Abstract
Purpose
To evaluate a new calculation model estimating the equivalent dose at 2 Gy (EQD2) taking into account dose gradient in high dose rate interstitial brachytherapy (HDRIB).
Material and methods
Forty dose-volume histograms (DVHs) of breast (20 pts) and prostate (20 pts) cancer dose distributions were reviewed. Physical prescribed doses (PPD) were 34 Gy (10f/5d) and 18 Gy (6f/2d) for breast (partial irradiation protocol) and prostate (boost after external irradiation) treatment, respectively. For each DVH, clinical target volume (CTV), V100, V150, V200, D90 and D100 were determined. Based on DVH segmentation, elementary doses (d) delivered to elementary volumes were determined, then multiplied by C (% of CTV receiving d). According to the linear quadratic model, EQD2 was calculated for different α/β ratios.
Results
For breast implant, median EQD2 (α/β = 4) was 42 Gy and 76 Gy (66-85) without and with dose gradient consideration, respectively. For prostate implant, median EQD2 (α/β = 1.5) was 39 Gy and 98 Gy (90-103) whether dose gradient was not or was taken into account, respectively.
Conclusions
This study pointed out that for brachytherapy, EQD2 calculation must take into account the dose gradient. Because this model is a mathematical one, it has to be cautiously applied. Nevertheless, it appears as a useful tool for EQD2 comparison between the same PPD delivered through EBRT or brachytherapy regarding trial result interpretation.
Keywords: brachytherapy, prostate cancer, breast cancer, dose gradient, equivalent dose
Purpose
Brachytherapy is a radiation technique that enables the delivery of a high dose, in a small volume, during a short period of time (for temporary implants). The continuing interest in brachytherapy stems from enhanced technological capabilities to place radiation sources accurately within and/or adjacent to tumors, usually allows a high tumor to normal tissue dose ratio and a reduction in the volume of irradiated normal tissue. Whatever the technical considerations (endocavitary, interstitial, low or high dose rate, temporary or permanent implants), the inevitable dose gradient in brachytherapy ensures non-uniform dosage within the target volume and consequently having its own biological effect [1]. The evaluation of the radiobiological impact of dose gradient in brachytherapy was already investigated through theoretical ways [2, 3]. Wheldon et al. [2] provided a radiobiological transformation of the dose-volume histogram (DVH), to be used in the assessment of treatment plans, in the comparison of treatment schedules and the analysis of side effects. The authors used the linear-quadratic equivalent dose at 2 Gy (EQD2) defined by Fowler [4]. More recently, Armpilia et al. [3] proposed to calculate the “equivalent” biological effective dose (BED) within non-uniformly irradiated tissues by considering the integrated cell kill within the volume of interest. The authors considered BED value at a single dose reference point multiplied by a multiplying factor (MF) to obtain the “equivalent” BED for the tissue volume enclosed by the surface containing that dose reference point. The authors specified that this method was developed for both spherical and cylindrical geometries (gynecological, lung, esophageal applications), but not designed for interstitial implants. In order to analyze the radiobiological impact of dose gradient, Wheldon and Armpilia proposed a mathematical model based on physical data [2, 3]. The present study proposes a new calculation method of the prescribed physical dose EQD2 taking into account the dose gradient, but based on clinical data obtained from breast and prostate high dose rate interstitial brachytherapy (HDRIB). The significance of this approach takes place in a double context of dose escalation and reduction of overall treatment time specifically for breast and prostate cancers. Indeed, for breast as well as prostate cancer, randomized clinical trials showed that the increase of total radiation dose led to significant increase of local control [5, 6]. Furthermore, in order to decrease treatment duration, randomized trials using hypofractionated protocols showed equivalent results in terms of local control and side effects for breast and prostate cancer [7, 8]. Currently, for breast cancer, ongoing phase III randomized trials investigated the role of dose escalation in young women [9] and hypofractionated regimen in the frame of accelerated and partial breast irradiation [10]. Those protocols allow the use of external beam radiation therapy (EBRT) as well as brachytherapy for delivering the same physical prescribed dose (PPD).
Aim of the study
The purpose of this study is to evaluate a new calculation method analyzing dose gradient effect on the equivalent dose at 2 Gy (EQD2) and to provide a tool for EQD2 comparison between the same PPD delivered through EBRT or brachytherapy.
