Abstract
Artemisia annua produces the antimalarial drug, artemisinin (AN), which is synthesized and stored in glandular trichomes (GLTs). In vitro-grown A. annua shoots produce more AN when they form roots. This may be a function not of the roots, but rather media components such as the phytohormones, α-naphthaleneacetic acid (NAA) and 6-benzylaminopurine (BAP), or salts and sucrose used to maintain either rooted or unrooted shoot cultures. We investigated how three main media components altered artemisinic metabolite production, pathway gene transcripts, and GLT formation in both mature and developing leaves in rooted and unrooted cultures. Although transcript levels of AN biosynthetic genes were not altered, AN levels were significantly different, and there were major differences in both artemisinic metabolite levels and trichomes in mature versus developing leaves. For example, NAA induced higher AN production in rooted shoots, but only in mature leaves. In developing leaves, BAP increased GLT density on the leaf surface. When both phytohormones were present, GLTs were larger on young developing leaves, but smaller on mature leaves. Furthermore, although other media components increased GLT density, their size decreased on young leaves, but there was no effect on mature leaves. Roots also appeared to drive conversion of artemisinic precursors towards end products. These results suggest that, while the presence of roots affects AN and trichome production, phytohormones and other media constituents used for in vitro culture of A. annua also exert an influence.
Keywords: Auxin, Cytokinin, Trichomes, Artemisinin, Terpene, Roots
Introduction
Artemisinin (AN, Fig. 1), a sesquiterpene lactone produced solely in glandular trichomes (GLTs) of Artemisia annua L. (Duke et al. 1994; Olsson et al. 2009), is currently the best treatment for malaria. AN is also effective against other human diseases like schistosomiasis, hepatitis B, and some cancers (Efferth 2009; Firestone and Sundar 2009). The plant is the only economically viable source of AN, and studies have generally focused on understanding the biochemistry with an aim to increasing in planta production.
Fig. 1.
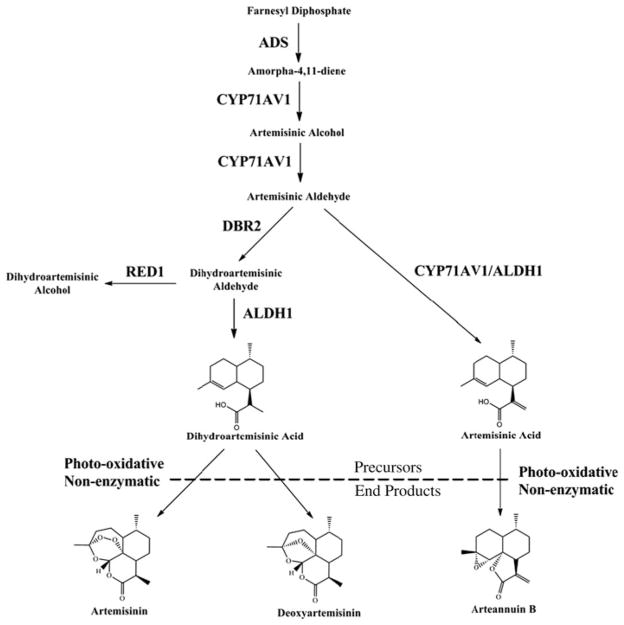
Artemisinin biosynthetic pathway. ADS amorphadiene synthase, CYP71AV1 monooxygenase, DBR2 double-bond reductase 2, ALDH1 aldehyde dehydrogenase, RED1 dihydroartemisinic aldehyde reductase
Ferreira and Janick (1996) previously suggested the roots may be playing some role in the production of AN in shoots; A. annua shoots grown in shooting medium (SHM) produced less AN than rooted shoots grown in rooting medium (RTM). The main difference between SHM and RTM is that SHM contains two phytohormones: the synthetic auxin α-naphthaleneacetic acid (NAA) and the cytokinin BAP. A. annua roots also seem to perceive signals that affect AN production in shoots as reported by Mannan et al. (2010) who showed that roots, but not shoots, were able to perceive an elicitation signal from DMSO and produced more AN in their shoots. Although these studies suggested that the roots might be playing a role in the AN biosynthetic pathway, no artemisinic metabolites were detected in the roots (Woerdenbag et al. 1991; Kim et al. 1992; Ferreira and Janick 1995; Gupta et al. 2002). Transcripts of key genes in the pathway were also barely detectable in roots (Teoh et al. 2006, 2009; Kim et al. 2008; Zhang et al. 2008; Olofsson et al. 2011).
GLT population and AN content in A. annua often correlate; more GLTs usually equals more AN (Kapoor et al. 2007; Arsenault et al. 2010a; Graham et al. 2010). Phytohormones also affect GLT development and/or AN production. For example, jasmonic acid (JA) increased populations of all trichomes, gibberellic acid (GA) only increased filamentous trichome numbers, yet both increased GLT size and AN production in planta (Liu et al. 2009; Maes et al. 2011; Banyai et al. 2011). Salicylic acid (SA) and abscisic acid (ABA) also increase AN production, but it is not clear if these can also induce GLT development (Jing et al. 2009; Guo et al. 2010; Pu et al. 2009). 6-benzylaminopurine (BAP), on the other hand, increased GLT numbers, but decreased their size; AN levels did not increase (Maes et al. 2011).
The first committed step toward production of AN and the other artemisinic metabolites is cyclization of farnesyl diphosphate to amorpha-4,11-diene by amorphadiene synthase (ADS, Fig. 1, Chang et al. 2000; Mercke et al. 2000; Wallaart et al. 2001). Next, amorpha-4,11-diene is catalyzed to artemisinic alcohol and then artemisinic aldehyde by a cytochrome P450 monooxygenase (CYP71AV1, CYP) and a cytochrome p450 reductase (CPR) (Ro et al. 2006; Teoh et al. 2006). From here, there are at least two possible routes. The first is production of artemisinic acid (AA) from artemisinic aldehyde by either CYP or an aldehyde dehydrogenase (ALDH1, Teoh et al. 2009). AA is then converted by photo-oxidation into arteannuin B (AB, Brown and Sy 2007). The second route leads to production of dihydroartemisinic aldehyde from artemisinic aldehyde, catalyzed by a double-bond reductase (DBR2, Zhang et al. 2008). Dihydroartemisinic aldehyde is then reduced to either the less desirable dihydroartemisinic alcohol via dihydroartemisinic aldehyde reductase (RED1, Rydén et al. 2010), which is not yet well studied in planta, or to dihydroartemisinic acid (DHAA) by ALDH1 (Teoh et al. 2009). Finally, DHAA can be converted into either AN or deoxyartemisinin (deoxyAN), also by a nonenzymatic photo-oxidation reaction (Wallaart et al. 1999; Brown and Sy 2004). For more detail see recent reviews (Covello 2008; Brown 2010; Nguyen et al. 2011).
This study focused on how roots of A. annua affect AN metabolite production in the shoots of the plant. Although there are other branches of the AN pathway (Brown 2010), for our purposes here, AA and DHAA are considered precursors to AB, and to AN/deoxyAN, respectively. AB, AN, and deoxyAN are the end products of these two branches of the AN biosynthetic pathway (Fig. 1). In particular, the roles of rooting in concert with the presence of BAP and NAA, the two phytohormones found in SHM, were compared with respect to GLTs, artemisinic pathway transcripts, and metabolites.
