuscular injection, subcutaneous injection, local injection directly into a tumor, and serosal cavity injection. In our current study, by establishing animal models of orthotopic lung cancer (10), we tried to observe the potential anti-tumor effect of the aerosolized OK432 (hereafter referred as a GAS preparation), which acts directly on lung tumors, and to evaluate the safety, effectiveness, and feasibility of this new administration mode in treating lung cancer.
Materials and methods
Cells and cell culture
The mouse Lewis lung carcinoma cell line was introduced from CAS Shanghai Institute of Cell Biology. It was cultured with DMEM medium containing 10% calf serum (Hyclone, USA) and grown in a 5% CO2 incubator at 37 °C and routinely sub-cultured. The cells in the exponential growth stage were made into single cell suspensions.
Animals
Fifty C57BL/6 mice, 4-5 weeks of age and weighing (18.8±1.53) g, were purchased from the Experimental Animal Center of Guangdong Province. They were raised in SPF environment at room temperature (25±2) °C and given aseptic full-price nutritional pellet feed and sterile water.
Methods
These 50 C57BL/6 mice were intraperitoneally injected with pentobarbital sodium (10 mg/kg) to induce anesthesia and fixed in the left lateral decubitus position after anesthesia. Then 100 µL Lewis single cell suspension (5×106 mL-1) prepared with the 1-mL injector was percutaneously inoculated into the upper margin of the sixth intercostal rib on the right anterior axillary line to a depth of about 5 mm rapidly and after that, the needle was promptly pulled out. The mice were maintained in the left lateral decubitus position after injection and observed until complete recovery.
Detection of survival time and gross observation
The survival status (body weight) and survival time were observed in the operated mice.
Spiral computed tomography (CT)
Spinal computed tomography (CT) was performed two weeks after the orthotopic implantation of tumor cells. Mice were fixed on a plane plate in a supine position following intraperitoneal injection of pentobarbital sodium (10 mg/kg) to induce anesthesia. The Toshiba Aquilion16-slice CT scanner was adopted to perform routine thin-slice plain CT scan from the mouse neck to abdomen, slice thickness 1 mm, reconstruction interval 0.5-0.8 mm, tube voltage 100 kV and tube current 90-110 mA.
Mice with CT-confirmed tumor cell implantation were divided into GAS group and control group (n=22 in each group)
In the GAS group, mice were put inside a 1000-mL glass container that was connected with a medical aerosolizer. The GAS preparation solution was dispensed with sterile water for injection to a concentration of 0.1 KE/mL, and then aerosolized continuously for 15 minutes. The procedure lasted 7 days. In the control group, mice were treated with equal volume of aerosolized normal saline.
Tissue embedding, pathological detection, and immunohistochemistry
After the aerosolization completed, two mice from each group were sacrificed by cutting off the neck under anesthesia to remove the intrathoracic heart, bilateral lungs, pleura, lymph nodes and mass etc, which were fixed in 10% formalin solution and embedded in paraffin to make into tissue sections, subjected to HE staining, and observed under a microscope. The remaining unstained tissue sections were stained using conventional S-P immunohistochemical method, with mouse CD3/CD4 as the primary and secondary antibodies; after having been added with diaminobenzidine (DAB) for color development and restained with hematoxylin, the sections were dehydrated, baked, and fixed.
Preparing of specimens for electron microscopy
The specimens for electron microscopy were prepared according to standard procedures: pre-fixation, post-fixation, dehydration, and embedding; tumor cells were located and selected under light microscope; ultra-thin sections (70-80 nm) were prepared by mechanical ultra-thin slicing. After have undergone double staining with uranyl acetate and lead citrate, the sections were observed and photoed under transmission electron microscope.
Statistical analysis
The median of survival time was calculated and the survival curves were drawn. The differences between these two groups were determined using Student t test. The survival was analyzed using Log Rank (Mantel-Cox) analysis, in which P<0.05 was regarded as statistically significant. Data were processed using SPSS 13.0 software package (SPSS Inc, Chicago, IL).