Material and methods
Patient features and dose-volume histogram analysis
Forty dose-volume histograms (DVHs) of breast (20 pts) and prostate (20 pts) cancer dose distributions based on multi-catheter HDRIB were retrospectively analyzed in the frame of an observational study. This analysis was approved by local institutional review boards.
Breast cancer patients were treated according to a prospective phase II national trial [11]. This protocol focused on the feasibility and reproducibility of an accelerated partial breast irradiation as sole post-operative irradiation in elderly women (> 70 years) presenting with T1-2 N0 disease, positive hormonal receptor status, with no lympho-vascular space invasion and clear surgical margins. Catheter implantation (Comfort catheterTM, Nucletron B.V., The Netherlands) was performed intra-operatively (Fig. 1A). Median number of inserted catheters was 10 [6–12]. CTV was defined based on 3 to 5 clips placed in the tumor bed. HDRIB delivered a total dose of 34 Gy in 10 fractions (twice daily) over 5 days (Fig. 1B).
Fig. 1.
High dose rate interstitial brachytherapy implant (A) and post-implant CT-scan (B) evaluating dose distribution analysis for breast cancer
For prostate cancer (PC), HDRIB was used as a prostate boost after EBRT for patient older than 60, presenting with a T2A disease, 10 < PSA < 20 ng/ml, Gleason score = 7 (intermediate risk PC) or T3A/B disease, PSA ≥ 20 ng/ml, Gleason score ≥ 8 (high-risk PC), according to the D'Amico classification criteria [12]. A total dose of 46 Gy in 23 fractions (2 Gy/fraction, 5 fractions/week) was firstly delivered to the prostate or pelvic area according to pelvic lymph node involvement risk. Then, prostate HDRIB was performed under general anesthesia (one session) allowing the placement of 17 catheters (Sharp needlesTM, Nucletron B.V., The Netherlands) inserted through a perineal template sutured to the perineum skin (Prostate templateTM, Nucletron B.V., The Netherlands) (Fig. 2A). HDRIB delivered a total dose of 18 Gy in 3 fractions (twice daily) over 2 days (Fig. 2B).
Fig. 2.
High dose rate interstitial brachytherapy implant (A) and post-implant CT-scan (B) evaluating dose distribution analysis (B) for prostate cancer
For breast and prostate patients, a post-implant CT-scan (image thickness 2.5 mm, including all the breast volume and the pelvic from S1 to the perineum for prostate implant) was performed the day after catheter implantation, permitting the delineation of CTV and organs at risk. Dose-volume adaptation was manually achieved (graphical optimization, PlatoTM, Nucletron B.V., The Netherlands) by dwell location and time variation (Fig. 1B and 2B).
For each DVH analysis, we determined the following parameters:
clinical target volume (CTV),
volume receiving 100% (V100), 150% (V150) and 200% (V200) of the prescribed physical dose (PPD),
dose delivered to 90% (D90) and 100% (D100) of the CTV.
The median values were determined. We calculated the equivalent dose at 2 Gy (EQD2) of PPD, taking into account or not the dose gradient for different αβ ratios. EQD2 calculation used α/β = 3, α/β = 4, α/β = 5 [7] and α/β = 1.5, α/β = 3, α/β= 5 [13, 14] for breast and prostate cancer, respectively.
Breast and prostate patients were treated with a high dose rate 192 Iridium source (10 Ci), using an after-loader machine (MicroSelectron™, Nucletron B.V., The Netherlands).
Calculation of equivalent dose at 2 Gy taking into account the dose gradient
For each DVH recorded on a worksheet:
-
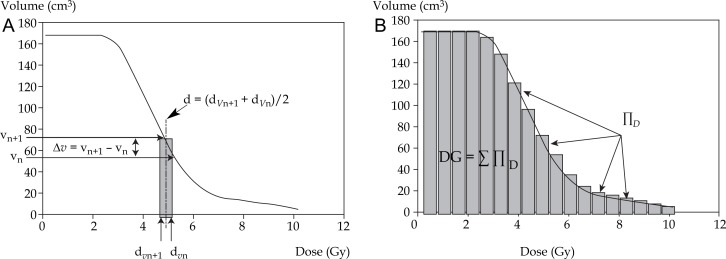
we calculated the elementary doses d delivered per fraction to the elementary volumes Δv (Fig. 3A):
d = (dVn + 1 + dVn)/2 and Δv = vn + 1 – vn
-
for each d, we determined C coefficient corresponding to the volume receiving d divided by the overall CTV (% of CTV receiving d):
C = Δv/CTV
-
for each d, we calculated D (10 fractions for breast cancer, 3 fractions for prostate cancer):
D = d × # fractions
for each D, we calculated the equivalent dose at 2 Gy (EQD2D). EQD2 was calculated with the equation EQD2 = D × [(d + α/β)/(2 + α/β)], as derived from the linear-quadratic model; D = total dose, d = dose per fraction, α = linear (first-order dose-dependent) component of cell killing, β = quadratic (second-order dose dependent) component of cell killing, α/β ratio = the dose at which both components are equal [2, 4].
