Abstract
Self-reported sexual behaviors are subject to bias. We previously developed a polymerase chain reaction for the detection of Y-chromosome sequences in vaginal fluid as a potential biomarker for recent sexual activity. In this study, we found menses results in lower Y-chromosome concentrations but with similar decay patterns as non-menstrual samples.
Keywords: biomarker, sexually transmitted infection, y-chromosome
Measures of sexual behavior are used in surveys and are particularly important in evaluating the efficacy of STI/HIV interventions.(1) Previous work has clearly shown that self-reported sexual behaviors are subject to bias.(2) As a result, biomarkers have been proposed as potential tools to minimize the bias and/or to validate sexual behavior reporting. However, use of STIs as biomarkers is expensive and not informative in low-incident populations. A consensus conference of the National Institute of Mental Health in 2000 recommended that disease-based biomarkers be reserved for Phase 3 studies of behavioral interventions.(3) Another area where biomarkers have become important is measuring condom efficacy.(4) Since 2000 there has been particular public health and policy interest in this issue, however, studying disease incidence in condom users is methodologically and ethically difficult.(5) These issues highlight the need for reliable biomarkers of sexual intercourse and prompted the search for new biomarkers of sexual activity. Ideally, a biological marker for sexual behavior would be highly sensitive (i.e. be present after unprotected intercourse), specific, use existing and accessible test technology, and be inexpensive. The specimen collection scheme should also be simple and easily implemented in field settings.
We previously developed and characterized a PCR-based assay for detection of spermatozoal Y-chromosome sequences (Yc) in vaginal fluid.(6-8) Since Yc is not found in women, we hypothesized it as a useful biomarker of sexual activity -- absence of Yc would indicate barrier efficacy (condom) or abstinence from vaginal intercourse. The assay is sensitive to 5 chromosomal copies of Yc DNA and can be performed on a self-administered vaginal swab (SAVS).(8) We found the Yc half-life (t1/2) in post-coital vaginal fluid to be 3.8 days and detectable for 15 days.(8) Furthermore, in 97% of couples who used male condoms, Yc was not detectable in vaginal fluid,(6) demonstrating that when properly used, condoms are very effective even when using highly sensitive measures.
We were concerned that the normal cyclical changes in vaginal ecology may affect Yc assay performance. To further characterize the sensitivity of the assay under different “washout” conditions, our objective in this study was to assess the performance of the Yc assay by detecting and quantifying post coitus, sperm-derived Yc DNA in vaginal fluid during menses and nonmenses intervals.
Fifty-one heterosexual women over the age of 18 were recruited in Baltimore, MD between May 2004 and July 2005 to perform a two-phase study. Inclusion criteria included: history of vaginal intercourse (sexually active), regular menstrual cycles, current use of an effective contraceptive or surgically sterile, current involvement with a monogamous male sex partner, and willing to have intercourse without a barrier (condom) followed by a two-week period of abstinence from sexual intercourse. Exclusion criteria included use of a vaginal douche in the prior two months, pregnancy, sexual partner with vasectomy and report of current use of condoms. Current users of injectable contraceptives (depomedroxyprogesterone acetate (DMPA)) were also excluded because menses was the mediating variable of interest and women using DMPA do not typically menstruate. Forty-five women (88%) completed the two-phase study with adequate collection of samples.
Each phase was two weeks in duration. Phase A was initiated two days prior to anticipated menses so that menses coincided with the first week of sample collection (Menses Cycle). Phase B was initiated one week after the participant's menses was completed to ensure that they would not have menses during the sample collection period (Non-menses Cycle). Based on our past experience, we anticipated most women to report use of oral contraceptives, which provided women an easy method to predict the approximate menstrual cycle and study start dates. The phases were not ordered for participants’ convenience, and therefore, the choice to start the study with the Menses Cycle or Non-menses Cycle was based on timing of the study's information session. Of total, 46% percent of participants completed the non-menses phase before the menses phase.
