Abstract
Endoscopy has an increasingly important role in the palliation of patients with pancreatic ductal adenocarcinoma. Endoscopic biliary drainage is still requested in the majority of patients who present with obstructive jaundice, and the increased use of self-expandable metallic stents has reduced the incidence of premature stent occlusion. First-line use of metallic stents is expected to be utilized more frequently as neoadjuvant protocols are improved. The efficacy of endoscopy for palliating gastroduodenal obstruction has advanced with the development of through-the-scope, self-expandable gastroduodenal stents. There have been advances in pain management, with endoscopic ultrasound-guided celiac plexus neurolysis reducing opiate requirements and pain for patients with unresectable malignancy. Future applications of endoscopy in pancreatic cancer may include fine needle injection of chemotherapeutic and other agents into the lesion itself. This review will summarize the evidence of endoscopy in the management of patients with pancreatic cancer.
Keywords: pancreatic cancer, endoscopic retrograde cholangiopancreatography, endoscopic ultrasound, stent
Introduction
Therapeutic endoscopic interventions for patients with pancreatic ductal adenocarcinoma (PDAC) have expanded from biliary drainage to include gastroduodenal stent placement for gastric outlet obstruction and endoscopic ultrasound (EUS) guided celiac plexus neurolysis for pain management. Future endoscopic therapies may include ultrasound-guided injection of chemotherapeutic or immunologic agents and local tumor ablation. The majority of this review will discuss techniques of endoscopic palliation and consider the evidence for each of these procedures. We will conclude by discussing potential future directions for endoscopy in the palliation of patients with PDAC.
Biliary obstruction
PDAC typically causes a distal bile duct obstruction which should be considered separately from perihilar obstruction (Klatskin tumor), where the efficacy and durability of endoscopic biliary drainage are lower. Furthermore, the impact of preoperative biliary drainage (PBD) on postoperative outcomes varies depending on the location of biliary obstruction. Perihilar obstruction will not be addressed in this review.
Endoscopic approaches to biliary drainage
Endoscopic retrograde biliary drainage is generally preferred in favor of percutaneous, transhepatic biliary drainage since patients prefer to avoid having a percutaneous drain when possible. When performed by experienced providers, endoscopic biliary drainage has favorable (80–90%) short term (< 90 day) success rates in the setting of distal bile duct obstruction, even when combined with diagnostic EUS and fine needle aspiration.1 Still, complications may occur in up to 10% of cases and include cholangitis, perforation, bleeding and post-ERCP pancreatitis. Biliary obstruction may be treated via plastic or metallic stents. Plastic stents are comparatively inexpensive and easily removed during future endoscopy or surgery. On the other hand, self-expandable metallic stents (SEMS) have a larger diameter than plastic stents since their diameter (8–10mm) are not constrained by the size of the working channel of the duodenoscope (4.2mm). While more expensive than plastic stents, SEMS are less likely to occlude, with a relative risk reduction compared to plastic stents after four months of 0.44, (95% CI 0.3, 0.63).2 [Editor's note: Metallic biliary endoprosthesis can be removed at surgery, if the patient comes to resection and the bile duct is divided for biliary reconstruction].
Locally advanced or metastatic PDAC
The goals of biliary drainage in the setting of locally advanced or metastatic PDAC are to palliate obstructive jaundice and normalize serum bilirubin prior to systemic chemotherapy. In addition to resolving jaundice and associated pruritis, biliary drainage improves anorexia, indigestion, and quality of life.3, 4Endoscopic biliary drainage is safer than surgical bypass, with endoscopic placement of a plastic stent having a lower relative risk of complications (0.60, 95% CI 0.45–0.81); on the other hand, biliary obstruction is more likely to recur with an endoscopic/plastic stent approach (relative risk (RR) 18.59, 5.33–64.86). As a result, for patients who are expected to live at least three to six months, SEMS are increasingly preferred over plastic stents due to their superior patency rates.5–9 Use of a SEMS in this setting minimizes short term morbidity while optimizing the durability of nonoperative biliary drainage. If a patient's life expectancy is shorter than 3 months, the role of biliary drainage altogether is questionable since palliation of jaundice will be limited. Nevertheless, in patients with such a short life expectancy, placement of a 10Fr plastic stent is reasonable.
Potentially resectable PDAC
In the United States, the majority of patients with potentially resectable PDAC undergo PBD despite evidence failing to demonstrate its impact on reducing postoperative complications.10–12 This is probably explained by the delayed timing of surgical consultation and pancreatoduodenectomy (PD), which often dictate PBD first and a less urgent surgical consultation second.12
What are the goals of biliary drainage in the preoperative setting?
The purpose of biliary drainage in a patient who is expected to undergo resectional surgery is twofold: first, to resolve jaundice; second, to permit administration of full-dose neoadjuvant chemotherapy. While hyperbilirubinemia may be a predictor of postoperative complications,13–16 the benefit of PBD is questionable.11, 12, 17–21 Experimental studies have demonstrated the benefits of biliary drainage in terms of improved nutritional status22, immune function23 and reducing endotoxinemia.24 In older studies, increased serum bilirubin has been correlated with a greater incidence of infectious, renal and nutritional complications, as well as postoperative mortality.25–29 Studies evaluating the role of PBD are typically limited to patients without marked hyperbilirubinemia (often defined as a serum total bilirubin ≥ 10–15mg/dL), where the potential benefits of PBD will be minimal. Furthermore, the duration between PBD and surgery is often less than 4 weeks despite evidence which shows normalization of hepatocyte function after 6 weeks of decompression.30
Although there have been several studies evaluating the benefit of PBD in patients with periampullary tumors and mild elevation in serum total bilirubin,11, 12, 17, 18, 21 there have been no prospective trials specifically evaluating the impact of PBD on patients with severe hyperbilirubinemia (i.e., “deep jaundice”). Therefore, the role for PBD in patients with marked hyperbilirubinemia and distal bile duct obstruction who are expected to undergo surgical resection remains uncertain.