-
for each EQD2D, we calculated ∏D:
∏D = EQD2D × C
-
finally, we calculated the total dose delivered to the whole CTV taking into account the dose gradient (DG) (Fig. 3B):
DG = Σ ∏D
Fig. 3.
DVH segmentation method for the calculation of elementary doses d (d = (dVn+1 + dVn)/2) delivered per fraction to elementary volumes Δv (Δv = vn+1 – vn) (A) and calculation of the total dose delivered to the whole CTV taking into account the dose gradient DG (DG = ∑∏D) (B)
Because the linear quadratic model may not be applicable for doses per fraction ≥ 8 Gy and in order to evaluate the accuracy of the EQD2 calculation method, the percentage of d ≥ 8 Gy median, d ≥ 8 Gy median and d median were calculated for breast and prostate implants [15, 16].
Due to the normal distribution of the different samples, statistical analysis was carried out using the Student t test. Data management and statistical calculations were performed with R 2.5.0 software (R Development Core Team, 2005).
Results
Dose-volume histogram parameters in breast and prostate implants are reported in Table 1. The results of EQD2 calculation for breast and prostate implants taking into account or not the dose gradient and according to the different α/β chosen ratios are reported in Table 2, respectively. For breast implant, median EQD2 (34 Gy) were 42 Gy and 76 Gy (66-85) with an α/β of 4 whether dose gradient was not or was taken into account, respectively. For prostate implant, median EQD2 (18 Gy) were 39 Gy and 98 Gy (90-103) with a α/β of 1.5, whether dose gradient was not or was taken into account, respectively.
Table 1.
Dose-volume histogram parameters in breast and prostate implants
| Breast | Prostate | |||
|---|---|---|---|---|
| Median | Min/Max | Median | Min/Max | |
| # catheters | 10 | 6-12 | 17 | – |
| CTV (cm3) | 84 | 53-119 | 45 | 41-60 |
| V100 (%) | 90 | 80-94 | 99 | 97-100 |
| V150 (%) | 35 | 24-46 | 44 | 35-68 |
| V200 (%) | 14 | 11-18 | 15 | 12-27 |
| D90 (%) | 100 | 89-104 | 115 | 113-123 |
| D100 (%) | 65 | 49-69 | 92 | 65-97 |
Min – minimum value, Max – maximum value, CTV – clinical target volume, V100 – volume receiving 100% of the prescribed physical dose, V150 – volume receiving 150% of the prescribed physical dose, V200 – volume receiving 200% of the prescribed physical dose, D90 – dose delivered to 90% of the CTV, D100 – dose delivered to 100% of the CTV
Table 2.
Equivalent dose at 2 Gy of the physical prescribed dose taking into account or not the dose gradient for α/β = 1.5, α/β= 3, α/β= 4 and α/β = 5 in breast and prostate implants
| Breast implant | Prostate implant | |||
|---|---|---|---|---|
| Median (Gy) | Min/Max | Median (Gy) | Min/Max | |
| DG not considered | ||||
| PPD | 34 | – | 18 | – |
| EQD2 | ||||
| α/β = 1.5 | – | – | 39 | – |
| α/β = 3 | 44 | – | 32 | – |
| α/β = 4 | 42 | – | – | – |
| α/β= 5 | 41 | – | 28 | – |
| DG considered | ||||
| PPD | 48 | 44-51 | 28 | 27-29 |
| EQD2 | ||||
| α/β= 1.5 | – | – | 98 | 90-103 |
| α/β= 3 | 81 | 71-91 | 76 | 71-80 |
| α/β= 4 | 76 | 66-85 | – | – |
| α/β= 5 | 72 | 63-80 | 63 | 59-66 |
DG – dose gradient, Min – minimum value, Max – maximum value, PPD – prescribed physical dose, EQD2 – PPD equivalent dose at 2 Gy dose without taking into account the dose gradient for α/β = 1.5, α/β = 3, α/β = 4 and α/β = 5
Discussion
This study proposes a calculation method of the EQD2 taking into account the dose gradient in case of HDRIB for breast and prostate cancer. Considering the present results, the EQD2 was significantly increased when the dose gradient was taken into account. Brachytherapy dose gradient appears as an important factor, which highly influences the PPD biological equivalence. Dose gradient is specific to brachytherapy whatever the dose rate (low, intermediate or high). However, according to our clinical practice for breast and prostate cancer treatment, this study focused on HDRIB.
