Abstract
Objective
To evaluate the efficacy and toxicity of the combination regimen of paclitaxel, cisplatin and 5-FU (PCF) as first-line or second-line therapy in patients with advanced gastric and esophagogastric junction (EGJ) adenocarcinoma in China.
Methods
The patients were treated with paclitaxel 150 mg/m2 on d1; fractionated cisplatin 15 mg/m2 and continuous infusion 5-FU 600 mg/(m2·d) intravenously on d1-d5 of a 21-d cycle until disease progression or unacceptable toxicities.
Results
Seventy-five patients have been enrolled, among which, 41 received PCF regimen as the first-line therapy (group A) and 34 received the regimen as the second-line therapy (group B) with the median age of 59 years old and Karnofsky performance status (KPS) score ≥80. Toxicities were analyzed in all 75 patients. Seventy-one patients were evaluable for efficacy. The median overall survival (mOS) was 12.0 months (95% CI: 7.9-16.2 months) in group A and 7.3 months (95% CI: 4.3-10.3 months) in group B, respectively. The median progression-free survival (mPFS) was 5.7 months (95% CI: 4.1-7.2 months) and 5.0 months (95% CI: 3.1-6.9 months), respectively. The response rate (CR+PR) was 40% (16/40; 95% CI: 24.9-56.7%) in group A and 22.6% (7/31; 95% CI: 9.6-41.1%) in group B. Major grade 3 or 4 adverse events include neutropenia (41.3%), febrile neutropenia (9.3%), nausea/anorexia (10.7%), and vomiting (5.3%). There was no treatment-related death.
Conclusions
The combination chemotherapy with PCF is active and tolerable as first-line and second-line therapy in Chinese patients with advanced gastric and EGJ adenocarcinoma. The response and survival of PCF are same as those of DCF, but the tolerance is much better.
Key Words: Advanced gastric cancer, esophagogastric junction (EGJ), adenocarcinoma, paclitaxel
Introduction
Advanced gastric cancer (AGC) and esophagogastric junction (GEJ) adenocarcinoma remain one of the most common causes of cancer death worldwide, especially in Asia. AGC patients have a poor prognosis (1-4). Incidence of gastric cancer in China is very high, and more than half of Chinese patients are in advanced stage at first diagnosis, with unresectable locally advanced or metastatic disease. Median survival of AGC is only 6-10 months. How to control the disease and improve the outcome of patients with local advanced or metastatic disease is still a major challenge (5,6). Taxanes (paclitaxel and docetaxel) have been studied in AGC as a single agent or combination (7-11). A large randomized phase III study (V325) showed increased efficacy with the combination of docetaxel, cisplatin and 5-FU (DCF). However, the toxicity profile of regimen (up to 82% of grade 3/4 hematological toxicity) made it hard to apply in clinic practice (11). It has also been noticed that Chinese patients tolerate to chemotherapy less well than Western populations (12,13). Domestic prospective multicenter studies are needed for Chinese AGC patients.
Since paclitaxel and docetaxel have similar anti-cancer activity in AGC with different toxicity profile, and fractionated cisplatin and infusional 5-FU may have less toxicity in the combination setting without compromising efficacy (13,14). We conducted this multicenter prospective study to investigate the effectiveness, tolerability and feasibility of the combination regimen of paclitaxel, cisplatin and 5-FU (PCF) in patients with AGC and EGJ as either first-line or second-line therapy. The study was supported by Gastric Cancer Specialty Committee of Chinese Anti-Cancer Association.