Endoscopic biliary drainage in a patient who may undergo surgery
Plastic stents are typically composed of polyethylene or polyurethane and derived from internal stents developed in the 1970s for deployment via a percutaneous approach.31 These stents are relatively inexpensive ($200–400 per stent), range in diameter from 7 to 11.5Fr and can be removed intraoperatively or during follow-up ERCP without difficulty. Since their diameter is limited by the size of the working channel of the endoscope, patency rates are limited: 10Fr plastic stents have median patency rates of approximately three months.32 After stent placement, bacterial translocation into the bile duct leads to the development of a biofilm along the internal surface of the stent, increasing the viscosity of bile (with formation of microscopic and occasionally macroscopic sludge) and its consequential precipitation (figure 1). Prophylactic antibiotics, stents coated with antimicrobial materials, variations in stent design, and deployment location (e.g., fully internalizing the stent in the bile duct) have failed to meaningfully improve patency rates in clinical studies.33–35 Increased diameter up to 10Fr is the only stent characteristic that significantly reduces the frequency of stent occlusion.36 Placement of multiple plastic stents in parallel increases the functional diameter across the stricture and permits biliary drainage between the stents (a.k.a., “wicking”). This technique is usually employed in the serial dilation of benign biliary strictures but probably improves patency rates compared to single plastic stents; prospective data evaluating this strategy in the setting of malignant, distal bile duct obstruction are lacking. For patients with resectable PDAC and biliary obstruction who are expected to undergo surgery within three months, a 10Fr plastic stent is definitely recommended over smaller diameter alternatives given their superior patency rates.
Figure 1. Occlusion of a plastic bile duct stent.
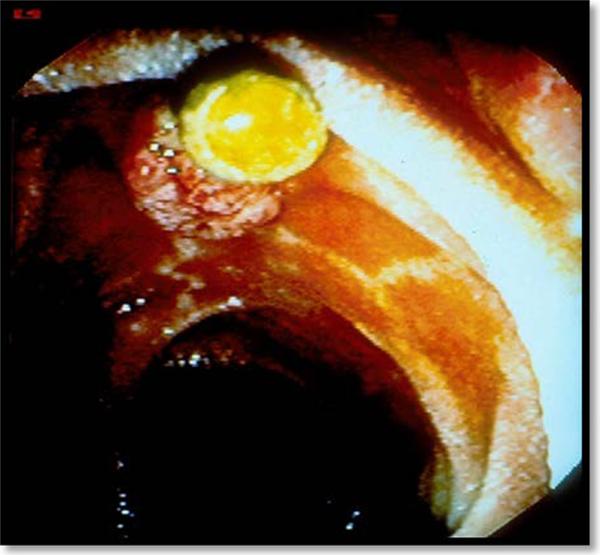
Due to their limited diameter, plastic stents may occlude after 2–3 months due to the development of a bacterial biofilm and precipitation of bile/sludge along the internal margin of the stent. For this reason, use of a 10Fr stent is preferred in the setting of malignant biliary obstruction when surgery is anticipated in the next three months.
Compared to plastic stents, SEMS have superior patency rates due to their greater diameter (8–10mm), and do not have to be replaced every 3 months. However, increased device cost (~ $2–3,000 each) and limited endoscopic or intraoperative removability are potential disadvantages of SEMS compared to PS. Original SEMS were composed of a stainless steel mesh that would embed into the bile duct mucosa, leading to a hyperplastic reaction and interfering with removal during subsequent endoscopy. To optimize patency and minimize ingrowth with tumor and hyperplastic tissue, newer designs utilize more flexible metal alloys and offer a partial or fully covered, nonporous membrane composed of a silicone-like material in certain models. These “covered” or “partially covered” SEMS can be removed during subsequent endoscopy more easily, although they are not currently FDA-approved for this use.37–40 In the setting of malignant biliary obstruction, clinical trials of these coated variants have shown similar or superior patency rates compared to uncovered SEMS for malignant biliary obstruction.41–44 However, numerous studies confirm the superior patency of SEMS when compared to PS, typically in patients with locally advanced or metastatic PDAC.5–9
Limited data suggest no impact on postoperative complications when patients undergo pancreatoduodenectomy after SEMS placement.45–47 If surgery remains a possibility, we recommend deploying the SEMS > 2cm below the hepatic bifurcation to minimize interference with the subsequent creation of a biliary-enteric anastomosis during surgery (figure 2A–B). Data do not strongly favor covered over uncovered SEMS in the setting of malignant obstruction. There is a theoretical risk of occluding the cystic duct with a covered SEMS in patients with an intact gallbladder; rates of cholecystitis are variable in the literature, but the majority of cases typically occur from malignant obstruction of the cystic duct insertion and are not due to the stent itself.48 That said we usually try to deploy the proximal margin of the stent below the cystic duct insertion whenever possible.