Materials and methods
Maintenance of in vitro cultures
Rooted A. annua shoots (clone Sam; voucher MASS 00317314) were grown in Magenta GA-7 boxes with RTM (Table S1) half-strength Murashige and Skoog basal medium with vitamins (MS, Phytotechnology Labs, 2 % (w/v) sucrose, and 5 g L−1 agargellan™ (Phytotechnology Labs), pH 5.8. Unrooted shoots were grown in Magenta boxes with SHM (Table S1) MS salts and vitamins, 0.25 μM NAA (Sigma-Aldrich), 2.5 μM N-6-benzylami-nopurine (BAP, Research Organics, Inc.), 3 % sucrose, and 5 g L−1 agargellan, pH 5.8. All cultures were grown at 25 °C in a growth chamber under continuous light at 70 μmol m−2 s−1 and subcultured every 2 weeks.
Effects of root development on artemisinic metabolites, gene expression, and trichomes
Unrooted shoots were grown in SHM for 2 weeks and then inoculated into either RTM or SHM (control) in Magenta boxes. Over 16 days, five plants were harvested periodically from each medium: one half for metabolite analysis and the other for transcript analysis. For GLT analysis, the second fully developed leaf from the shoot apical meristem of rooted shoots and a random fully developed leaf from unrooted shoots of 16-day-old cultures were used. There is no obvious shoot apical meristem in shoot cultures, so it cannot be used as a developmental locus. Primers used for analysis are presented in Table S2 with 18S used as internal reference. Gene expression was calculated using the 2−ΔΔCT comparative method (Cikos et al. 2007) and presented as fold change relative to day 0. Details are provided in Arsenault et al. (2010a).
Effects of NAA and BAP on artemisinic metabolites and trichomes in rooted shoots
Rooted shoots were inoculated into tubes containing 10 mL of either RTM, RTM + 0.25 μM NAA, RTM + 2.5 μM BAP, RTM + 0.25 μM NAA and 2.5 μM BAP or SHM (Table S1). After inoculation, the height of each plantlet was marked on the outside of the tube to demarcate between pre-existing (mature) and new (young) shoot growth. Cultures were grown as above and harvested after 21 days. At the mark on the tube, shoots were separated into three sections and fresh weights measured: upper (young) and lower (mature) shoots, and their roots. Samples were extracted and metabolites analyzed as further described. The experiment was repeated for GLT analysis.
Effects of BAP and NAA on artemisinic metabolites in rooted shoots with roots excised
Cultures approximately 2 weeks post-root emergence were used. Roots were excised and then shoots were inoculated into tubes containing 10 mL of either RTM or RTM + 2.5 μM BAP or 0.25 μM NAA. After 21 days, cultures were harvested, extracted, and analyzed as further described.
Metabolite extraction and analysis
Fresh shoots (leaves + stems) were extracted with HPLC grade pentane (Fisher Scientific, ~10 mL g FW−1), sonicated in a chilled water bath (Fisher Scientific) for 30 min, extracts decanted, dried under N2, and stored at −20 °C until analysis. Extracts were transferred to vials, dried, and then resuspended in pentane for AN, AB, and deoxyAN GC/MS analyses: HP-5MS column (30 m × 0.25 mm × 0.25 μm) in an Agilent 7890A GC system coupled to an Agilent 5975C MSD with triple-axis detector and the following oven program: injection at 250 °C, detection at 280 °C, initial temperature at 120 °C held for 2 min, ramp to 300 °C at 5 °C min−1, and then held at 300 °C for 5 min, with He at 1 mL min−1.
For AA and DHAA analyses, extracts were transferred to vials as above and resuspended in 20 μL of 1:1 (v/v) pyridine (CHROMASOLV® Plus, Sigma-Aldrich) and N,O-bis(tri-methylsilyl)trifluoroacetamide (BSTFA, Restek) solution plus 50 μL pentane (Zhang et al. 2010). Samples were analyzed using GC/MS injection at 250 °C, detection at 280 °C, initiation temperature at 125 °C, and ramp to 300 °C at 5°C min−1 (Zhang et al. 2010) with 1 mL min−1 He.
Metabolites were identified via mass spectra compared to external standards (AN, Sigma-Aldrich Chemical; AB and AA, a gift from Dr. Nancy Acton of the Walter Reed Army Research Institute, Silver Spring, MD) and mass spectra of Zhang et al. (2010). DHAA, identified using the Zhang et al. (2010) mass spectra, is labile under long term storage making true standards unreliable so it was quantified based on an AA standard and expressed as AA equivalents. DeoxyAN was identified using the NIST library (2008); no deoxyAN standard was available so it was quantified based on an AN standard and expressed as AN equivalents.
Trichome analysis
To compare GLTs between 16 day rooted and unrooted shoots, the second fully formed leaf from rooted shoots and a fully developed leaf from unrooted shoots were taken for analysis. To compare young and mature leaves in rooted shoots, the second and ninth fully formed leaves from the apical meristem were chosen, respectively. Each leaf was placed on a glass slide with three drops of Type F immersion liquid (Leica Microsystems CMs GmbH) with silicon vacuum grease at each corner below the cover slip to level the leaf. Clear nail polish sealed the cover slip to secure the sample and prevent evaporation. Using a confocal microscope (Leica TCS SP5; Broadband Confocal, Leica Microsystems) three pictures of each leaf were taken, stacked, and pictures exported to ImageJ (http://rsbweb.nih.gov/ij/) for GLT counts and size area measurements. In three pictures, the GLTs mm−2 were counted, and the area of six randomly chosen GLTs were taken for GLT size analysis. Leaf area occupied by GLTs was:
Statistical methods
All experiments had ≥3 replicates. Metabolites and GLT data were averaged and analyzed using Student’s t test. Real-time PCR data were analyzed using the Mann–Whitney U test (Yuan et al. 2006).
Results
Artemisia annua shoots grown in RTM produce more AN than shoots grown in SHM, so kinetics of artemisinic metabolite production, GLTs, and transcript levels in rooting and unrooted shoots were measured first. Subsequently these same characteristics were measured in shoots ± roots grown in vitro with variations in phytohormones and nutrient levels. Comparisons were also made between young and mature shoots.
Effects of rooting on artemisinic metabolites, gene transcript and glandular trichomes
When unrooted shoots (shoots maintained in SHM) were inoculated into either RTM or SHM (control) and then grown for 16 days, roots first emerged at about 8 days in RTM (Fig. 2, arrow). After 16 days, shoots grown in RTM developed long roots and shoots elongated (Fig. 2). In contrast, shoots grown in SHM remained rootless and produced shrubby plantlets (Fig. 2). Shoot mass of unrooted shoots increased, reaching 0.18 g FW on day 16, while shoot mass of rooted shoots barely changed by day 16 (0.08 g FW). Once roots emerged on day 8, root mass of rooted shoots increased significantly by day 16 (0.17 g FW), and total biomass of rooted shoots was about 50 % greater than unrooted shoots.
Fig. 2.