In the present study, dose gradient impact on EQD2 appeared more important for prostate HDRIB. However, as shown in Table 1, for prostate implant compared to breast implant, the CTV was smaller and the number of implanted catheters was higher leading to better DVH parameters in terms of target volume coverage. Furthermore, breast implant was performed post-operatively on a clinical target volume (adjuvant intent), while prostate implant targeted a gross tumor volume. Whatever the implanted volume and the technical parameters of irradiation, for equivalent dose calculation, the linear quadratic (LQ) model uses dose parameters (total dose, dose per fraction), fractionation sensitivity parameters (α/β ratio), but does not consider the third fundamental component which is the irradiated volume receiving a certain dose per fraction. This third component appears crucial especially in case of brachytherapy, due to the important dose gradient. Indeed, within the CTV which receives the prescribed physical dose (isodose envelope), there are many smaller volumes which receive higher doses. In order to provide more accurate results for clinical implications, EQD2 calculation of the dose delivered through brachytherapy must take into account all those high doses delivered to small volumes. In other words, each (dose/volume) entity could have its own EQD2 and its own biological consequence in terms of local control.
The present mathematical results are consistent with the already obtained clinical results observed with brachytherapy boost, compared to full radiation dose delivered through external beam radiation therapy (EBRT). Kestin et al. [17] reported the results of a matched-pair analysis of HDRIB boost versus EBRT-alone for locally advanced prostate cancer. After a follow-up of 30 months, the authors observed that 5-year biochemical control rates for EBRT (46 Gy, 23f) + HDRIB (21 Gy, 2f) versus EBRT-alone (66 Gy, 33f) were 67% vs. 44%, respectively (p < 0.001). Use of EBRT-alone was considered as an independent prognostic factor for biochemical failure in multivariate analysis. Two prospective randomized trials comparing HDRIB plus EBRT with EBRT-alone in intermediate or high-risk prostate cancer confirmed these results [18, 19]. Sathya et al. [18] compared biochemical or clinical failure (BCF) rates obtained after EBRT (40 Gy, 20f) + low-dose rate brachytherapy (35 Gy) or EBRT-alone (66 Gy, 33f). With a median follow-up of 8.2 years, BCF rates were 29% vs. 61% in the EBRT + HDRIB and EBRT-alone arm, respectively (p = 0.0024). However, in this study focusing only on the prescribed physical dose, radiation dose in the EBRT-alone arm was significantly lower than the minimal recommended dose (70 Gy) and to EBRT + HDRIB arm. Hoskin et al. [19] compared EBRT (35.75 Gy, 13f) + HDRIB (17 Gy, 2f) with EBRT-alone (55 Gy, 20f). With a median follow up of 30 months, a significant improvement in actuarial biochemical relapse-free survival was seen in favor of the combined brachytherapy schedule (p = 0.03). Those results can be explained by technical and biological considerations. Indeed, clinically significant post-EBRT prostate cancer local recurrence occurs at the site of the primary tumor [20]. Consequently, better local control can be achieved not only by dose-escalation delivered to the whole prostate, but also in boosting the radiation dose within the primary tumor [20]. Prostate HDRIB allows performing a concomitant boost (dose painting approach), which appears biologically efficient due to the dose gradient which is delivered in the primary tumor because of brachytherapy technique (dwell-time position optimization) (Fig. 4).
Fig. 4.