Figure 2A–B. Distal bile duct obstruction: Deployment of a self-expandable metallic stent (SEMS).
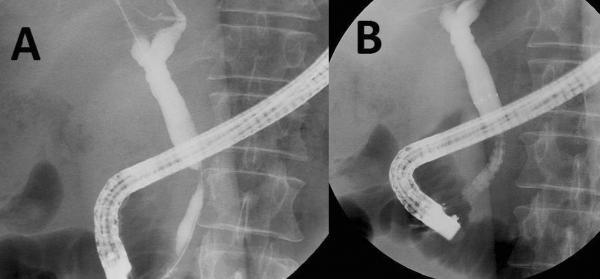
Cholangiogram confirms a distal bile duct stricture (A). A self-expandable metallic stent (SEMS) is deployed (note contrast flow immediately following deployment), with the proximal margin of the stent > 2cm below the hepatic bifurcation (B). This permits safe creation of a choledochojejunostomy if surgery is performed at a later date.
Accurate staging of PDAC is challenging: in a recent landmark prospective, randomized clinical trial comparing PBD followed by surgery, 30% of all patients deemed resectable preoperatively could only undergo palliative bypass at the time of surgical exploration.12 Postoperative complications were particularly high in this subgroup that had previously undergone PBD. Based on the recent evidence, if surgical resection is definitely planned within two months and PBD is requested, placement of a single, 10Fr plastic stent is recommended. However, if surgical resection is delayed indefinitely or neoadjuvant therapy is planned, we recommend first-line placement of a fully covered SEMS in an effort to minimize the probability of having to perform a second ERC with stent placement later.46 Despite compelling models favoring these recommendations, SEMS are currently only FDA approved for use in patients with unresectable, malignant biliary obstruction.2, 7, 49 Our practice is outlined in figure 3.
Figure 3. Recommended algorithm for biliary drainage in patients with PDAC.
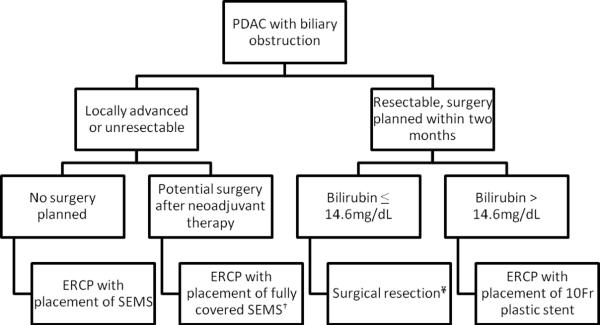
PDAC = pancreatic ductal adenocarcinoma; SEMS = self-expandable metallic stents; PDAC
†SEMS are currently approved by the FDA for use in patients who have an inoperable, malignant bile duct stricture.
¥Surgical resection without preoperative biliary drainage is reasonable if the procedure can be arranged in a timely fashion and the patient has no significant symptoms related to biliary obstruction (e.g., cholangitis, pruritis refractory to medical therapy).
Gastroduodenal obstruction
Historically, nonsurgical palliation of gastric outlet obstruction was limited to balloon dilation and intraluminal tumor ablative techniques such as bipolar current and argon plasma coagulation. These approaches conferred limited short term benefit and often required re-intervention. Advances in device development, particularly the advent of SEMS, has extended the role of endoscopy to the palliation of gastric outlet obstruction in patients with PDAC. Since patients with PDAC and concomitant gastric outlet obstruction often have unresectable disease, an endoscopic intervention may obviate the need for surgery altogether. The flexibility of the SEMS deployment system and use of fluoroscopy permits palliation of strictures that cannot be traversed endoscopically (figure4A–E). Trials comparing surgical bypass with endoscopic deployment of a gastroduodenal stent have confirmed the superiority of endoscopy in terms of symptom relief, length of hospitalization and costs.50–54 Similar to biliary drainage, surgical bypass of the gastroduodenal obstruction (typically open or laparoscopic gastrojejunostomy) confers a more durable benefit at the cost of greater short term morbidity and longer time for symptom resolution.55
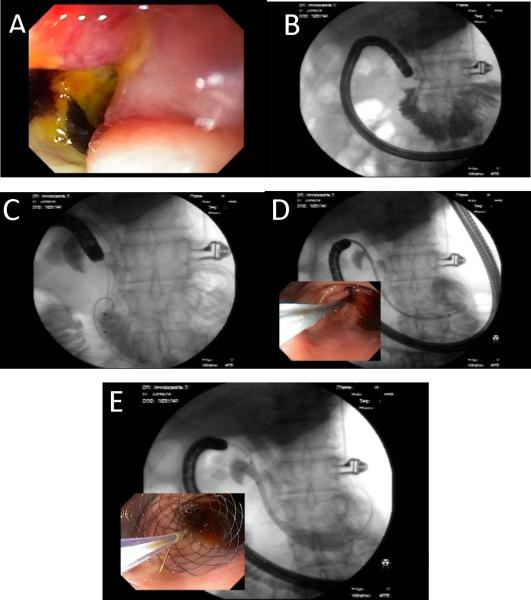
Figure 4A–E. Endoscopic deployment of a gastroduodenal stent.