Prior to initiation, participants were trained to collect the SAVS (9;10) and were provided instructions on the handling and storage of specimens. At the beginning of each Phase, subjects had unprotected intercourse and then abstained for 14 days. Participants obtained SAVS at one day postcoital and then every other day for 14 days, totaling seven swabs in each phase. Subjects were instructed to store the swabs in their freezer immediately after collection and to transfer the samples on ice to the study site at the end of each phase. Subjects kept a daily coital log which included yes/no check-off prompts for penile/vaginal intercourse, the use of condoms, diaphragms, spermicide, lubricants, contraception, receptive oral sex, digital penetration by male partner, douching, menses and the use of tampons or pads. Participants were not instructed to abstain from intercourse prior to the first day of the study schedule. The participants marked all samples with the date so any mistimed samples were temporally ordered. Participants were instructed that if they missed a scheduled sample day, to collect the swab the next day and to mark the swab with the correct date. They would then resume the study on the original sampling schedule. The protocol was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine. All participants provided written informed consent.
SAVS were extracted for total DNA as previously described.(7) Briefly, vaginal fluid was recovered from the SAVS by placing the swab in 0.5 mL of saline for 25 minutes, and total DNA was extracted via a two-step extraction process involving 1) lyses of the male and female vaginal epithelial cells, and removal of DNA derived from epithelial cells while preserving intact sperm cells, and 2) lyses of sperm cells containing DNA followed by purification of the Yc DNA. This two-step process ensured that detectable Yc DNA sequences were derived from sperm cells, and not from male epithelial cells that might have been deposited during intercourse (11). Detection of Yc sequences was performed on the LightCycler system with primers and probes specific to a fragment of the SRY gene with positive and negative controls. PCR products were quantified using the absolute quantification module of the LightCycler system with the assistance of a standard concentration curve.
Because of the potential for intrinsic biological variability, we estimated Yc clearance from the same subject during the menstrual and non-menstrual phase. In this design, subjects served as their own control. The sample size was based on the results of pilot data. A 95% confidence interval for the slope, with a width of ± 0.03 required a sample of size 50.
The statistical methods used are similar to those employed previously in longitudinal evaluations of the Y-chromosome biomarker.(8) Briefly, the mean trends of log (Yc) over time were described with smoothed non-parametric curve (lowess, locally weighted smoothing). These plots are completed for the whole population as well as for individuals. Time trends and impact of menses were evaluated using generalized linear models with random effects. For Yit denoting the concentration of log (Yc) for individual i = (1,2,..., n) at time point t = (0,1,2,...T), the model fitted to the data can be written as
where α is the initial concentration measured at time t =0, a is the random effect for the intercept, β1 is the exponential decay rate with a random component b, t is time measured as days post-coitus and βj , j=2,..., p being the effects of additional covariates xj. In this model, a and b represent the random subject-specific effects where we assume that (ai, bi) are independently distributed as multivariate normal with mean 0 and arbitrary variance-covariance matrix. The errors {εit} are modeled as independent and identically distributed normal random variables with constant variance σ2. The mean half-life, adjusted for the effect of all other covariates in the model, was estimated as (t1/2 = ln(0.5)/β1). The heterogeneity among the women in both initial levels and rate of decline can be accounted for in the models by including explanatory variables and random effects for individual factors that were not measured. The final model included time and menses as well as random intercept and random slope. Observations with undetectable values of Yc were assigned a value of 0.5 so that the log could be defined. Analysis was performed using SAS and STATA programs.