High dose rate interstitial brachytherapy boost for prostate cancer with positive biopsies located within the right base. While a prescribed physical dose of 18 Gy is delivered to the whole prostate gland, the primary tumor site receives a physical dose of 27 Gy (V150) as a concomitant boost
For breast cancer, the EORTC 22881/10882 trial provides consistent results regarding the impact of brachytherapy dose gradient on prescribed physical dose EQD2 [5]. This randomized trial investigated the impact of a boost radiation dose of 16 Gy on local control, fibrosis and overall survival for patients with stage I-II breast cancer who underwent breast-conserving therapy. Regarding the boost technique, EBRT (photons or electrons) as well as low pulse dose rate interstitial brachytherapy was used according to the investigator clinical practice. With a median follow-up of 5.11 years, Poortmans et al. [21] reported the influence of the boost technique on local control. In the boost arm, 5-year local recurrence rates were 4.8%, 4.0% and 2.5% for photon, electron or brachytherapy boost technique, respectively (p = 0.09). In this trial, whatever the boost technique, the boost dose was fixed to 16 Gy. According to our EQD2 calculation method, for a prescribed physical dose of 34 Gy, the EQD2 (including dose gradient) was 76 Gy (α/β = 4) more than twice the PPD. Cautiously extrapolating this approach for the EORTC 22881/10882 trial, 16 Gy PPD should be approximately equivalent to 32 Gy for a total dose delivered to the tumor bed of 82 Gy (50 Gy + 32 Gy) in case of brachytherapy boost (dose gradient effect) compared to 66 Gy (50 Gy + 16 Gy) for photon or electron boost. Currently, the Young boost trial [9] investigated the local control rate after EBRT by applying two different boost doses, for patients aged 50 or younger who underwent breast-conserving therapy. In this trial, the boost technique included EBRT (photons and/or electrons) or low pulse dose rate brachytherapy for delivering a PPD of 16 Gy (conventional arm) versus 24.8 Gy (experimental arm). However, due to the brachytherapy dose gradient effect, a randomized patient that received a boost of 16 Gy delivered through brachytherapy would effectively receive an EQD2 about 30 Gy, compared to another randomized patient to receive a boost of 24.8 Gy delivered through EBRT. Differences in terms of biological effect between brachytherapy and EBRT could have a potential impact on local control and it would likely to lead to misinterpretation of Young boost trial results, unless subgroup analyses were conducted from the outset.
The ongoing NASB PB-39/RTOG 0413 trial randomizes a post-operative whole (conventional) versus accelerated and partial breast irradiation (APBI) for localized breast cancer [NASB PB]. APBI techniques include EBRT (PPD = 38.5 Gy/10f/5d) and HDRIB/Mammosite® (PPD = 34 Gy/ 10f/5d). EQD2 of 38.5 Gy PPD delivered through EBRT was set to 50 Gy (α/β = 4) while, according to our results, EQD2 of 34 Gy delivered through HDRIB and taking into account the dose gradient was estimated to be 76 Gy (α/β = 4). As well as in the Young boost breast trial, in the NASB PB-39/ RTOG 0413 trial, differences in terms of biological effect between HDRIB and EBRT could have a potential impact on the future results. Rosenstein et al. [22] analyzed the dose fractionation schedules currently used in ongoing APBI trials by comparing their biologically effective dose (BED) values to those of three standard (S50 = 50 Gy, S60 = 60 Gy and S66 = 66 Gy) whole breast protocols applied after conservative surgery. The authors used a modified BED equation taking into account cell proliferation that may take place during treatment. The authors concluded that APBI protocol BED values were consistently lower than the BEDs for either S60 or S66, leading to a risk of lower tumor control for the PBI regimens compared with standard whole breast EBRT using an additional boost dose to the tumor bed. However, in their BED comparison analysis, Rosenstein et al. [22] did not take into account the impact of dose gradient for APBI protocols using brachytherapy.
Antonucci et al. [23] recently updated the results of a matched-pair analysis with 10-year follow-up comparing APBI (low/high dose rate brachytherapy) versus whole-breast irradiation (EBRT). The authors reported 10-year local recurrence rates of 4% and 5% for whole-breast irradiation and APBI, respectively (p = 0.48). Although there is no published mature data regarding local control for APBI using EBRT. Jagsi et al. [24] recently reported cosmetic results. The authors applied the APBI NASB PB-39/RTOG 0413 protocol delivering a PPD of 38.5 Gy (10f/5d) through a highly sophisticated EBRT technique (intensity-modulated radiotherapy with active breathing control). With a median follow-up of 2.5 years, the authors noticed 7 patients who developed unacceptable cosmetic results (leading to early study closure) and considered hypofractionated schedule used for external beam APBI and prescribed by the ongoing NASB PB-39/RTOG 0413 trial as suboptimal.