An obstructing malignant stricture is visualized in the duodenal sweep (A) and demarcated using fluoroscopy (B). A previous biliary metallic stent is only seen on fluoroscopy. A balloon catheter is used to advance a 0.035” stiff guidewire across the stricture, aided by a combination of endoscopy and fluoroscopy (C). The endoscope is withdrawn to the antrum, where the stent catheter is advanced over the guidewire and centered across the stricture (D). The stent is deployed by slowly withdrawing its sheath, allowing its proximal margin to flare in the antrum (E).Reproduced, permission pending, from Cote GA and Edmundowicz SA56
Gastroduodenal stent placement may be complicated by perforation, bleeding and stent migration. In addition, if biliary drainage is not assured prior to deployment of the enteral stent, secondary bile duct obstruction may ensue.56, 57 Since the gastroduodenal stent usually traverses the major papilla, we recommend endoscopic biliary drainage prior to deployment of a gastroduodenal stent whenever possible. If this is not technically feasible, it is possible to attempt endoscopic biliary drainage through the interstices of the enteral stent; however, success rates with this approach are lower, based on limited observation and experience.57
In certain cases the enteral stent may not fully expand in the first 24–72 hours due to inadequate radial forces opposing the obstructing tumor or severe angulation of the obstructed bowel loop. Balloon dilation may facilitate initial expansion but has limited durability. Placement of a second stent within the first is typically reserved for cases of stent migration or stent re-stenosis; risk factors for migration include patients who have a robust response to systemic chemotherapy whereas re-stenosis from tumor ingrowth is more likely to occur in those with longer survival, occurring in up to 18% of patients over time.58 Restenosis may also occur from food particles obstructing the lumen; we generally advise patients to remain on a liquid diet for several days after deployment to permit expansion of the stent. Thereafter, a low residue diet is advisable indefinitely. Covered gastroduodenal stents have been developed in an effort to reduce re-stenosis rates; these have higher migration rates and are not currently available in the United States.
EUS-guided celiac plexus neurolysis
Despite the mantra of “painless jaundice” being the textbook presentation for patients with pancreatic cancer, many individuals present with significant upper abdominal and back pain as a result of their disease. For patients who are not expected to undergo imminent surgical resection, early and aggressive titration of analgesics and opiates to achieve pain control should be a priority for the treating physician. EUS-guided celiac plexus neurolysis with injection of combination local anesthetic (e.g., bupivacaine) and highly concentrated alcohol may facilitate pain management with fewer side effects than opiates.59 The procedure is technically straightforward since the celiac axis is typically located within a few centimeters of the gastric wall. When the celiac ganglia can be visualized, the efficacy of the injection is superior, having 15-fold greater odds of response(figure 5).60 Otherwise, an FNA needle is inserted anterior to the celiac axis in one or two locations followed by injection under endosonographic guidance. Some advocate a broader region of injection to include the space anterior to the superior mesenteric artery.61 There are no other factors associated with a better response, but direct tumor invasion of the celiac axis corresponds with reduced efficacy.62
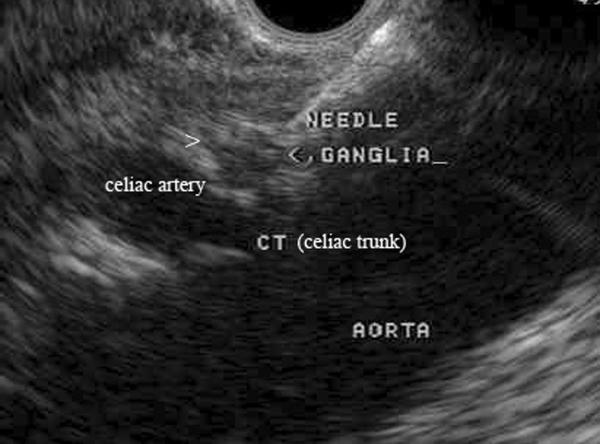
Figure 5. EUS-guided celiac neurolysis.
A 22 gauge needle is inserted into a celiac ganglion identified by endoscopic ultrasound. Factors associated with a better response include direct injection of celiac ganglia (when visualized) and absence of tumor invasion of the celiac plexus.
Severe complications from EUS-guided neurolysis are rarely reported but may include hemorrhage and spinal cord infarction.63 More common sequelae are a transient increase in pain for several days post-procedure and self-limited diarrhea. Patients may develop self-limited hypotension within hours of the injection that can be managed with an intravenous fluid bolus. Pain reduction can be expected in 75–85% of patients within two weeks of the procedure, and a minority of patients can stop opiates altogether.59, 64, 65 Early use of EUS-guided neurolysis is associated with less pain up to three months later compared, is to medical management alone while also reducing opiate requirements.66 Given its reasonable efficacy and favorable safety profile, we recommend early consideration of EUS-guided neurolysis for patients with unresectable PDAC who have abdominal pain requiring regular use of opiates. In select cases, this can be performed during the initial diagnostic procedure when a preliminary cytology confirms malignancy.
New directions
Targeted therapy
PDAC represents a systemic disease, yet much of its morbidity and mortality derive from complications related to its regional spread. Therefore, efforts to control its growth locally present an opportunity for endoscopy as the vehicle to deliver therapies. A current example is the use of endoscopically-directed markers to localize radiation therapy. Potential future applications include the endoscopic delivery of local chemotherapeutic and immunologic agents.