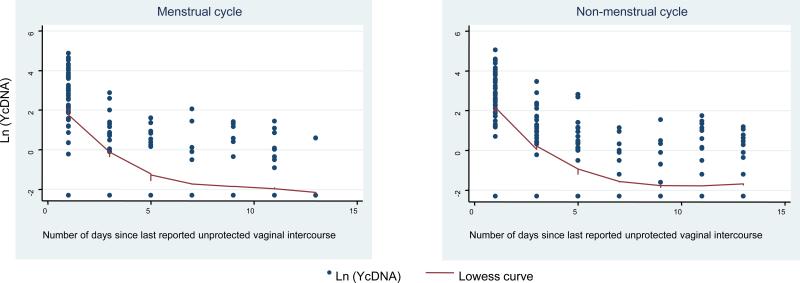
Overall, the estimated average Yc decay slope was -0.30 per day (95% confidence interval (CI) -0.34, -0.26). The random effects model demonstrated that while the Yc concentrations were lower in the menses cycle as compared to the non-menses interval by an average of -0.26 (95% CI: -0.48, -0.04), the interaction between time and menses status was not statistically significant, indicating the Yc clearance times were similar (Figure 1). Substantial heterogeneity between the women in both initial Yc levels and rate of decline were also observed. (Figure 2) Table 1 displays the proportion of specimens that were Yc-positive at each sampling point after exposure. The study also confirmed our previously calculated Yc half-life (t1/2) in post-coital vaginal fluid during the Non-menses Cycle to be 3.8 days and detectable for at least 14 days (8).
Figure 1.
Average decline over time in Y-chromosome DNA during menses and non-menses intervals. The fitted line is based on a nonparametric lowess trend line.
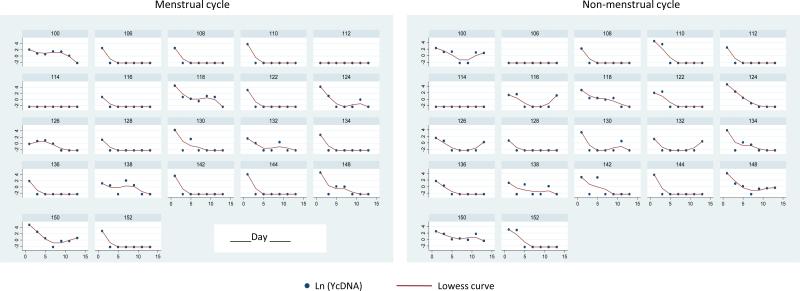
Figure 2.
Decline over 14 days in Y-chromosome DNA during menses and non-menses intervals by participant. Displayed are participants with even identification numbers and more than one observation. This figure points to the heterogeneity among the women in both initial levels and rate of decline. This heterogeneity was accounted for in the models by including additional explanatory variables and random effects for individual factors that were not measured.
Table 1.
Number (%) of specimens that were detectable for Y-chromosome by number of postcoital days and Menses/Non-menses phase of study
| Non-menses cycle | Menses cycle | |||
|---|---|---|---|---|
| number of days post coital | N | % | N | % |
| 1 | 39 | 86.7 | 39 | 86.7 |
| 3 | 22 | 48.9 | 16 | 35.6 |
| 5 | 16 | 35.6 | 10 | 22.2 |
| 7 | 8 | 17.8 | 6 | 13.3 |
| 9 | 6 | 13.3 | 6 | 13.3 |
| 11 | 10 | 22.2 | 11 | 24.4 |
| 13 | 10 | 22.2 | 1 | 2.2 |
In this study, we investigated whether the presence of menses shortens the residence time of sperm-derived Yc sequence in the vagina following unprotected intercourse. We found that post-coital Yc levels tended to be lower during menses compared to non-menses intervals, but the decline patterns and rates of decline were similar. We speculate that Yc clearance times were similar between menses and non-menses intervals because of Yc adherence to the vaginal wall, although this remains to be investigated. Our study also provides additional evidence (6;8;11) that Yc DNA from SAVS represents a viable and robust biomarker of recent unprotected sexual activity in women. The study also confirmed our previously calculated decay curves despite the switch to RT-PCR in this analysis, as compared to our original studies in which we used gels that have much less precision.(8). RT-PCR does not require post-amplification manipulations which have been shown to introduce variability when comparing one sample to another.
Further research is necessary to characterize the Yc assay in the presence of other extrinsic factors. Vaginal douching, a highly prevalent feminine hygiene practice (12) particularly among women at-risk for STDs (13;14), and other sexual behaviors including receptive oral sex, digital penetration and rectal sex should be evaluated in their effect on Yc clearance. Our current work has been among heterosexual women, but future research should also include other populations such as adolescents and men who have sex with men.
Summary.