Materials and methods
Patients
Eligibility criteria of the patients had histologically confirmed advanced gastric or EGJ adenocarcinoma, including unresectable, metastatic or relapsed disease after initial resection. Eligibility criteria included at least one measurable lesion of longest diameter measured by spiral computer tomography (CT) or magnetic resonance imaging (MRI) ≥1 cm or by regular CT ≥2 cm and it should be imageologically diagnosed within 4 weeks before the inclusion day. Patients were ≥18 years old with life expectancy of at least 3 months, their Karnofsky performance status (KPS) score ≥80, and no other malignancy. Patients had adequate hematological, renal and hepatic function (within 7 d of enrollment to the study): hemoglobin ≥9.5 g/dL, white blood cell (WBC) count ≥4.0×109/L, neutrophil count ≥2.0×109 /L, and platelets ≥100.0×109/L. Total bilirubin ≤1.0× UNL, creatinine ≤1.0× UNL, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤2.5× UNL (AST and ALT ≤5× UNL in patients with hepatic metastasis), and normal electrocardiogram (ECG)/cardiac function. Patients in group A as first-line therapy included those who had disease relapsed and metastasized in more than 6 months after adjuvant chemotherapy and at least 4 weeks after the previous radiotherapy on untargeted lesions. The patients in group B as second-line therapy were those who had failed in their first-line non-taxane therapy.
Exclusion criteria included: (I) patients allergic to taxanes; (II) the maximum diameter of the tumor ≥10 cm, (III) hepatic metastasis area ≥50% of hepatic total area, (IV) pulmonary metastasis area ≥25% of pulmonary total area, (V) preexisting peripheral neuropathy, (VI) brain metastases or bony metastasis, and (VII) active infection and other serious underlying medical conditions that would impair the ability of the patient to receive the planned treatment. Participants signed informed consents before they entered the study, which was approved by the Ethical Committee of Peking University Cancer Hospital.
Chemotherapy protocol
Paclitaxel (AnzataxTM, Hospira Inc., Australia) 150 mg/m2 was administered intravenously for 3 h on d1 of the 21-d cycle (initial 22 patients had the dose of paclitaxel of 175 mg/m2, which then was modified to 150 mg/m2 based on toxicity, details in result session). Fractionated cisplatin of 15 mg/(m2·d) was infused intravenously over 1 h and continuous infusional 5-FU 600 mg/(m2·d) was given in d1-d5 of each cycle. The patients received standard intravenous hypersensitivity prophylaxis, including dexamethasone 10 mg, cimetidine 300 mg and diphenhydramine 40 mg (intramuscular injection) 30 min before administration of paclitaxel. Treatment continued until disease progression, unacceptable toxicity or consent withdrawal.
All patients received physical examination, full blood count and serum chemistry analyses. Chest X-ray, ECG, abdominal CT scan and/or MRI were also performed before entering the study.
Adverse effects and dose modification
Toxicity was reported by using a National Cancer Institute-Common Toxicity Criteria (NCI-CTC) version 2.0 (15) toxicity scales. If hematological toxicity ≥ grade 3 and peripheral neuropathy ≥ grade 2 occurred during the previous treatment, the dose of paclitaxel could be decreased by 20% based on the treating physicians’ description for the following treatment. For patients with grade 3 diarrhea lasting for more than 7 d despite the administration of loperamide, grade 3 mucositis lasting for more than 5 d or grade 4 mucositis, a 20% reduction in the daily dose of 5-FU was required. Chemotherapy of the next cycle could be started only after all the adverse effects recovered to grade 0-1 as judged according to NCI-CTG classification criteria. Dose escalation after dose reduction was not permitted. Granulocyte colony stimulating factor (G-CSF) was given if the neutropenia ≥ grade 3 after chemotherapy. If the toxicity level did not improve to grade 0-1 after 3 weeks, the treatment stopped.
Assessment and statistics
Response was evaluated every two cycles of treatment by using Response Evaluation Criteria in Solid Tumors (RECIST). All responses were evaluated by an independent review committee consisting of 2 medical oncologists and 1 radiologist. Of the lesions observed prior to treatment, a maximum of five measurable lesions from each metastasized organ up to a total of 10 lesions were selected as target lesions. In cases of partial or complete response, a confirmative CT scan was performed 4 weeks later. Complete response (CR) was defined as the complete disappearance of all evaluable lesions, persisting for >4 weeks. Partial response (PR) was defined as a ≥30% reduction in the sum of the products of the largest perpendicular diameters in all measurable lesions for ≥4 weeks, without the development of new lesions. Progressive disease (PD) was defined as an increase in a previous lesion by >20%, or the development of any new lesion. Stable disease (SD) was defined as any change in a previous lesion that did not fit into either the PR or PD categories.