Stereotactic radiation permits the administration of higher concentrations to the targeted lesion while minimizing collateral tissue damage and systemic side effects. EUS-guided placement of fiducial markers into pancreatic tumors is technically feasible with a safety profile on par with fine needle aspiration; these markers can now be deployed using a 22 gauge needle, reducing technical complexity for lesions in the pancreatic head and uncinate.67–70
EUS-guided tumor ablation via photodynamic therapy and radiofrequency probes along with injection of chemotherapeutic or immunologic agents may permit targeted therapy with fewer side effects compared to systemic chemotherapy.71 The strongest evidence is limited to precancerous cysts of the pancreas treated with injection of ethanol in combination with paclitaxel.72 Early experience with intratumoral injection of TNFerade™, a biologic that combines tumor necrosis factor-alpha with an adenovirus vector, shows promise in improving the radiosensitivity of PDAC.73
Pancreatic duct stenting
PDAC causes pain through several mechanisms, one of which is posited to be obstruction of the pancreatic duct. Similar to “obstructive” chronic pancreatitis where the object of endoscopic or surgical therapy is often drainage of the pancreatic duct, placement of a larger diameter (10Fr) pancreatic duct stent may improve pain and exocrine insufficiency related to PDAC.74 Clinical trials are lacking, but this would be a logical intervention at the time of endoscopic biliary decompression in patients with pancreatic head lesions. The added risks of attempting pancreatic duct stent placement, particularly incomplete drainage, should be considered in any clinical trial evaluating this palliative intervention. [Editor's note: Pancreatic duct stenting is currently rarely used in this setting, and should not be considered standard of care.]
Summary
With future advances in nonsurgical therapies for PDAC, endoscopy is expected to serve an important role in the palliation of biliary, gastroduodenal and perhaps pancreatic duct obstruction. Endoscopic palliation is expected to increase, as endoscopic approaches are logical conduits for the delivery of local agents to the primary tumor. In addition to safety and efficacy, the cost implications of any new endoscopic interventions will significantly influence their implementation, considering the anticipated changes in the U.S. health care system. The added procedure-related costs should be offset by reductions in the length and frequency of hospitalization as well as measurable improvements in quality of life. For patients with PDAC, the decision to proceed with any endoscopic intervention should derive from a multidisciplinary discussion that includes the patient as well as experts in surgery, medical and radiation oncology, and gastroenterology.
Acknowledgments
Conflicts of Interest and Source of Funding: Gregory Coté is currently receiving grants from the National Institute of Health (5R21DK090708-02 and 1K23DK095148-01) and has received honoraria from Boston Scientific Corp. and Olympus America. Stuart Sherman has received honoraria from Olympus America, Boston Scientific Corp., Cook Medical and Repligen Corporation.
Abbreviations
- PDAC
pancreatic ductal adenocarcinoma
- EUS
endoscopic ultrasound
- PBD
preoperative biliary drainage
- ERCP
endoscopic retrograde cholangiopancreatography
- SEMS
self-expandable metallic stents
- RR
relative risk
- PD
pancreatoduodenectomy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross WA, Wasan SM, Evans DB, et al. Combined EUS with FNA and ERCP for the evaluation of patients with obstructive jaundice from presumed pancreatic malignancy. Gastrointest Endosc. 2008;68:461–6. doi: 10.1016/j.gie.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 2.Moss AC, Morris E, Leyden J, et al. Malignant distal biliary obstruction: a systematic review and meta-analysis of endoscopic and surgical bypass results. Cancer Treat Rev. 2007;33:213–21. doi: 10.1016/j.ctrv.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Ballinger AB, McHugh M, Catnach SM, et al. Symptom relief and quality of life after stenting for malignant bile duct obstruction. Gut. 1994;35:467–70. doi: 10.1136/gut.35.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira-Lima JC, Jakobs R, Maier M, et al. Endoscopic stenting in obstructive jaundice due to liver metastases: does it have a benefit for the patient? Hepatogastroenterology. 1996;43:944–8. [PubMed] [Google Scholar]
- 5.Moss AC, Morris E, Mac Mathuna P. Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database Syst Rev. 2006:CD004200. doi: 10.1002/14651858.CD004200.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Arguedas MR, Heudebert GH, Stinnett AA, et al. Biliary stents in malignant obstructive jaundice due to pancreatic carcinoma: a cost-effectiveness analysis. Am J Gastroenterol. 2002;97:898–904. doi: 10.1111/j.1572-0241.2002.05606.x. [DOI] [PubMed] [Google Scholar]