Results
The growth of orthotopically implanted tumor tissues
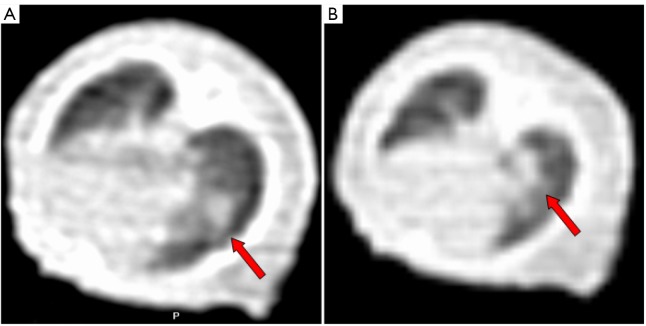
Intrathoracic implantation via puncture succeeded in all the 50 nude mice. Spiral CT scan was performed at day 7 and 14 on tumor-bearing nude mice after the intrathoracic injection of lung tumor cell suspension into the right chest for two consecutive weeks to observe intrathoracic tumor growth in mice dynamically. Figure 1 shows the results of spiral CT of the orthotopically implanted tumor of lung cancer for the same nude mouse. As shown by the CT findings in week 2, the intrathoracic tumor metastasis rate of nude mice was 88% (44/50).
Figure 1.
Spinal CT findings of a tumor-bearing nude mouse. A. Small right lung nodules two weeks after intrathoracic injection of tumor cell suspension; B. the gradual growth of intrathoracic nodules (arrow), which was confirmed as mediastinal metastasis by gross anatomy
Change of body weight and survival after injection
Figure 2 shows that the mean body weight gain stopped in both GAS group and control group following intrathoracic inoculation of tumor cells; after the initiation of nebulization 14 days later, the body weight declined in the control group but increased in GAS group (P=0.031).
Figure 2.
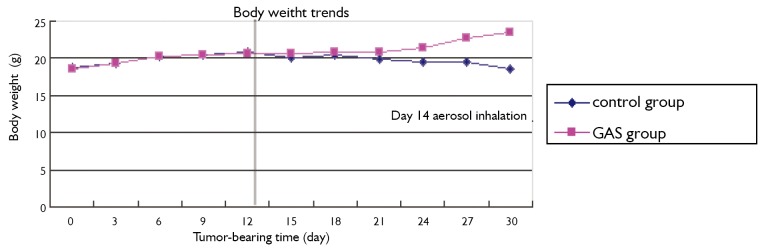
Weight trends of nude mice following intrathoracic inoculation of tumor cells
Overall survival
As shown in Figure 3, the survival time is (41.1±2.31) days in GAS group and (31.8±1.47) days in control group (P<0.001).
Figure 3.
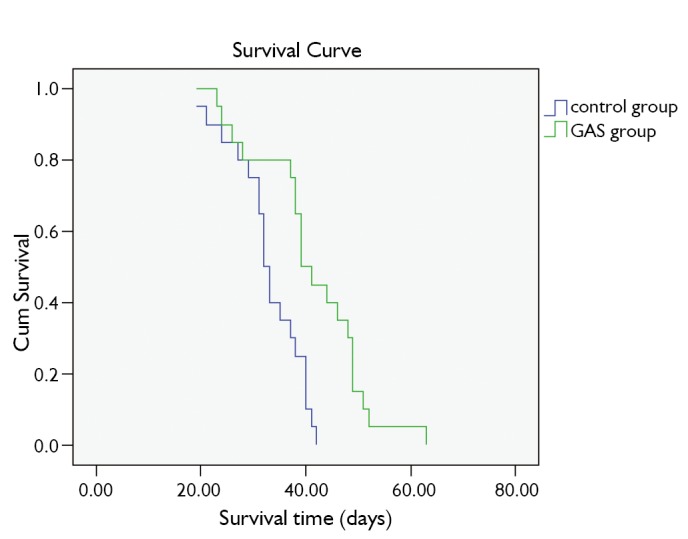
Survival curves of tumor-bearing nude mice in GAS group and control group
Histological findings
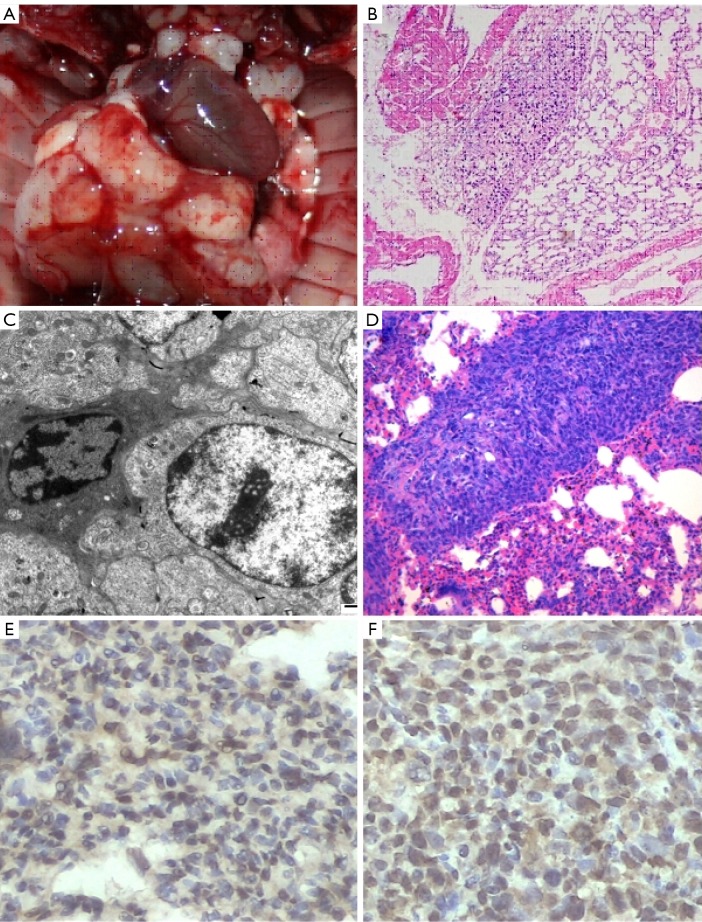