The primary endpoint was overall survival (OS), and secondary endpoints were progression-free survival (PFS), response rate (RR) and toxicity. All enrolled patients were included in the intent-to-treat analysis. Survival time was analyzed by software Kaplan-Meier of SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). After the treatment, all subjects were followed up every 3 months until their death.
Results
Characteristics of patients
Seventy-five patients from ten centers were enrolled in this trial from November 2004 to April 2007 and their baseline characteristics are shown in Table 1. Among them, 54 were male and 21 female with median age of 59 years (range, 32-75 years). All patients had KPS score ≥80 and 53 had KPS score ≥90 (70.7%). Forty-one patients were treated as first-line therapy and 34 patients were treated as second-line therapy. Among the 34 patients, 10 had 5-FU/cisplatin-based and 24 had oxaliplatin-based regimens as first-line therapy. The total number of 289 cycles was delivered. The median number of treatment cycles was four cycles (range, 2-6 cycles).
Table 1. Basic characteristics of the patients.
| Characteristic | Total (n=75) | Group A (n=41) | Group B (n=34) |
|---|---|---|---|
| Age (year) | |||
| Median | 59 | 60 | 57 |
| Range | 32-75 | 32-75 | 38-70 |
| Sex | |||
| Male | 54 | 30 | 24 |
| Female | 21 | 11 | 10 |
| KPS score | |||
| 100 | 2 | 1 | 1 |
| 90 | 51 | 30 | 21 |
| 80 | 22 | 10 | 12 |
| Primary site | |||
| Esophagogastric junction | 21 | 12 | 9 |
| Body of stomach | 32 | 22 | |
| Gastric antrum | 22 | 7 | 10 |
| Local progression/relapse | 11 | 3 | 15 |
| Metastasis | 64 | 38 | 8 |
| Initial therapy | 26 | ||
| Surgery | 0 | 18 | |
| No surgery | 41 | 16 | |
| Received before adjuvant treatment | 0 | 14 | |
| Adjuvant radiotherapy | 0 | 0 |
A total of 75 patients were treated as scheduled, and the tumor response was evaluated in 71 cases. Three patients who received only 1 cycle of chemotherapy were not assessable (two withdraw from the study because of financial and personal reasons). One patient developed grade IV hematological toxicity. The following bone marrow biopsy revealed bone marrow metastasis. Therefore, the investigator decided to drop her off the study with continuing the similar treatment with modified dosage.
Efficacy
The efficacy data are shown in Table 2. Among all evaluable 71 patients, 1 achieved CR and 22 had PR. The overall response rate (CR+PR) was 32.4% (23/71, 95% CI: 21.7-44.5%). In addition, 38 patients had SD and 10 patients had their disease progression with disease control rate (CR+PR+SD) of 85.9%.
Table 2. Best overall response rates in two groups.
| Tumor response | Total (n=75) | Group A (n=41) | Group B (n=34) |
|---|---|---|---|
| Number of evaluable patients | 71 | 40 | 31 |
| Confirmed CR | 1 | 1 | 0 |
| Confirmed PR | 22 | 15 | 7 |
| SD | 38 | 20 | 18 |
| PD | 10 | 4 | 6 |
| Overall response rate (%) | 32.4 | 40.0 | 22.6 |
| mOS (months) | 12.0 | 7.3 | |
| mPFS (months) | 5.7 | 5.0 |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; mOS, median overall survival; mPFS, median progression-free survival
Among the 40 evaluable patients treated as first-line therapy (group A), 1 CR, 15 PR, and 20 SD were reported with the overall response rate of 40% (16/40, 95% CI: 24.9-56.7%). In the 31 evaluable patients as second-line treatment (group B), there were 0 CR, 7 PR, and 18 SD with the response rate of 22.6% (7/31, 95% CI: 9.6-41.1%).