- 7.Yeoh KG, Zimmerman MJ, Cunningham JT, et al. Comparative costs of metal versus plastic biliary stent strategies for malignant obstructive jaundice by decision analysis. Gastrointest Endosc. 1999;49:466–71. doi: 10.1016/s0016-5107(99)70044-1. [DOI] [PubMed] [Google Scholar]
- 8.Davids PH, Groen AK, Rauws EA, et al. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488–92. doi: 10.1016/0140-6736(92)92752-2. [DOI] [PubMed] [Google Scholar]
- 9.Prat F, Chapat O, Ducot B, et al. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1–7. doi: 10.1016/s0016-5107(98)70291-3. [DOI] [PubMed] [Google Scholar]
- 10.Lillemoe KD. Preoperative biliary drainage and surgical outcome. Ann Surg. 1999;230:143–4. doi: 10.1097/00000658-199908000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Gurusamy KS, Lin H, et al. Preoperative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev. 2008:CD005444. doi: 10.1002/14651858.CD005444.pub2. [DOI] [PubMed] [Google Scholar]
- 12.van der Gaag NA, Rauws EA, van Eijck CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129–37. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 13.Povoski SP, Karpeh MS, Jr., Conlon KC, et al. Preoperative biliary drainage: impact on intraoperative bile cultures and infectious morbidity and mortality after pancreaticoduodenectomy. J Gastrointest Surg. 1999;3:496–505. doi: 10.1016/s1091-255x(99)80103-6. [DOI] [PubMed] [Google Scholar]
- 14.Eshuis WJ, van der Gaag NA, Rauws EA, et al. Therapeutic delay and survival after surgery for cancer of the pancreatic head with or without preoperative biliary drainage. Ann Surg. 2010;252:840–9. doi: 10.1097/SLA.0b013e3181fd36a2. [DOI] [PubMed] [Google Scholar]
- 15.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 16.Gerhards MF, van Gulik TM, de Wit LT, et al. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma--a single center experience. Surgery. 2000;127:395–404. doi: 10.1067/msy.2000.104250. [DOI] [PubMed] [Google Scholar]
- 17.Pitt HA, Gomes AS, Lois JF, et al. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985;201:545–53. doi: 10.1097/00000658-198505000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatfield AR, Tobias R, Terblanche J, et al. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet. 1982;2:896–9. doi: 10.1016/s0140-6736(82)90866-2. [DOI] [PubMed] [Google Scholar]
- 19.Lai EC, Mok FP, Fan ST, et al. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994;81:1195–8. doi: 10.1002/bjs.1800810839. [DOI] [PubMed] [Google Scholar]
- 20.Wig JD, Kumar H, Suri S, et al. Usefulness of percutaneous transhepatic biliary drainage in patients with surgical jaundice--a prospective randomised study. J Assoc Physicians India. 1999;47:271–4. [PubMed] [Google Scholar]
- 21.McPherson GA, Benjamin IS, Hodgson HJ, et al. Pre-operative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg. 1984;71:371–5. doi: 10.1002/bjs.1800710522. [DOI] [PubMed] [Google Scholar]
- 22.Gouma DJ, Coelho JC, Schlegel JF, et al. The effect of preoperative internal and external biliary drainage on mortality of jaundiced rats. Arch Surg. 1987;122:731–4. doi: 10.1001/archsurg.1987.01400180113022. [DOI] [PubMed] [Google Scholar]
- 23.Clements WD, McCaigue M, Erwin P, et al. Biliary decompression promotes Kupffer cell recovery in obstructive jaundice. Gut. 1996;38:925–31. doi: 10.1136/gut.38.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saitoh N, Hiraoka T, Uchino R, et al. Endotoxemia and intestinal mucosal dysfunction after the relief of obstructive jaundice by internal and external drainage in rats. Eur Surg Res. 1995;27:11–8. doi: 10.1159/000129367. [DOI] [PubMed] [Google Scholar]
- 25.Lacaine F, Fourtanier G, Fingerhut A, et al. Surgical mortality and morbidity in malignant obstructive jaundice: a prospective multivariate analysis. Eur J Surg. 1995;161:729–34. [PubMed] [Google Scholar]
- 26.Armstrong CP, Dixon JM, Taylor TV, et al. Surgical experience of deeply jaundiced patients with bile duct obstruction. Br J Surg. 1984;71:234–8. doi: 10.1002/bjs.1800710326. [DOI] [PubMed] [Google Scholar]
- 27.Blamey SL, Fearon KC, Gilmour WH, et al. Prediction of risk in biliary surgery. Br J Surg. 1983;70:535–8. doi: 10.1002/bjs.1800700910. [DOI] [PubMed] [Google Scholar]
- 28.Dixon JM, Armstrong CP, Duffy SW, et al. Upper gastrointestinal bleeding. A significant complication after surgery for relief of obstructive jaundice. Ann Surg. 1984;199:271–5. doi: 10.1097/00000658-198403000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon JM, Armstrong CP, Duffy SW, et al. Factors affecting mortality and morbidity after surgery for obstructive jaundice. Gut. 1984;25:104. doi: 10.1136/gut.25.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koyama K, Takagi Y, Ito K, et al. Experimental and clinical studies on the effect of biliary drainage in obstructive jaundice. Am J Surg. 1981;142:293–9. doi: 10.1016/0002-9610(81)90296-8. [DOI] [PubMed] [Google Scholar]
- 31.Pereiras RV, Jr., Rheingold OJ, Huston D, et al. Relief of malignant obstructive jaundice by percutaneous insertion of a permanent prosthesis in the biliary tree. Ann Intern Med. 1978;89:589–3. doi: 10.7326/0003-4819-89-5-589. [DOI] [PubMed] [Google Scholar]