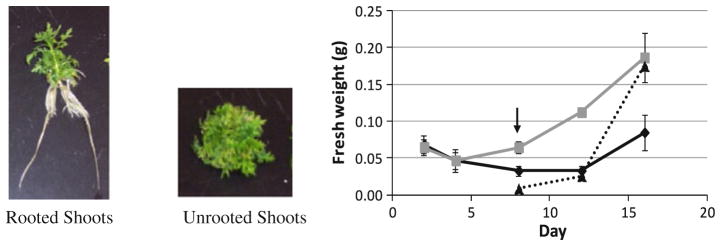
A. annua shoots grown in either rooting (RTM) or shooting medium (SHM) for 16 days. Grey solid line unrooted shoots, black solid line shoots of rooted shoots, black dotted line roots of rooted shoots. Arrow indicates appearance of roots, n ≥ 4
After 4 days, both AN and deoxyAN were significantly greater in rooted shoots than unrooted shoots (Fig. 3a, b). By day 8 when roots were first visible, rooted shoots had significantly greater amounts of all three end products than unrooted shoots (Fig. 3a–c). In contrast, neither of the measured precursors, AA and DHAA, was significantly different. When the artemisinic metabolites were measured at 16 days, more AN, deoxyAN, and AB were found in rooted shoots (390.3, 171.7, and 199.1 μg g FW−1, respectively) than in unrooted shoots (131.3, 68.4, and 88.6 μg g FW−1, respectively), but DHAA and AA levels in both cultures remained about the same (Fig. 3a–e). Compared to unrooted shoots, rooted shoots accumulated more end products (AN, deoxyAN, AB) than precursors (DHAA, AA) of the AN biosynthetic pathway (Fig. 3). Interestingly, when measured right after inoculation, shoot cultures subcultured into either fresh SHM or fresh RTM consistently dropped in artemisinic content from that measured at 14–16 days, a response for which we have no explanation except that it seems to be a subculture stress response.
Fig. 3.
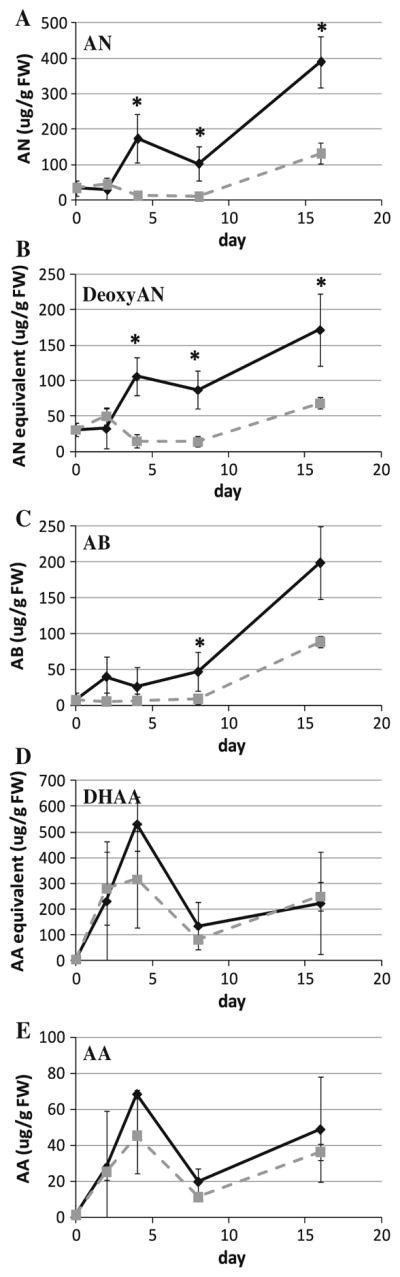
Metabolite levels in shoots as roots develop. a Artemisinin (AN), b deoxyartemisinin (deoxyAN), c arteannuin B (AB), d dihydroartemisinic acid (DHAA), e artemisinic acid (AA). Solid line RTM, dashed line SHM, n ≥ 4, *p ≤ 0.05
Next, transcript levels of three genes in the pathway were measured. ADS and CYP71AV1 (CYP) are the first two enzymes in the AN biosynthetic pathway; therefore it was important to determine how rooting affects transcript levels of these genes. DBR2 was also measured, because it functions only in the branch that leads to AN and deoxyAN production. Differences detected in dbr2 transcripts help determine, if there is a shift to either the AA–AB branch or the DHAA–AN–deoxyAN branch of the pathway in response to rooting. As in the previous experiment, unrooted shoots were inoculated into either RTM or SHM (control). Transcripts of ads, cyp, and dbr2 were measured using qPCR on days 2, 4, 8, 12, and 16 after inoculation. Although ads transcripts on day 4, 8, 12, and 16 increased relative to day 0, there was no difference between rooted and unrooted shoots (Fig. 4a). Similar to the response for ads, transcripts of cyp and dbr2 also showed no differences between rooted and unrooted shoots (Fig. 4b, c). Although transcripts clearly increase early in all cultures, there is no significant difference between rooted and unrooted shoots at any measured time point suggesting that rooting does not affected transcript levels of these genes, and that the shift in AN level must be due to something else.
Fig. 4.
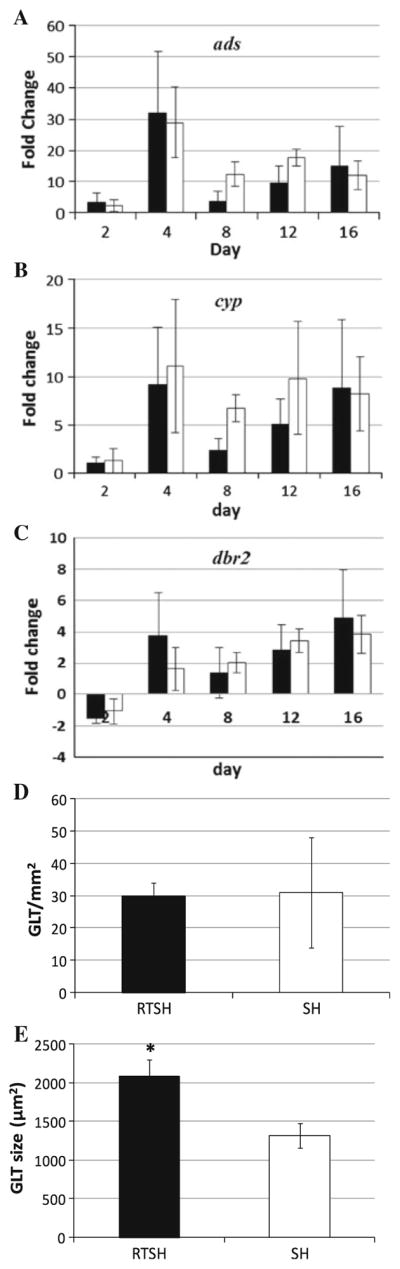
Changes in transcript levels of a ads, b cyp, and c dbr2 and GLTs in shoots as roots develop in A. annua. For transcript levels black bar unrooted shoots inoculated into RTM, white bar unrooted shoots inoculated into SHM, n ≥ 3. d GLT population density, e GLT size, RTSH rooted shoots, SH unrooted shoots, n ≥ 4, *p ≤ 0.05
AN is produced and stored in the GLTs. Since rooting did not seem to affect transcript levels of the AN pathway genes, we considered that GLTs instead may be affected. Using confocal microscopy, the number and size of trichomes were measured on 16-day-old in vitro cultured rooted and unrooted shoots. There was no significant difference in GLT populations on leaves of rooted and unrooted shoots (29.9 and 30.9 GLT mm−2, respectively; Fig. 4d). On the other hand, GLTs of rooted shoots were significantly larger than those of unrooted shoots (2,081 and 1,317 μm2, respectively; Fig. 4e). It appeared that roots or media composition may, therefore, affect the size, but not necessarily the number, of GLTs on leaves of A. annua.