The median follow-up time was 25.8 months. At the time of this analysis, 74 patients (98%) presented with progressive disease, and 64 (85%) of the 75 enrolled patients were dead. The median OS (mOS) was 12.0 months (95% CI: 7.9-16.2 months) in group A, and 7.3 months (95% CI: 4.3-10.3 months) in group B, and the difference in mOS between the two groups was statistically significant (P=0.013) (Figure 1). The median PFS (mPFS) was 5.7 months (95% CI: 4.1-7.2 months) in group A, and 5.0 months (95% CI: 3.1-6.9 months) in group B, and there was no significant difference between the two groups (P=0.249) (Figure 2).
Figure 1.
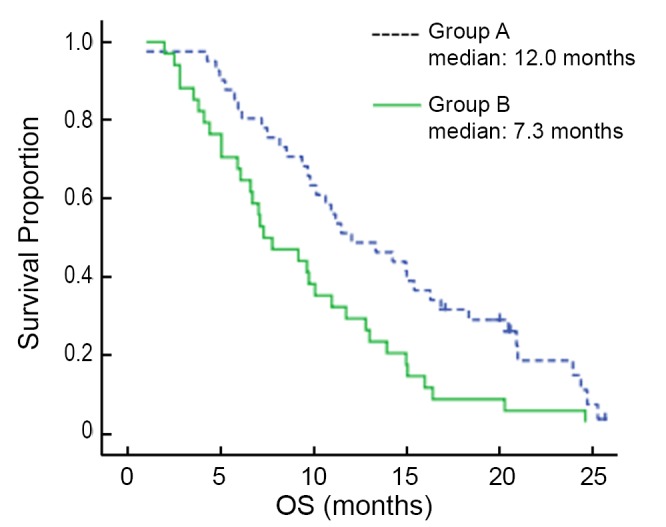
Kaplan-Meier estimates of OS among chemotherapy as first-line and second-line AGC patients treated with paclitaxel, cisplatin and fluorouracil
Figure 2.
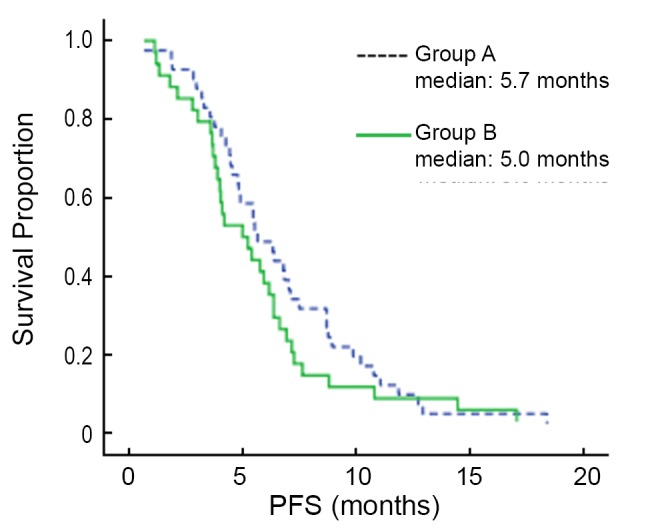
Kaplan-Meier estimates of PFS among chemotherapy as first-line and second-line AGC patients treated with paclitaxel, cisplatin and fluorouracil
Drug dose adjustment and toxicity
The chemotherapy-related toxicities in the 75 patients were analyzed (Table 3). The main adverse event was hematological toxicity. The initially designed dose of paclitaxel was 175 mg/m2. Twelve of 22 patients (54.5%) were treated at that dose level developed grade III/IV neutropenia. Considering severe neutropenia related to paclitaxel, the dose of paclitaxel was adjusted to 150 mg/m2 based on the recommendation of the data safety monitoring committee. After the dose adjustment, the rest of 75 patients were treated at the 150 mg/m2 dose level. Among them, 19 (35.9%) developed grade III/IV neutropenia. All together, the overall grade III/IV neutropenia was 41.3% (31/75). There were 28 patients (37%) receiving G-CFS before starting the following cycle of chemotherapy to prevent treatment delay. Other hematologic toxicity of grade III/IV was anemia (2.7%).