- 32.van Berkel AM, Bruno MJ, Bergman JJ, et al. A prospective randomized study of hydrophilic polymer-coated polyurethane versus polyethylene stents in distal malignant biliary obstruction. Endoscopy. 2003;35:478–82. doi: 10.1055/s-2003-39666. [DOI] [PubMed] [Google Scholar]
- 33.Leung JW, Liu YL, Desta TD, et al. In vitro evaluation of antibiotic prophylaxis in the prevention of biliary stent blockage. Gastrointest Endosc. 2000;51:296–303. doi: 10.1016/s0016-5107(00)70358-0. [DOI] [PubMed] [Google Scholar]
- 34.Rees EN, Tebbs SE, Elliott TS. Role of antimicrobial-impregnated polymer and Teflon in the prevention of biliary stent blockage. J Hosp Infect. 1998;39:323–9. doi: 10.1016/s0195-6701(98)90298-5. [DOI] [PubMed] [Google Scholar]
- 35.Tringali A, Mutignani M, Perri V, et al. A prospective, randomized multicenter trial comparing DoubleLayer and polyethylene stents for malignant distal common bile duct strictures. Endoscopy. 2003;35:992–7. doi: 10.1055/s-2003-44601. [DOI] [PubMed] [Google Scholar]
- 36.Speer AG, Cotton PB, MacRae KD. Endoscopic management of malignant biliary obstruction: stents of 10 French gauge are preferable to stents of 8 French gauge. Gastrointest Endosc. 1988;34:412–7. doi: 10.1016/s0016-5107(88)71407-8. [DOI] [PubMed] [Google Scholar]
- 37.Kahaleh M, Tokar J, Le T, et al. Removal of self-expandable metallic Wallstents. Gastrointest Endosc. 2004;60:640–4. doi: 10.1016/s0016-5107(04)01959-5. [DOI] [PubMed] [Google Scholar]
- 38.Shin HP, Kim MH, Jung SW, et al. Endoscopic removal of biliary self-expandable metallic stents: a prospective study. Endoscopy. 2006;38:1250–5. doi: 10.1055/s-2006-944969. [DOI] [PubMed] [Google Scholar]
- 39.Familiari P, Bulajic M, Mutignani M, et al. Endoscopic removal of malfunctioning biliary self-expandable metallic stents. Gastrointest Endosc. 2005;62:903–10. doi: 10.1016/j.gie.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 40.Kahaleh M, Behm B, Clarke BW, et al. Temporary placement of covered self-expandable metal stents in benign biliary strictures: a new paradigm? (with video) Gastrointest Endosc. 2008;67:446–54. doi: 10.1016/j.gie.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 41.Cho JH, Kim YJ, Kim HM, et al. A Comparison of Outcomes Among Secondary Covered, Uncovered Metalic and Plastic Stent for Occluded Biliary Metallic Stent for Malignant Distal Biliary Obstruction. Gastrointestinal Endoscopy. 2009;69:Ab136–Ab136. [Google Scholar]
- 42.Isayama H, Komatsu Y, Tsujino T, et al. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729–34. doi: 10.1136/gut.2003.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kullman EP, Soderlund C, Linder S, et al. Covered (cSEMS) Versus Uncovered (uSEMS) Nitinol Self-Expandable Metal Stent in the Palliative Treatment of Malignant Distal Biliary Obstruction: Preliminary Results from a Randomized Controlled Multicenter Trial. Gastrointestinal Endoscopy. 2009;69:AB132–AB132. doi: 10.1016/j.gie.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 44.Yoon WJ, Lee JK, Lee KH, et al. A comparison of covered and uncovered Wallstents for the management of distal malignant biliary obstruction. Gastrointest Endosc. 2006;63:996–1000. doi: 10.1016/j.gie.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 45.Wasan SM, Ross WA, Staerkel GA, et al. Use of expandable metallic biliary stents in resectable pancreatic cancer. Am J Gastroenterol. 2005;100:2056–61. doi: 10.1111/j.1572-0241.2005.42031.x. [DOI] [PubMed] [Google Scholar]
- 46.Aadam AA, Evans DB, Khan A, et al. Efficacy and safety of self-expandable metal stents for biliary decompression in patients receiving neoadjuvant therapy for pancreatic cancer: a prospective study. Gastrointest Endosc. 2012;76:67–75. doi: 10.1016/j.gie.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 47.Decker C, Christein JD, Phadnis MA, et al. Biliary metal stents are superior to plastic stents for preoperative biliary decompression in pancreatic cancer. Surg Endosc. 2011;25:2364–7. doi: 10.1007/s00464-010-1552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isayama H, Kawabe T, Nakai Y, et al. Cholecystitis after metallic stent placement in patients with malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2006;4:1148–53. doi: 10.1016/j.cgh.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Chen VK, Arguedas MR, Baron TH. Expandable metal biliary stents before pancreaticoduodenectomy for pancreatic cancer: a Monte-Carlo decision analysis. Clin Gastroenterol Hepatol. 2005;3:1229–37. doi: 10.1016/s1542-3565(05)00886-4. [DOI] [PubMed] [Google Scholar]
- 50.Del Piano M, Ballare M, Montino F, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc. 2005;61:421–6. doi: 10.1016/s0016-5107(04)02757-9. [DOI] [PubMed] [Google Scholar]
- 51.Espinel J, Sanz O, Vivas S, et al. Malignant gastrointestinal obstruction: endoscopic stenting versus surgical palliation. Surg Endosc. 2006;20:1083–7. doi: 10.1007/s00464-005-0354-8. [DOI] [PubMed] [Google Scholar]
- 52.Fiori E, Lamazza A, Volpino P, et al. Palliative management of malignant antro-pyloric strictures. Gastroenterostomy vs. endoscopic stenting. A randomized prospective trial. Anticancer Res. 2004;24:269–71. [PubMed] [Google Scholar]