Effects of NAA and BAP on artemisinic metabolites and trichomes of rooted shoots
Although roots may play a role in the AN biosynthetic pathway, it is also possible that the phytohormones, BAP and NAA, in SHM are inhibiting the pathway and causing lower levels of AN, deoxyAN, and AB in unrooted shoots. To determine if either roots and/or phytohormones are important in artemisinic metabolite production, 2-week-old rooted shoots were inoculated into RTM ± NAA, ± BAP, or ± both phytohormones (Fig. S1A–D). Another difference between RTM and SHM is that RTM only contains half-strength MS salts and vitamins plus 2 % sucrose, while SHM contains full strength MS salts and vitamins plus 3 % sucrose. To separate hormonal responses from responses to other medium constituents, 2-week-old rooted shoots were inoculated into SHM and compared to the rooted shoots inoculated into RTM + NAA + BAP (Fig. S1D, E). Cultures were grown for 3 weeks and then biomass, artemisinic metabolites, and GLTs were measured and compared in the young (post inoculation) and mature (pre inoculation) leaves of the shoots. It was assumed that if there was an effect from either phytohormone or other medium constituents (MS salts and sucrose concentration), then it would occur in the new growth that developed post inoculation into the various media formulations. Data are summarized in Fig. S2.
After 3 weeks, rooted shoots in RTM and RTM + NAA showed similar growth morphology (Fig. S1A, B): many leaves, lengthened internodes, and long roots. On the other hand, rooted shoots grown in RTM + BAP (Fig. S1C), RTM + NAA + BAP (Fig. S1D) and SHM (Fig. S1E) did not grow as tall as those grown in either RTM (Fig. S1A) or RTM + NAA (Fig. S1B). In the former medium, roots (Fig. S1C–E) were also thick and short; however, the mass of the roots was no different from those in RTM or RTM + NAA (Fig. S1F).
Artemisinic end products, AN, deoxyAN, and AB, were also measured in both young and mature growth, and compared to growth in RTM there was no change in AN in the young growth of rooted shoots grown in RTM with either or both phytohormones (Fig. 5a). DeoxyAN increased in young growth only when BAP was present (Fig. 5b). AB on the other hand, increased in young growth when grown in RTM + NAA, + BAP or Both (Fig. 5c). There was more of the AN precursor, DHAA, in young growth in cultures with BAP (Fig. 5d).
Fig. 5.
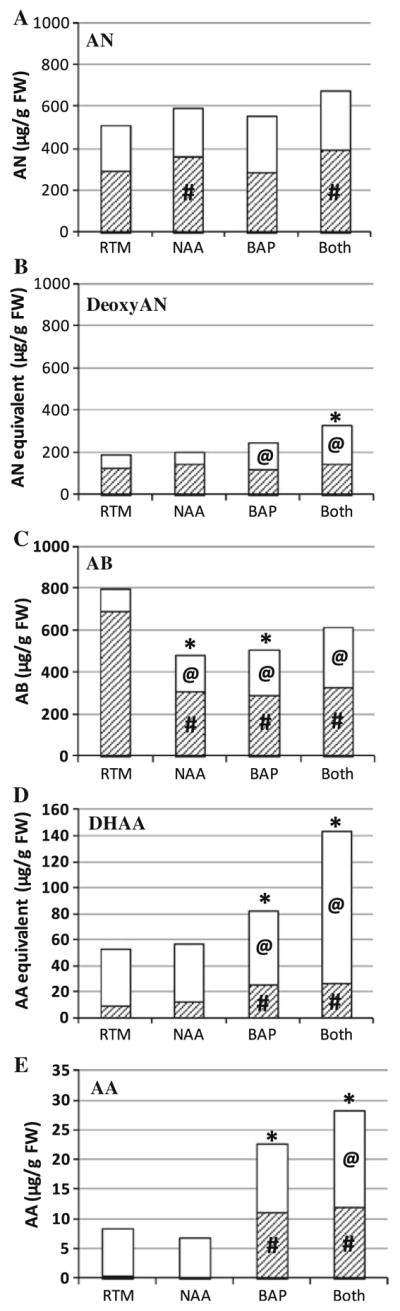
Metabolite levels of rooted shoots after growing in RTM with NAA ± BAP when compared to correlating leaves in RTM controls. End products: a AN, b deoxyAN, c AB; precursors: d DHAA, e AA. Shaded mature leaves, white young leaves, n ≥ 7. For comparison to mature leaves in RTM #p ≤ 0.05, for comparison to young leaves in RTM @p ≤ 0.05, for comparison to entire plant in RTM *p ≤ 0.05. RTM rooting medium; NAA, RTM + 0.25 μM NAA; BAP, RTM + 2.5 μM BAP; Both, RTM + 0.25 μM NAA + 2.5 μM BAP
One might assume that any effect of the phytohormones or other medium constituents would be visible mainly in the new growth, but that was not the case. When compared to the mature growth of cultures grown in RTM, there was more AN in the mature growth of cultures grown in RTM + NAA and in RTM + NAA + BAP (Fig. 5a), but there was no effect on deoxyAN (Fig. 5b). In contrast AB decreased in mature growth of cultures grown in RTM + NAA ± BAP (Fig. 5c). The precursors, AA and DHAA, both increased when BAP was present ± NAA (Fig. 5d, e).
Overall, cultures grown in the presence of NAA and/or BAP had significantly less AB in their total shoot biomass when grown in either NAA or BAP, but not Both (Fig. 5c); total AN was not affected (Fig. 5a). Total deoxyAN only increased if both phytohormones were present (Fig. 5b). Total AA and DHAA levels also increased whenever BAP was present (Fig. 5d, e).
To determine if the other media constituents (MS salts, vitamins, and sucrose) had any effect on artemisinic metabolite levels of rooted shoots, we compared cultures grown in RTM + NAA + BAP to cultures grown in SHM. While the higher concentration of MS salts, vitamins, and sucrose in SHM increased the production of AB in mature growth (Table S3), it did not affect any of the other metabolites in either new or mature growth.
Effects of NAA, BAP, and other media constituent on trichomes
Since AN and the other artemisinic metabolites are stored in the GLTs and it appears that phytohormones are playing a role in the AN biosynthetic pathway, it is possible that GLT development was also affected. As in the previous experiment, 2-week-old rooted shoots were inoculated into the five test media and after 3 weeks, two fully expanded leaves were analyzed. The second fully formed leaf from the shoot apical meristem was taken as representative of young leaves developed post inoculation; the ninth fully formed leaf, below the inoculation line, was taken as representative of mature leaves. Each set of leaves was viewed under a confocal microscope and pictures taken and stacked to provide a top down view of the maximum area of each GLT; this enabled measurement of GLT expansion that may have occurred as leaves expanded throughout development. Views of glandular and filamentous trichomes are shown in Fig. 6a. Data are summarized in Fig. S2.