Figure 4A shows the image of the gross specimen of tumor-bearing nude mice, within which the right lung mass is clearly visible and there are visible metastatic foci in contralateral lung tissue, mediastinal tissue and chest wall. Figure 4B is the diagram for HE staining of histopathological sections of lung tissue in nude mice. The tumor tissue is located in the central region of the visual field, which developed accompanying alveolar tissue and blood vessels. The irregular tumor cell alignment, nuclear hyperchromatism, and obvious heteromorphism are visible after magnifying.
Figure 4.
A. Gross anatomy of the thoracic cavity of tumor-bearing nude mice; B. Microscopic features of the pathological sections of tumor developed within the mouse lung (HE staining); C. Pathological sections of lung tumor after treatment with aerosolized GAP preparation in nude mice (HE staining); D. Electron microscopy of lung tumor after treatment with aerosolized GAP preparation in nude mice; E. Staining for CD3 after treatment shows the aggregation of massive CD3-positive T lymphocytes (arrow) around the tumor cells; F. Staining for CD4 after treatment shows the aggregation of massive CD4-positive T lymphocytes (arrow) around the tumor cells
In the GAS group, marked necrosis of tumor cells was seen under transmission electron microscope: pale and swollen cytoplasm, swollen mitochondria, grey-white cellular matrix, rupture of cristae, and loss of nucleus. Figure 4C shows the features of early apoptotic granulocytes. In Figure 4D, massive necrosis of tumor cells is visible around alveoli, in which the aggregation of T lymphocytes and red blood cells is observed. Tumor cells with dense and deeply staining cytoplasm and marginal collection of heterochromatin are visible at the left and right sides. Staining for CD3/CD4 showed that there were massive CD3+/CD4+ T lymphocytes around the tumor cells.