Table 3. Hematological and non-hematological adverse events (NCI-CTCAE version 2.0).
| Type of adverse event (n=75) | All grades |
Grade III, IV |
|||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Hematologic toxicity | |||||
| Neutropenia | 74 | 98.6 | 31 | 41.3 | |
| Febrile neutropenia | 7 | 9.3 | 7 | 9.3 | |
| Thrombocytopenia | 11 | 14.6 | 0 | 0 | |
| Anemia | 42 | 56.0 | 2 | 2.7 | |
| Nonhematologic toxicity | |||||
| Fatigue/malaise | 50 | 66.7 | 0 | 0 | |
| Nausea/anorexia | 68 | 90.7 | 8 | 10.7 | |
| Vomiting | 46 | 61.3 | 4 | 5.3 | |
| Diarrhea | 12 | 1.3 | 0 | 0 | |
| Alopecia | 69 | 92.0 | 0 | 0 | |
| Stomatitis | 16 | 21.3 | 0 | 0 | |
| Neurosensory toxicity | 36 | 48.0 | 2 | 2.6 | |
| AST/ALT | 8 | 10.6 | 0 | 0 | |
| Bilirubin elevation | 5 | 6.7 | 0 | 0 | |
| Creatinine | 2 | 2.6 | 0 | 0 | |
NCI-CTCAE, National Cancer Institute-Common Terminology Criteria for Adverse Events
The major non-hematological toxicities greater than NCI-CTC grade III were nausea/anorexia (8/75, 10.7%), vomiting (4/75, 5.3%) and neurosensory toxicity (2/75, 2.6%). Mild alopecia was observed in 69 patients (92.0%). There was no treatment-related death.
Discussion
Systemic chemotherapy is widely accepted as palliative treatment for patients with unresectable advanced or recurrent and metastatic gastric or EGJ adenocarcinoma, leading to improved quality of life and prolonged survival time. However, there has been no standard chemotherapy regimen worldwide (5). Fluoropyrimidine [fluorouracil (5-FU), capecitabine, and S-1] and cisplatin-based regimens were considered as standard therapy for patients with AGC for a long time, but the randomized trials had not shown a significant improvement in OS (14,16). In the previous studies, taxanes (paclitaxel and docetaxel) have been administered as single agent or in combination (commonly with cisplatin, or/and 5-FU) in the treatment of gastric or EGJ adenocarcinoma (17-21). V325 trial, a randomized phase III trial of DCF vs. CF as first-line therapy in patients with advanced or locally recurrent gastric cancer demonstrated benefit of adding docetaxel to the CF regimen with mOS of 9.2 months in the DCF group vs. 8.6 months in the CF group. However, the high incidence of hematologic toxicities (82% grade 3 or 4 neutropenia; 65% grade 3 or 4 leukopenia; and 29% complicated neutropenia) limited its application in clinic management. Efforts have been tried to keep the efficacy of the combination therapy and decrease the toxicity by modification. Moreover, Chinese patients appear to be less tolerable to chemotherapy than Western counterparts for unknown reasons. We designed this prospective study to modify DCF regimen as replacing docetaxel with paclitaxel in combination with fractionated low-doses cisplatin and continuous 5-FU infusion. It has reported that paclitaxel and docetaxel have equivocal efficacy in gastric cancer treatment (13), with different toxicity profile. The peripheral neuropathy is the dose-limiting toxicity of paclitaxel, while severe neutropenia is usually associated with docetaxel. The fractional administration of cisplatin and 5-FU showed satisfied activity and good tolerance, especially low incidence of gastrointestinal toxicity (21,22).