Fig. 6.
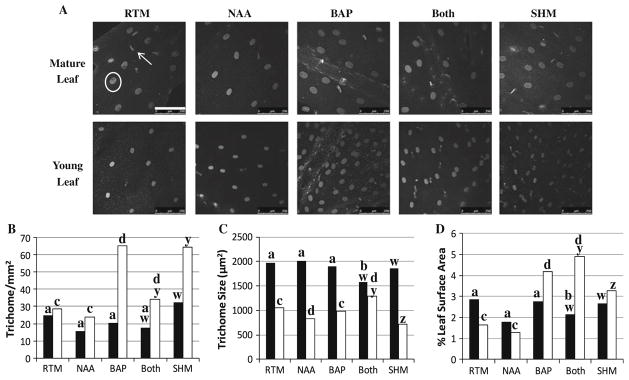
Glandular trichome development of rooted shoots in RTM with NAA ± BAP or in SHM. a GLTs under a confocal microscope; in RTM mature leaf image white circle glandular GLT (GLT), white arrow filamentous trichome, bar 250 μm. b GLT population density, c GLT size, d percent leaf surface occupied by GLTs. Black mature leaves, white young leaves. For comparison between mature leaves in RTM versus NAA, BAP or Both a, b; for young leaves c, d. For comparison between mature leaves in SHM and Both w, x; for young leaves y, z. RTM rooting medium, SHM shooting medium; NAA, RTM + 0.25 μM NAA; BAP, RTM + 2.5 μM BAP; Both, RTM + 0.25 μM NAA + 2.5 μM BAP
When rooted shoots were transferred into fresh RTM, GLT numbers increased only in young leaves and only in the presence of BAP (Fig. 6b). GLT size decreased slightly in young leaves when NAA was present (Fig. 6c). In contrast GLT size on mature leaves showed no response to either NAA or BAP; however, when both phytohormones were present, size decreased nearly 25 % (Fig. 6c). For comparison, the average GLT size of young leaves was 1,056 μm2 compared to 1,966 μm2 for mature leaves of plants grown in RTM (Fig. 6c).
Percent leaf area occupied by GLTs was also calculated to measure overall impact of each phytohormone on GLT development (Fig. 6d). In rooted shoots grown in RTM, the relative area occupied by GLTs in mature leaves was twice that of young leaves, but in RTM + BAP + NAA (Both) the results were reversed. Area occupied by GLTs in young leaves was twice that of mature leaves. Although SHM and RTM + NAA + BAP (Both) each contained BAP, there was no difference in overall leaf area occupied by GLTs.
Other media constituents also affected GLTs, but only in size (Fig. 6c). In SHM, where there was a greater amount of salts, vitamins, and sucrose, there was no change in GLT density in either young or mature leaves. On the other hand, young leaves on plants growing in SHM had smaller GLTs than plants grown in RTM + NAA + BAP (Both, Fig. 6c) and this resulted in less leaf coverage (Fig. 6d). In contrast, there was no difference in GLT population density, size or leaf surface area occupied by GLTs on mature leaves.
Effects of BAP and NAA on rooted shoots with their roots excised
Both BAP and NAA affected production of artemisinic metabolites, so we considered that shoots with already formed root systems may be predisposed to responding to media conditions. In other words, is there a preset root signal? It was posited, therefore, that if phytohormones had a greater influence than roots, then in the presence of phytohormones, shoots that had their roots excised should yield greater artemisinic compounds than rooted shoots. For example, if BAP, but not the roots, plays a critical role in the AN pathway, then the metabolite levels should be similar to those found in shoots with intact roots grown with BAP.
Compared to growth in the RTM control (shoots with roots left intact at inoculation), BAP-treated cultures had less shoot, but increased root mass (Table S4). In contrast, in shoots with excised roots, BAP increased shoot mass compared to RTM controls; root mass was undetectable (Table S4). NAA had no effect on either root or shoot mass for either type of inoculum.
In shoots with intact roots neither BAP nor NAA had any effect on AN or deoxyAN production compared to RTM controls (Fig. 7a). Each phytohormone; however, decreased AB content by about 50 % (Fig. 6a). BAP also increased the two precursors, AA and DHAA (Fig. 7a). Compared to AN, the DHAA level in all three culture conditions was low, <20 % of the corresponding AN. Overall, all cultures had high levels of end products, but precursors were ≤ 10 % of the total end products (Fig. 7c).
Fig. 7.
Metabolite levels in shoots after growing in RTM with NAA or BAP when inoculated either as rooted shoots or shoots that had their roots excised prior to inoculation. a individual metabolites in shoots with roots intact with NAA or BAP, b individual metabolites in shoots with roots excised grown in medium containing NAA or BAP, c comparison of total end products (AN + deoxyAN + AB) versus total precursors (AA + DHAA) versus sum of all five metabolites in shoots where inoculum was rooted shoots, d comparison of total end products (AN + deoxyAN + AB) versus total precursors (AA + DHAA) versus sum of all five metabolites in shoots where inoculum was shoots with roots excised. *p ≤ 0.05 when compared to RTM, n ≥ 6. Black RTM, white RTM + 2.5 μM BAP, hatched RTM + 0.25 μM NAA. AN artemisinin, deoxyAN deoxyartemisinin, AB arteannuin B, AA artemisinic acid, DHAA dihydroartemisinic acid, AX all artemisinic metabolites
When shoots with excised roots were instead used, BAP reduced AN, deoxyAN, and AB compared to shoots grown in either RTM or in RTM + NAA (Fig. 7b). There was, however no significant change in either AA or DHAA (Fig. 7b). NAA had no effect on any of the measured metabolites. Compared to AN, shoots grown in all three culture conditions had high DHAA levels (Fig. 7b), which differed from shoots with intact roots (Fig. 7a). In contrast to shoots with intact roots (Fig. 7c), the total level of precursors found in shoots with excised roots was about the same as the total amount of end products (Fig. 7d).
Discussion
To determine if A. annua roots have any regulating effect on AN production that is also not confounded by BAP or NAA, roots were removed from rooted shoots, inoculated into media ± BAP or NAA, and compared to shoots with intact roots. Comparisons were also made to controls grown in RTM, which lacks phytohormones. Although the total level of sesquiterpenes does not appear to differ between these experimental treatments, end products AN, AB, and deoxyAN significantly decreased when shoots with excised roots were grown in RTM + BAP. Roots, therefore, were crucial to the continued production of AN in the media tested, and especially in the presence of BAP. Without roots, BAP actually inhibited AN production. This was surprising considering that the root apical meristem is a primary site for cytokinin production (Kende 1965) and transported up and through the shoot via the xylem (Sachs and Thimann 1967); rootless shoots provided with exogenous BAP should have produced at least an equivalent amount of artemisinic metabolites as their RTM controls. Contrary to our results, Sa et al. (2001) showed that an ipt transgenic line (A1-1) of A. annua produced about 60 % more cytokinin and 60 % more AN than the untransformed control. In our study in the BAP medium, roots did not form on excised shoots, while in NAA medium, shoots developed new roots. Consequently roots were present at harvest and as expected, compared to NAA-free controls (RTM), AN content was similar. Interestingly, the A1-1 transgenic line had a 60 % decrease in root mass, so together with our results this suggests that the ratio of auxin (being produced in the shoot) to cytokinin (supplied in the medium) is crucial for proper function of the artemisinic pathway.