- 53.Johnsson E, Thune A, Liedman B. Palliation of malignant gastroduodenal obstruction with open surgical bypass or endoscopic stenting: clinical outcome and health economic evaluation. World J Surg. 2004;28:812–7. doi: 10.1007/s00268-004-7329-0. [DOI] [PubMed] [Google Scholar]
- 54.Mehta S, Hindmarsh A, Cheong E, et al. Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surg Endosc. 2006;20:239–42. doi: 10.1007/s00464-005-0130-9. [DOI] [PubMed] [Google Scholar]
- 55.Jeurnink SM, Steyerberg EW, van Hooft JE, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490–9. doi: 10.1016/j.gie.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 56.Cote GA, Edmundowicz SA. Gastroduodenal and Colonic Endoprostheses. In: Ginsberg GG, Gostout CJ, Kochman ML, Norton ID, editors. Clinical Gastrointestinal Endoscopy. 2nd ed. Elsevier Saunders; St. Louis, Missouri: 2012. pp. 749–754. [Google Scholar]
- 57.Telford JJ, Carr-Locke DL, Baron TH, et al. Palliation of patients with malignant gastric outlet obstruction with the enteral Wallstent: outcomes from a multicenter study. Gastrointest Endosc. 2004;60:916–20. doi: 10.1016/s0016-5107(04)02228-x. [DOI] [PubMed] [Google Scholar]
- 58.Dormann A, Meisner S, Verin N, et al. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004;36:543–50. doi: 10.1055/s-2004-814434. [DOI] [PubMed] [Google Scholar]
- 59.Arcidiacono PG, Calori G, Carrara S, et al. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011:CD007519. doi: 10.1002/14651858.CD007519.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ascunce G, Ribeiro A, Reis I, et al. EUS visualization and direct celiac ganglia neurolysis predicts better pain relief in patients with pancreatic malignancy (with video) Gastrointest Endosc. 2011;73:267–74. doi: 10.1016/j.gie.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 61.Sakamoto H, Kitano M, Kamata K, et al. EUS-guided broad plexus neurolysis over the superior mesenteric artery using a 25-gauge needle. Am J Gastroenterol. 2010;105:2599–606. doi: 10.1038/ajg.2010.339. [DOI] [PubMed] [Google Scholar]
- 62.Iwata K, Yasuda I, Enya M, et al. Predictive factors for pain relief after endoscopic ultrasound-guided celiac plexus neurolysis. Dig Endosc. 2011;23:140–5. doi: 10.1111/j.1443-1661.2010.01046.x. [DOI] [PubMed] [Google Scholar]
- 63.Fujii L, Clain JE, Morris JM, et al. Anterior spinal cord infarction with permanent paralysis following endoscopic ultrasound celiac plexus neurolysis. Endoscopy. 2012;44(Suppl 2):E265–6. doi: 10.1055/s-0032-1309708. [DOI] [PubMed] [Google Scholar]
- 64.LeBlanc JK, Al-Haddad M, McHenry L, et al. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: one injection or two? Gastrointest Endosc. 2011;74:1300–7. doi: 10.1016/j.gie.2011.07.073. [DOI] [PubMed] [Google Scholar]
- 65.Wiechowska-Kozlowska A, Boer K, Wojcicki M, et al. The efficacy and safety of endoscopic ultrasound-guided celiac plexus neurolysis for treatment of pain in patients with pancreatic cancer. Gastroenterol Res Pract. 2012;2012:503098. doi: 10.1155/2012/503098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wyse JM, Carone M, Paquin SC, et al. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541–6. doi: 10.1200/JCO.2010.32.2750. [DOI] [PubMed] [Google Scholar]
- 67.Ammar T, Cote GA, Creach KM, et al. Fiducial placement for stereotactic radiation by using EUS: feasibility when using a marker compatible with a standard 22-gauge needle. Gastrointest Endosc. 2010;71:630–3. doi: 10.1016/j.gie.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 68.Pishvaian AC, Collins B, Gagnon G, et al. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412–7. doi: 10.1016/j.gie.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 69.Sanders MK, Moser AJ, Khalid A, et al. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest Endosc. 2010;71:1178–84. doi: 10.1016/j.gie.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 70.Varadarajulu S, Trevino JM, Shen S, et al. The use of endoscopic ultrasound-guided gold markers in image-guided radiation therapy of pancreatic cancers: a case series. Endoscopy. 2010;42:423–5. doi: 10.1055/s-0029-1243989. [DOI] [PubMed] [Google Scholar]
- 71.Seo DW. EUS-Guided Antitumor Therapy for Pancreatic Tumors. Gut Liver. 2010;4(Suppl 1):S76–81. doi: 10.5009/gnl.2010.4.S1.S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oh HC, Seo DW, Song TJ, et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology. 2011;140:172–9. doi: 10.1053/j.gastro.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 73.Hecht JR, Farrell JJ, Senzer N, et al. EUS or percutaneously guided intratumoral TNFerade biologic with 5-fluorouracil and radiotherapy for first-line treatment of locally advanced pancreatic cancer: a phase I/II study. Gastrointest Endosc. 2012;75:332–8. doi: 10.1016/j.gie.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costamagna G, Alevras P, Palladino F, et al. Endoscopic pancreatic stenting in pancreatic cancer. Can J Gastroenterol. 1999;13:481–7. doi: 10.1155/1999/123210. [DOI] [PubMed] [Google Scholar]