Y-chromosomal DNA can be considered a robust biomarker of recent unprotected sexual activity in women during menstruation.
Acknowledgments
Sources of support: This work was supported by NIH grant HD-43674
References
- 1.Mauck CK, Doncel GF. Biomarkers of semen in the vagina: applications in clinical trials of contraception and prevention of sexually transmitted pathogens including HIV. Contraception. 2007;75:407–419. doi: 10.1016/j.contraception.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Zenilman JM, Weisman CS, Rompalo AM, et al. Condom use to prevent incident STDs: the validity of self-reported condom use. Sex Transm Dis. 1995;22:15–21. doi: 10.1097/00007435-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Pequegnat W, Fishbein M, Celentano D, et al. NIMH/APPC workgroup on behavioral and biological outcomes in HIV/STD prevention studies: a position statement. Sex Transm Dis. 2000;27:127–132. doi: 10.1097/00007435-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Macaluso M, Lawson L, Akers R, et al. Prostate-specific antigen in vaginal fluid as a biologic marker of condom failure. Contraception. 1999;59:195–201. doi: 10.1016/s0010-7824(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 5.Warner L, Macaluso M, Austin HD, et al. Application of the case-crossover design to reduce unmeasured confounding in studies of condom effectiveness. American Journal of Epidemiology. 2005;161:765–773. doi: 10.1093/aje/kwi094. [DOI] [PubMed] [Google Scholar]
- 6.Ghanem KG, Melendez JH, Neil-Solis C, et al. Condom use and vaginal Y-chromosome detection: the specificity of a potential biomarker. Sex Transm Dis. 2007;34:620–623. doi: 10.1097/01.olq.0000258318.99606.d9. [DOI] [PubMed] [Google Scholar]
- 7.Melendez JH, Giles JA, Yuenger JD, et al. Detection and quantification of Y-chromosomal sequences by real-time PCR using the LightCycler system. Sex Transm Dis. 2007;34:617–619. doi: 10.1097/01.olq.0000258336.65285.31. [DOI] [PubMed] [Google Scholar]
- 8.Zenilman JM, Yuenger J, Galai N, et al. Polymerase chain reaction detection of Y chromosome sequences in vaginal fluid: preliminary studies of a potential biomarker for sexual behavior. Sex Transm Dis. 2005;32:90–94. doi: 10.1097/01.olq.0000149668.08740.91. [DOI] [PubMed] [Google Scholar]
- 9.van de WJ, Altini L, Jones H, et al. Two methods of self-sampling compared to clinician sampling to detect reproductive tract infections in Gugulethu, South Africa. Sex Transm Dis. 2006;33:516–523. doi: 10.1097/01.olq.0000204671.62529.1f. [DOI] [PubMed] [Google Scholar]
- 10.Boskey ER, Atherly-Trim SA, O'Campo PJ, et al. Acceptability of a self-sampling technique to collect vaginal smears for gram stain diagnosis of bacterial vaginosis. Womens Health Issues. 2004;14:14–18. doi: 10.1016/j.whi.2003.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayntz-Press KA, Sims LM, Hall A, et al. Y-STR profiling in extended interval (> or = 3 days) postcoital cervicovaginal samples. J Forensic Sci. 2008;53:342–348. doi: 10.1111/j.1556-4029.2008.00672.x. [DOI] [PubMed] [Google Scholar]
- 12.Brotman RM, Klebanoff MA, Nansel T, et al. Why Do Women Douche? A Longitudinal Study with Two Analytic Approaches. Ann Epidemiol. 2008;18:65–73. doi: 10.1016/j.annepidem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Foxman B, Aral SO, Holmes KK. Interrelationships among douching practices, risky sexual practices, and history of self-reported sexually transmitted diseases in an urban population. Sex Transm Dis. 1998;25:90–99. doi: 10.1097/00007435-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Scholes D, Stergachis A, Ichikawa LE, et al. Vaginal douching as a risk factor for cervical Chlamydia trachomatis infection. Obstet Gynecol. 1998;91:993–997. doi: 10.1016/s0029-7844(98)00095-7. [DOI] [PubMed] [Google Scholar]