Neither precursors nor end products changed in the shoots grown ± NAA. Again this was likely, because at harvest all shoots had formed roots. Without roots, precursor levels, especially DHAA, remained high, and in total were equivalent to the concentration of end products. On the other hand, in the presence of roots, the ratio of total end products to precursors was at least tenfold, if there were rooted shoots then DHAA was low. Overall roots were thus needed to drive conversion of precursors to end products including DHAA to AN, and BAP appeared to limit this process.
When measuring metabolite production in shoots during root development, end products also accumulated only when roots were present. The high levels of end products were not accompanied by a significant increase in AN gene transcripts, suggesting that roots and/or media may not regulate the pathway at the level of transcription, but possibly by another unidentified mechanism, perhaps post translation. GLT size in rooted shoots also was larger than in unrooted shoots. At first glance this seemed to indicate that the presence of roots induced an accumulation of metabolites in the GLT causing it to expand. However, the presence of two potent phytohormones, NAA and BAP, in SHM had to be investigated, because they could confound interpretations.
To our knowledge, not much is known about the effect of auxins on the AN biosynthetic pathway. Although Martinez and Staba (1988) reported that the AN content of unrooted shoots was unaffected by the auxins, 2,4-D and IAA, Woerdenbag et al. (1993) later reported that NAA at 0.2 mg L−1 yielded the maximum level of AN. In our study comparing young to mature shoot tissue, NAA increased AB only in young shoots, suggesting that NAA might be driving the conversion of AA to AB. AA, however was not detected in the mature growth, suggesting that it was already converted to AB. In the mature growth whenever NAA was present, AN increased compared to RTM controls. To determine if AN levels could be increased in field-grown plants, we subsequently sprayed mature leaves of potted plants with NAA, but there was no change in AN or other artemisinic metabolite content (data not shown), suggesting the NAA response in the older growth was only perceived via root exposure. A similar root-isolated response was previously reported (Mannan et al. 2010).
Reactive oxygen species (ROS) are thought to be involved in the AN biosynthetic pathway, specifically the last step from AA → AB and DHAA → AN or DHAA → deoxyAN (Fig. 1, Brown and Sy 2004, 2007; Covello 2008; Mannan et al. 2010). In maize, auxin induced ROS in roots (Joo et al. 2001), and in coleoptiles (Schopfer et al. 2002). Although those auxin levels were at least 20 times greater than used in this study, the results are consistent with our NAA response in mature A. annua shoots, suggesting a possible ROS stimulation of AN. On the other hand, the level of AB remained low compared to that in NAA-free controls. Considering that AB formation from AA also involves ROS (Brown and Sy 2007), this suggests that at least in the mature portion of the shoot that was already developed at inoculation, the NAA effect may not be ROS related.
In contrast to observed effects of NAA on mature rooted shoots, BAP did not stimulate AN production, but it did increase the sum metabolites (DHAA + deoxyAN + AN) of that pathway. Interestingly, however, while BAP stimulated AA production in mature growth, AB decreased. In young growth, on the other hand, AB increased in response to BAP. Together with the effects of NAA on mature growth, it appears that the AA → AB transition is also inhibited by both phytohormones, but that only NAA stimulates AN production. The data further suggest that, in the DHAA–AN–deoxyAN branch, NAA may be the key player in the DHAA–AN sub-branch, while BAP appears to be more influential in the DHAA–deoxyAN sub-branch.
Although in the mature leaves BAP did not independently affect either glandular GLT populations or GLT size compared to RTM leaves, in RTM + BAP + NAA, GLT size decreased. When metabolites were measured, they also declined overall about 20 %, consistent with the decline in GLT size. Furthermore, compared to RTM controls, when both NAA and BAP were present, AB declined ~50 %, but AA and AN/DHAA increased. Together these results suggest that in the mature growth, NAA is likely inhibiting production of AB, but enhancing production of AN. BAP also inhibits AB production in old growth. Like BAP, NAA seems to be regulating the AN biosynthetic pathway directly and not through development of GLTs, where artemisinic metabolites are synthesized and stored.
Sa et al. (2001) constitutively expressed isopentenyl transferase in A. annua, and both cytokinin and AN levels increased, suggesting that cytokinins play a role in AN biosynthesis. Maes et al. (2011) later reported that when plants were sprayed with BAP, artemisinic metabolites did not increase despite increased populations of GLTs. In our study the young growth of rooted shoots grown in RTM + BAP showed an increase in the DHAA precursors and two of the end products, deoxyAN and AB, but there was no change in AN or AA. In mature growth of the same cultures, BAP inhibited AB production by about 60 % while also increasing production of DHAA and AA, but without affecting GLTs or AN. Together these results suggest that BAP shifts artemisinic metabolism and GLT development, but mainly in young growth.
In the current study increasing the concentration of salts, vitamins, and sucrose affected the AN biosynthetic pathway in mature shoots, but mainly by the AB route and none of the other artemisinic metabolites was affected. Different concentrations of sugars (i.e., sucrose, glucose, and fructose) alter production of artemisinic metabolites over time. For example, in unrooted shoots, sucrose levels of 1–3 % (w/v) induced optimum production of AN (Woerdenbag et al. 1993; Liu et al. 2003). In A. annua seedlings, glucose was shown to stimulate AN production, while fructose inhibited AN production and as the ratio of glucose to fructose increased, so also did AN production (Wang and Weathers 2007; Arsenault et al. 2010b). Nutrients like potassium and nitrogen can also increase AN production (Ferreira 2007; Davies et al. 2009). Our study shows that factors such as vitamins, salts, and sucrose also play a role in AN production, but not necessarily as pathway regulators.
Lommen et al. (2006) previously noted that levels of the AN precursor, DHAA, were highest in young leaves, declining as leaves matured. As DHAA declined, AN increased with highest levels measured in mature and in senesced (brown) leaves (Lommen et al. 2006). We observed similar metabolite patterns in A. annua rooted shoots. Graham et al. (2010) further noted that maximum AN levels were in leaves at node 11 and older, suggesting full maturity of GLTs. Studies have shown correlations between AN content and GLT population (Kapoor et al. 2007; Arsenault et al. 2010a; Graham et al. 2010). The current study also shows a correlation between end product accumulation and GLT size. For AN at least, its accumulation in mature leaves seems linked to the expansion of the GLTs.
Conclusions
When roots were present, artemisinic precursors were low in shoots and end products high, suggesting that roots were needed to drive the final reactions from DHAA to AN and deoxyAN and from AA to AB. DHAA in particular was high and AN low when roots were absent, but when roots were present, DHAA was low and AN high. NAA induced higher AN production, but only in mature growth of rooted plants; however, NAA had no effect on AN production when applied to leaves. In the absence of roots, BAP inhibited AN production, but in the presence of roots, BAP had no effect. Overall, there was no difference in the amount of total end products of rooted shoots grown with either phytohormone compared to those grown in hormone-free RTM. While NAA decreased GLT size, it did so only in young leaves, where BAP, on the other hand, increased the number of GLTs. When both phytohormones were present, GLT number and size increased in young leaves, but their size decreased in mature leaves. Furthermore, an increase in other media constituents resulted in decreased size and area of GLT coverage, but only in young leaves. Together these results have furthered our understanding of the role of roots as well as NAA and BAP in glandular GLT development and the production of AN in A. annua.