Although our investigation is not a large scale randomized trial, our results demonstrated encouraging with a mOS of 12.0 months in first-line setting and 7.3 months in second-line therapy in Chinese AGC or EGJ patients and much better adverse effects and tolerability compared to the DCF regimen. The incidence of grade 3/4 neutropenia of was 41.3% (35.9% after the dose of paclitaxel adjusted to 150 mg/m2). The incidences of other non-hematologic grade 3/4 toxicities in V325, such as stomatitis (21%), diarrhea (19%), neurosensory (8%), nausea (14%) and vomiting (14%), were lower in this study as 0%, 0%, 2.6%, 10.7% and 5.3% respectively.
In addition, many patients did not respond to first-line chemotherapy or have progression of their disease within some months from the end of first-line treatment. There was lack of established second-line chemotherapy regimens for patients with AGC (23-26). The combination of paclitaxel, cisplatin and fluorouracil has not been systemically studied in patients with advanced and metastatic gastric cancer as second-line setting. This study indicated that PCF is effective for those patients who had not experienced taxane-based chemotherapy with an impressive response rate of 22.6%, and overall survival of 7.3 months. Importantly, all patients in second-line therapy were previously treated with fluorouracil and platinum (either cisplatin or oxaliplatin).
In summary, the combination chemotherapy of paclitaxel with fractional infusion of 5-FU and cisplatin showed encouraging efficacy and tolerability profile in the treatment of Chinese patients with advanced gastric or EGJ adenocarcinoma as either the first-line or the second-line treatment. Response and survival of PCF are same as DCF, but the tolerance is much better. PCF is especially promising in AGC who failed first-line treatment of platinum-based therapy, and requires further investigation in larger scale study, especially in combination with target-oriented agents (e.g., combining with antiangiogenic biologics or trastuzumab in patients with positive HER-2).
Acknowledgements
This study was funded by Hospira Inc., Australia. Numerous individuals from the ten centers participated to complete this study. The representative authors are grateful for everyone’s effort and all patients.
The authors thank Weijing Sun, MD, Department of Medicine, Hematology-Oncology Division, University of Pennsylvania, Philadelphia, USA for his assistance with this paper.
Disclosure: The authors declare no conflict of interest.
References
- 1.Hwang J, Cho SH, Shim HJ, et al. Phase II study of paclitaxel, cisplatin, and 5-fluorouracil combination chemotherapy in patients with advanced gastric cancer. J Korean Med Sci 2008;23:586-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006;12:354-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50 [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108 [DOI] [PubMed] [Google Scholar]
- 5.Glimelius B, Ekström K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997;8:163-8 [DOI] [PubMed] [Google Scholar]
- 6.Foukakis T, Lundell L, Gubanski M, et al. Advances in the treatment of patients with gastric adenocarcinoma. Acta Oncol 2007;46:277-85 [DOI] [PubMed] [Google Scholar]
- 7.Cascinu S, Graziano F, Cardarelli N, et al. Phase II study of paclitaxel in pretreated advanced gastric cancer. Anticancer Drugs 1998;9:307-10 [DOI] [PubMed] [Google Scholar]
- 8.Chao Y, Li CP, Chao TY, et al. An open, multi-centre, phase II clinical trial to evaluate the efficacy and safety of paclitaxel, UFT, and leucovorin in patients with advanced gastric cancer. Br J Cancer 2006;95:159-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Einzig AI, Neuberg D, Remick SC, et al. Phase II trial of docetaxel (Taxotere) in patients with adenocarcinoma of the upper gastrointestinal tract previously untreated with cytotoxic chemotherapy: the Eastern Cooperative Oncology Group (ECOG) results of protocol E1293. Med Oncol 1996;13:87-93 [DOI] [PubMed] [Google Scholar]