Discussion
The past decades have witnessed a tremendous progress in lung cancer research. However, the increases in the effectiveness rate and survival rate remain slow, making lung cancer one of the most challenging malignancies worldwide (11). Multidisciplinary treatment of lung cancer, based on immuno-biological therapies in combination with other means, has appeared promising (12,13). Although the GAS preparations have been commonly used in clinical practices as BRMs and their efficacies have been well established, their administration modes only include intramuscular injection, subcutaneous injection, local injection directly into a tumor, and serosal cavity injection. Furthermore, their side effects after injection hamper their anti-tumor roles. For lung cancer, how to choose the most suitable route of administration for the application of immunomodulatory drugs has become a research priority. Currently the majority of anticancer drugs are administered orally or intravenously; however, such modes of administration cannot ensure that the drugs are concentrated in target cancer cells; furthermore, after having been metabolized in liver and kidney and circulated in blood, these drugs can have strong systemic effect and bring various side effects to the normal tissues. Therefore, how to improve the effectiveness of the administration mode has long been a research focus in oncology. The direct contact between the respiratory tract and environmental air, the rich capillary network in bronchiolar-alveolar regions, and the blood-gas diffusion/exchanges processes in lung provide a good physiological basis for aerosolized administration, which makes it possible to treat lung cancer with the aerosolized anti-tumor drugs. This administration mode allows the gathering of high-concentration aerosolized particles (about 5-mm in diameter) in the respiratory tract; these particles can directly deposit and act on the surface of respiratory tract, and thus increase the local drug concentration in the respiratory tract, reduce the hepatic degradation (if administered orally or intravenously), prevent systemic side effects, and ultimately improve the efficacy (14).
For the aerosolized medications for lung cancer and/or even pulmonary metastases, there have been may animal model-based studies and clinical reports, including the application of aerosolized diindolylmethane for the treatment of lung cancer in mouse models, application of aerosolized 5-fluorouracil for prolonging the survival in patients with advanced lung cancer, and even the application of cisplatin for patients with chemotherapy-refractory bronchial lung cancer or lung metastases (15-18). Anecdotal clinical observations have shown that aerosolized GAS preparations have shown certain efficacies in treating advanced lung cancer: it may help to improve immune functions, shrink tumors, improve mental status, and prolong survival; furthermore, although some patients suffered from fever after drug administration, other adverse events are rare. However, supporting data from animal experiments remain scarce. In our current study, we observed the efficacy of aerosolized GAS preparation in treating C57 mice orthotopically inoculated with Lewis lung cancer cells with an attempt to evaluate the feasibility, safety, and effectiveness of this mode for lung cancer.
During the establishment of animal models, lung carcinoma cell suspensions were administered via intrathoracic injection, which has been proven to be one of the simplest and most efficient ways for establishing orthotopic lung cancer mouse models (10). Also in our study, we used spinal CT to observe the growth of masses inside the lungs of tumor-bearing mice in a dynamic way; in fact, spinal CT is useful not only for confirming the success (or not) of tumor implantation inside chest but also for observing the in vivo growth of masses as time goes. Furthermore, spinal CT-based dynamic tracking/scanning also provides an objective and feasible way to detect the efficacies of anti-tumor drugs in animal experiments.
Based on these orthotopic lung cancer mouse models, the efficacy of aerosolized GAS preparation was studies, which allows us to control drug intake more conveniently, to analyze the tumor tissues in a more visible way, and to assess its anti-tumor efficacy from multiple perspectives. Studies have also shown that GAS preparations can exert their anti-tumor effectiveness by stimulating megakaryocytes, natural killer cells, and T lymphocytes [mainly T helper-1 (TH1) cells] (19,20). In our current experiment, after the administration of an aerosolized GAS preparation, obvious necrosis was seen in the orthotopic lung cancer tissues, and electron microscopy showed pale and swollen cytoplasm, swollen mitochondria, grey-white cellular matrix, rupture of cristae, and loss of nucleus. Meanwhile, the aggregation of T lymphocytes was also visible around the necrotic tumor cells. Immunohistochemistry showed that CD3+ and CD4+ T cells are the main lymphocytes around the tumor cells; the inhalation of aerosolized GAS preparation can reach the effective blood concentration around the tumor and thus exert anti-tumor effect via T lymphocytes.