Supplementary Material
Key message.
Rooting of Artemisia annua increases trichome size on leaves and helps drive the final steps of the biosynthesis of the sesquiterpene antimalarial drug, artemisinin.
Acknowledgments
We thank Dr. Patrick Arsenault, University of Pennsylvania Medical School, for much including a critical reading of the manuscript; Victoria Huntress and Prof. Luis Vidali helped with confocal imaging; Prof. Humi Mayer, WPI Math department, assisted data analysis and Praphapan Lasin helped with plants. The project described was supported by Award Number NIH-2R15GM069562-03 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Footnotes
Communicated by P. Lakshmanan.
Electronic supplementary material The online version of this article (doi:10.1007/s00299-012-1355-4) contains supplementary material, which is available to authorized users.
References
- Arsenault PR, Vail D, Wobbe KK, Erickson K, Weathers PJ. Reproductive development modulates gene expression and metabolite levels with possible feedback inhibition of artemisinin in Artemisia annua L. Plant Physiol. 2010a;154:958–968. doi: 10.1104/pp.110.162552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault PR, Vail DR, Wobbe KK, Weathers PJ. Effect of sugars on artemisinin production in A. annua L.: transcription and metabolite measurements. Molecules. 2010b;15:2302–2318. doi: 10.3390/molecules15042302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banyai W, Kirdmanee C, Mii M, Supaibulwatana K. Enhancement of artemisinin content and biomass in Artemisia annua by exogenous GA3 treatment. Plant Growth Regul. 2011;63:45–54. [Google Scholar]
- Brown GD. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao) Molecules. 2010;15:7603–7698. doi: 10.3390/molecules15117603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Sy LK. In vivo transformations of dihydroartemisinic acid in Artemisia annua plants. Tetrahedron. 2004;60:1139–1159. [Google Scholar]
- Brown GD, Sy LK. In vivo transformations of artemisinic acid in Artemisia annua plants. Tetrahedron. 2007;63:9548–9566. [Google Scholar]
- Chang YJ, Song SH, Park SH, Kim SU. Amorpha-4,11-diene synthase of Artemisia annua: cDNA isolation and bacterial expression of a terpene synthase involved in artemisinin biosynthesis. Arch Biochem Biophys. 2000;383:178–184. doi: 10.1006/abbi.2000.2061. [DOI] [PubMed] [Google Scholar]
- Cikos S, Bukovska A, Koppel J. Relative quantification of mRNA: comparison of methods currently used for real-time PCR data analysis. BMC Mol Biol. 2007;20:113–123. doi: 10.1186/1471-2199-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covello PS. Making artemisinin. Phytochemistry. 2008;69:2881–2885. doi: 10.1016/j.phytochem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Atkinson CJ, Burns C, Woolley JG, Hipps NA, Arroo RRJ, Dungey N, Robinson T, Brown P, Flockart I, Hill C, Smith L, Bentley S. Enhancement of artemisinin concentration and yield in response to optimization of nitrogen and potassium supply to Artemisia annua. Ann Bot Lond. 2009;104:315–323. doi: 10.1093/aob/mcp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke MG, Paul RN, Elsohly HN, Sturtz G, Duke SO. Localization of artemisinin and artemisitene in foliar tissues of glanded and glandless biotypes of Artemisia annua L. Int J Plant Sci. 1994;155:185–209. [Google Scholar]
- Efferth T. Herbal drugs: ethnomedicine to modern medicine. Springer; Berlin: 2009. Artemisinin: a versatile weapon from traditional Chinese medicine; pp. 173–194. [Google Scholar]
- Ferreira JFS. Nutrient deficiency in the production of artemisinin, dihydroartemisinic acid, and artemisinic acid in Artemisia annua L. J Agric Food Chem. 2007;55:1686–1694. doi: 10.1021/jf063017v. [DOI] [PubMed] [Google Scholar]
- Ferreira JFS, Janick J. Floral morphology of Artemisia annua with special reference to trichomes. Int J Plant Sci. 1995;156:807–815. [Google Scholar]
- Ferreira JFS, Janick J. Roots as an enhancing factor for the production of artemisinin in shoot cultures of Artemisia annua. Plant Cell Tissue Organ Cult. 1996;44:211–217. [Google Scholar]
- Firestone GL, Sundar SN. Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev Mol Med. 2009;11:e32. doi: 10.1017/S1462399409001239. [DOI] [PubMed] [Google Scholar]
- Graham IA, Besser K, Blumer S, Branigan CA, Czechowski T, Elias L, Guterman I, Harvey D, Isaac PG, Khan AM, Larson TR, Li Y, Pawson T, Penfield T, Rae AM, Tathbone DA, Reid S, Ross J, Smallwood MF, Segura V, Townsend T, Vyas D, Winzer T, Bowles D. The genetic map of Artemisia annua L. identifies loci affecting yield of the antimalarial drug artemisinin. Science. 2010;327:328–331. doi: 10.1126/science.1182612. [DOI] [PubMed] [Google Scholar]
- Guo XX, Yang XQ, Yang RY, Zeng QP. Salicylic acid and methyl jasmonate but not Rose Bengal enhance artemisinin production through involving burst of endogenous singlet oxygen. Plant Sci. 2010;178:390–397. [Google Scholar]
- Gupta SK, Singh P, Baipai P, Ram G, Singh D, Gupta MM, Jain DC, Khanuja SP, Kumar S. Morphogenetic variation for artemisinin and volatile oil in Artemisia annua. Indian Crop Prod. 2002;16:217–224. [Google Scholar]
- Jing F, Zhang L, Li M, Tang Y, Wang Y, Wang Y, Wang Q, Pan Q, Wang G, Tang K. Abscisic acid (ABA) treatment increases artemisinin content in Artemisia annua by enhancing the expression of genes in artemisinin biosynthesis pathway. Biologia. 2009;64:312–323. [Google Scholar]
- Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in roots gravitropism. Plant Physiol. 2001;126:1055–1060. doi: 10.1104/pp.126.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor RR, Chaudhary V, Bhatnagar AK. Effects of arbuscular mycorrhiza and phosphorus application on artemisinin concentration in Artemisia annua L. Mycorrhiza. 2007;17:581–587. doi: 10.1007/s00572-007-0135-4. [DOI] [PubMed] [Google Scholar]
- Kende H. Kinetin-like factors in root exudates of sunflowers. Proc Nat Acad Sci USA. 1965;53:1302–1307. doi: 10.1073/pnas.53.6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NC, Kim JG, Lim HJ, Hahn RT, Kim SU. Production of secondary metabolites by tissue cultures of Artemisia annua L. J Korean Agric Chem Soc. 1992;35:99–105. [Google Scholar]
- Kim SH, Chang YJ, Kim SU. Tissue specificity and developmental pattern of amorpha-4,11-diene synthase (ADS) proved by promoter-driven GUS expression in the heterologous plant Arabidopsis thaliana. Planta Med. 2008;74:188–193. doi: 10.1055/s-2008-1034276. [DOI] [PubMed] [Google Scholar]