- 10.Sakamoto J, Morita S, Yumiba T, et al. A phase II clinical trial to evaluate the effect of paclitaxel in patients with ascites caused by advanced or recurrent gastric carcinoma: a new concept of clinical benefit response for non-measurable type of gastric cancer. Jpn J Clin Oncol 2003;33:238-40 [DOI] [PubMed] [Google Scholar]
- 11.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991-7 [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Shen L, Li J, et al. A phase II trial of paclitaxel and cisplatin in patients with advanced squamous-cell carcinoma of the esophagus. Am J Clin Oncol 2008;31:29-33 [DOI] [PubMed] [Google Scholar]
- 13.Hu B, Yu JR, Wen ZZ, et al. Capecitabine combined with cisplatin as first-line therapy in Chinese patients with advanced gastric carcinoma-a phase II clinical study. Zhonghua Zhong Liu Za Zhi 2008;30:940-3 [PubMed] [Google Scholar]
- 14.Park SH, Lee WK, Chung M, et al. Paclitaxel versus docetaxel for advanced gastric cancer: a randomized phase II trial in combination with infusional 5-fluorouracil. Anticancer Drugs 2006;17:225-9 [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute. Cancer Therapy Evaluation Program, Common Toxicity Criteria. Version 2.0. Bethesda, MD: National Cancer Institute 1999. [Google Scholar]
- 16.Lee JL, Kang YK, Kang HJ, et al. A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer 2008;99:584-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takiuchi H, Goto M, Imamura H, et al. Multi-center phase II study for combination therapy with paclitaxel/doxifluridine to treat advanced/recurrent gastric cancer showing resistance to S-1 (OGSG 0302). Jpn J Clin Oncol 2008;38:176-81 [DOI] [PubMed] [Google Scholar]
- 18.Roth AD, Maibach R, Martinelli G, et al. Docetaxel (Taxotere)-cisplatin (TC): an effective drug combination in gastric carcinoma. Swiss Group for Clinical Cancer Research (SAKK), and the European Institute of Oncology (EIO). Ann Oncol 2000;11:301-6 [DOI] [PubMed] [Google Scholar]
- 19.Constenla M, Garcia-Arroyo R, Lorenzo I, et al. Docetaxel, 5-fluorouracil, and leucovorin as treatment for advanced gastric cancer: results of a phase II study. Gastric Cancer 2002;5:142-7 [DOI] [PubMed] [Google Scholar]
- 20.Paridaens R, Biganzoli L, Bruning P, et al. Paclitaxel versus doxorubicin as first-line single-agent chemotherapy for metastatic breast cancer: a European Organization for Research and Treatment of Cancer Randomized Study with cross-over. J Clin Oncol 2000;18:724-33 [DOI] [PubMed] [Google Scholar]
- 21.Lo SS, Khorana AA, Javle M, et al. A phase II study of weekly docetaxel in combination with capecitabine in advanced gastric and gastroesophageal adenocarcinomas. Oncology 2010;78:125-9 [DOI] [PubMed] [Google Scholar]
- 22.Ohtsu A, Shimada Y, Shirao K, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol 2003;21:54-9 [DOI] [PubMed] [Google Scholar]
- 23.Catalano V, Graziano F, Santini D, et al. Second-line chemotherapy for patients with advanced gastric cancer: who may benefit? Br J Cancer 2008;99:1402-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baize N, Abakar-Mahamat A, Mounier N, et al. Phase II study of paclitaxel combined with capecitabine as second-line treatment for advanced gastric carcinoma after failure of cisplatin-based regimens. Cancer Chemother Pharmacol 2009;64:549-55 [DOI] [PubMed] [Google Scholar]
- 25.Rosati G, Bilancia D, Germano D, et al. Reduced dose intensity of docetaxel plus capecitabine as second-line palliative chemotherapy in patients with metastatic gastric cancer: a phase II study. Ann Oncol 2007;18:vi128-32 [DOI] [PubMed] [Google Scholar]
- 26.Koizumi W, Akiya T, Sato A, et al. Second-line chemotherapy with biweekly paclitaxel after failure of fluoropyrimidine-based treatment in patients with advanced or recurrent gastric cancer: a report from the gastrointestinal oncology group of the Tokyo cooperative oncology group, TCOG GC-0501 trial. Jpn J Clin Oncol 2009;39:713-9 [DOI] [PubMed] [Google Scholar]