The aerosolization time is also an important issue. Our pre-experiment had shown that more tumor-bearing nude mice died due to the tumor progression 3 weeks after tumor cell implantation; therefore, in our current experiment, the aerosolization was initiated 2 weeks after the inoculation of tumor cells and was performed for 7 consecutive days, so as to minimize bias related to the death of mice due to tumor progression during aerosolization. Analysis of survival showed that the GAS group had significantly superior survival time and body weight than the control group, which further confirmed the anti-tumor efficacy of the GAS preparation. Meanwhile, no mouse died during aerosolization, which further demonstrated the safety of the GAS preparation in that dosage.
In conclusion, as shown in the orthotopic lung cancer mouse model, the inhalation of aerosolized GAS preparation is feasible, safe, and effective for treating lung cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A.Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29 [DOI] [PubMed] [Google Scholar]
- 2.Voon PJ, Chul Cho B, Yeo WL, et al. The role of epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of advanced stage non-small cell lung cancer. J Thorac Dis 2010;2:144-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirker R, Filipits M.Cetuximab in non-small cell lung cancer. Transl Lung Cancer Res 2012;1:54-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li YQ, Shi AH, Li FH, et al. Phase I study to determine MTD of docetaxel and cisplatin with concurrent radiation therapy for stage III non-small cell lung cancer. Chin J Cancer Res 2011;23:129-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang P, Xu XY, Liu XJ, et al. The value of delayed 18F FDG-PET imaging in diagnosis of solitary pulmonary nodules: A preliminary study on 28 patients. Quant Imaging Med Surg 2011;1:31-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hennon MW, Demmy TL. Video-assisted thoracoscopic surgery (VATS) for locally advanced lung cancer. Ann Cardiothorac Surg 2012;1:37-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamoto H, Shoin S, Koshimura S, et al. Studies on the anticancer and streptolysin S-forming abilities of hemolytic streptococci. Jpn J Microbiol 1967;11:323-6 [DOI] [PubMed] [Google Scholar]
- 8.Hojo H, Hashimoto Y.Cytotoxic cells induced in tumor-bearing rats by a streptococcus preparation (OK-432). Gann 1981;72:692-9 [PubMed] [Google Scholar]
- 9.Luh KT, Yang PC, Kuo SH, et al. Comparison of OK-432 and mitomycin C pleurodesis for malignant pleural effusion caused by lung cancer. A randomized trial. Cancer 1992;69:674-9 [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Liu J, Guan Y, et al. Establishment of an orthotopic lung cancer model in nude mice and its evaluation by spiral CT. J Thorac Dis 2012;4:141-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winter H, van den Engel NK, Rusan M, et al. Active-specific immunotherapy for non-small cell lung cancer. J Thorac Dis 2011;3:105-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SH, Zhu Y, Ozden O, et al. SIRT2 is a tumor suppressor that connects aging, acetylome, cell cycle signaling, and carcinogenesis. Transl Cancer Res 2012;1:15-21 [PMC free article] [PubMed] [Google Scholar]
- 13.Murala S, Alli V, Kreisel D, et al. Current status of immunotherapy for the treatment of lung cancer. J Thorac Dis 2010;2:237-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol 2003;56:588-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichite N, Chougule M, Patel AR, et al. Inhalation delivery of a novel diindolylmethane derivative for the treatment of lung cancer. Mol Cancer Ther 2010;9:3003-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Chen HT, Roa W, et al. Farnesol for aerosol inhalation: nebulization and activity against human lung cancer cells. J Pharm Pharm Sci 2003;6:95-100 [PubMed] [Google Scholar]
- 17.Zhou C, Manegold C.Chemotherapy of lung cancer: A global perspective of the role of ifosfamide. Transl Lung Cancer Res 2012;1:61-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittgen BP, Kunst PW, van der Born K, et al. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin Cancer Res 2007;13:2414-21 [DOI] [PubMed] [Google Scholar]
- 19.Oshimi K, Kano S, Takaku F, et al. Augmentation of mouse natural killer cell activity by a streptococcal preparation, OK-432. J Natl Cancer Inst 1980;65:1265-9 [PubMed] [Google Scholar]
- 20.Misaki T, Watanabe Y, Iida Y, et al. Recruitment of T lymphocytes and induction of tumor necrosis factor in thyroid cancer by a local immunotherapy. Cancer Immunol Immunother 1992;35:92-6 [DOI] [PMC free article] [PubMed] [Google Scholar]