- Liu CZ, Guo C, Wang Y, Ouyang F. Factors influencing artemisinin production from shoot cultures of Artemisia annua L. World J Microb Biot. 2003;19:535–538. [Google Scholar]
- Liu S, Tian N, Huang J, Liu Z. Isolation and identification of novel genes involved in artemisinin production from flowers of Artemisia annua using suppression subtractive hybridization and metabolite analysis. Planta Med. 2009;74:1542–1547. doi: 10.1055/s-0029-1185809. [DOI] [PubMed] [Google Scholar]
- Lommen WJM, Schenk E, Bouwmeester HJ, Verstappen FWA. Trichome dynamics and artemisinin accumulation during development and senescence of leaves of Artemisia annua leaves. Planta Med. 2006;72:336–345. doi: 10.1055/s-2005-916202. [DOI] [PubMed] [Google Scholar]
- Maes L, Van Nieuwerburgh FCW, Zhang Y, Reed DW, Pollier J, Vande Casteele SRF, Covello PS, Deforce DLD, Goossens A. A dissection of the phytohormonal regulation of trichome formation and biosynthesis of the antimalarial compound artemisinin in Artemisia annua plants. New Phytol. 2011;189:176–189. doi: 10.1111/j.1469-8137.2010.03466.x. [DOI] [PubMed] [Google Scholar]
- Mannan AA, Liu C, Arsenault PR, Towler MJ, Vail DR, Lorence A, Weathers PJ. DMSO triggers the generation of ROS leading to an increase in artemisinin and dihydroartemisinic acid in Artemisia annua shoot cultures. Plant Cell Rep. 2010;29:143–152. doi: 10.1007/s00299-009-0807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez BC, Staba EJ. The production of artemisinin in Artemisia annua L. tissue cultures. Adv Cell Cult. 1988;6:69–87. [Google Scholar]
- Mercke P, Bengtsson M, Bouwmeester HJ, Posthumus MA, Brodelius PE. Molecular cloning, expression, and characterization of amorpha-4, 11-diene synthase, a key enzyme of artemisinin biosynthesis in Artemisia annua L. Arch Biochem Biophys. 2000;381:173–180. doi: 10.1006/abbi.2000.1962. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Arsenault PR, Weathers PJ. Trichomes + roots + ROS = artemisinin: regulating artemisinin biosynthesis in Artemisia annua L. In Vitro Cell Dev Biol Plant. 2011;47:329–338. doi: 10.1007/s11627-011-9343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson L, Engström A, Lundgren A, Brodelius P. Relative expression of genes of terpene metabolism in different tissues of Artemisia annua L. BMC Plant Biol. 2011;11:45. doi: 10.1186/1471-2229-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson ME, Olofsson LM, Lindahl AL, Lundgren A, Brodelius M, Brodelius PE. Localization of enzymes of artemisinin biosynthesis to the apical cells of glandular secretory trichomes of Artemisia annua L. Phytochemistry. 2009;70:1123–1128. doi: 10.1016/j.phytochem.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Pu GB, Ma DM, Chen JL, Ma LQ, Wang H, Li GF, Ye HC, Liu BY. Salicylic acid activates artemisinin biosynthesis in Artemisia annua L. Plant Cell Rep. 2009;7:1127–1135. doi: 10.1007/s00299-009-0713-3. [DOI] [PubMed] [Google Scholar]
- Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MCY, Withers ST, Shiba Y, Sarpong R, Keasling JD. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- Rydén AM, Ruyter-Spira C, Quax WJ, Osada H, Muranaka T, Kayser O, Bouwmeester H. The molecular cloning of dihydroartemisinic aldehyde reductase and its implication in artemisinin biosynthesis in Artemisia annua. Planta Med. 2010;76:1778–1783. doi: 10.1055/s-0030-1249930. [DOI] [PubMed] [Google Scholar]
- Sa G, Ma M, Ye HC, Liu BY, Li GF, Chong K. Effects of ipt gene expression on the physiological and chemical characteristics of Artemisia annua L. Plant Sci. 2001;160:691–698. doi: 10.1016/s0168-9452(00)00453-2. [DOI] [PubMed] [Google Scholar]
- Sachs T, Thimann KV. The role of auxins and cytokinins in the release from apical dominance. Am J Bot. 1967;45:135–144. [Google Scholar]
- Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A. Evidence that hydroxyl radicals mediate auxin-induce extension growth. Planta. 2002;214:821–828. doi: 10.1007/s00425-001-0699-8. [DOI] [PubMed] [Google Scholar]
- Teoh KH, Polichuk DR, Reed DW, Nowak G, Covello PS. Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett. 2006;580:1411–1416. doi: 10.1016/j.febslet.2006.01.065. [DOI] [PubMed] [Google Scholar]
- Teoh KH, Polichuk DR, Reed DW, Covello PS. Molecular cloning of an aldehyde dehydrogenase implicated in artemisinin biosynthesis in Artemisia annua. Botany. 2009;87:638–642. [Google Scholar]
- Wallaart TE, Van Uden W, Lubberink HGM, Woerdenbag HJ, Pras N, Quax WJ. Isolation and identification of dihydroartemisinic acid from Artemisia annua and its possible role in the biosynthesis of artemisinin. J Nat Prod. 1999;62:430–433. doi: 10.1021/np980370p. [DOI] [PubMed] [Google Scholar]
- Wallaart TE, Bouwmeester HJ, Hille J, Popping L, Maijers NC. Amorpha-4,11-diene synthase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta Med. 2001;66:460–465. doi: 10.1007/s004250000428. [DOI] [PubMed] [Google Scholar]
- Wang Y, Weathers PJ. Sugars proportionately affect artemisinin production. Plant Cell Rep. 2007;26:1073–1081. doi: 10.1007/s00299-006-0295-2. [DOI] [PubMed] [Google Scholar]
- Woerdenbag H, Pras N, Bos R, Visser JF, Hendriks H, Malingré TM. Analysis of artemisinin and related sesquiterpenoids from Artemisia annua L. by combined gas chromatography/mass spectrometry. Phytochem Anal. 1991;2:215–219. [Google Scholar]
- Woerdenbag HJ, Lüers JfJ, Uden WV, Pras N, Malingré TM, Alfermann AW. Production of the new antimalarial drug artemisinin in shoot cultures of Artemisia annua L. Plant Cell Tissue Organ Cult. 1993;32:247–257. [Google Scholar]
- Yuan JS, Reed A, Chen F, Stewart CNJ. Statistical analysis of real-time PCR data. Bioinformatics. 2006;7:85–90. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Teoh KH, Reed DW, Maes L, Goossens A, Olson DJH, Ross ARS, Covello PS. The molecular cloning of artemisinic aldehyde 11(13) reductase and its role in glandular trichome-dependent biosynthesis of artemisinin in Artemisia annua. J Biol Chem. 2008;283:21501–21508. doi: 10.1074/jbc.M803090200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Nowak G, Reed DW, Covello PS. The production of artemisinin precursors in tobacco. Plant Biotechnol J. 2010;9:445–454. doi: 10.1111/j.1467-7652.2010.00556.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.