As shown in this study, one of the limitations of the described EQD2 calculation method taking into account the dose gradient, is the proportion of elementary volumes with high doses per fraction. Indeed, it is not certain that the LQ model is still applicable at very low (< 1 Gy) or high (> 8 Gy) doses per fraction [15, 16]. In this analysis, the median proportion of the dose per fraction > 8 Gy was 40% and 70% for breast and prostate cancer, respectively. The d median calculated from the global DVH was 7 Gy and 15 Gy for breast and prostate cancer, respectively. This calculation method could be considered as more accurate for breast implant.
The different methods used to calculate the equivalent dose remain based on mathematical approach. Nevertheless, these theoretical models represent helpful tools to calculate the correct dose per fraction in case of hypofractionated regimen for EBRT or HDRIB. However, local control represents the most relevant clinical end point. In this frame, we recently analyzed local control results after HDRIB used in a pre-operative intent for cervix carcinoma [25]. The prescribed physical dose was 39 Gy in 9 fractions over 5 days. Assuming an α/β = 10 for tumor control, the calculated EQD2 was 46 Gy (without taking into account the dose gradient effect). This dose could seem insufficient compared to the standard dose of 60 Gy usually delivered through low dose rate brachytherapy [26]. However, we observed that 91% of the patients achieved a complete pathological response after surgery suggesting that this dose was likely to be sufficient and its efficacy could be explained, in part, by the brachytherapy dose gradient effect [25].
Conclusions
Based on clinical data, this study emphasizes the point that for brachytherapy, EQD2 calculation must take into account the dose gradient. Considering the limitations of the present EQD2 calculation method, it has to be cautiously applied. Even if this model leads to considerably very high EQD2 (> 100 Gy with dose gradient effect), the published results for breast and prostate cancer clinical series confirmed that these dose regimens achieved good local control rates without increasing complication rates. Nevertheless, this model appears as a useful tool for EQD2 comparison between the same PPD delivered through EBRT or brachytherapy. This notion has to be considered in clinical trial design and result interpretation.
References
- 1.Dale RG, Jones B. The clinical radiobiology of brachytherapy. Br J Radiol. 1998;71:465–483. doi: 10.1259/bjr.71.845.9691890. [DOI] [PubMed] [Google Scholar]
- 2.Wheldon TE, Deehan C, Wheldon EG, et al. The linear-quadratic transformation of dose-volume histograms in fractionated radiotherapy. Radiother Oncol. 1998;46:285–295. doi: 10.1016/s0167-8140(97)00162-x. [DOI] [PubMed] [Google Scholar]
- 3.Armpilia C, Dale RG, Sandilos P, et al. Radiobiological modeling of dose-gradient effects in low dose rate, high dose rate and pulsed brachytherapy. Phys Med Biol. 2006;51:4399–4411. doi: 10.1088/0031-9155/51/17/018. [DOI] [PubMed] [Google Scholar]
- 4.Fowler JF. The linear-quadratic model and progress in radiotherapy. Br J Radiol. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 5.Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25:3259–3265. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 6.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 7.START Trialists’ Group. Bentzen SM, Agrawal RK, et al. The UK Standardization of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollack A, Hanlon AL, Horwitz EM, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64:518–526. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young boost. Radiation dose intensity study in breast cancer in young women: a randomized phase III trial of additional dose to the tumor bed. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=120 (on line access 09/01/12).
- 10.NSABP B-39/RTOG 0413. A Randomized Phase III Study of Conventional Whole Breast Irradiation (WBI) Versus Partial Breast Irradiation (PBI) for Women with Stage 0, I, or II Breast Cancer. http://www.rtog.org/members/protocols/0413/0413.pdf (09/01/12). [PubMed]
- 11.Hannoun-Levi JM, Marsiglia H, Belkacemi Y, et al. Accelerated and Partial Breast Irradiation in Elderly Women: Gerico-03 Phase II Trail results. Int J Radiat Oncol Phys Biol. 2009;75:S222. [Google Scholar]
- 12.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 13.Fowler JF, Ritter MA, Chappell RJ, et al. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys. 2003;56:1093–1104. doi: 10.1016/s0360-3016(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 14.Williams SG, Taylor JM, Liu N, et al. Use of individual fraction size data from 3756 patients to directly determine the alpha/ beta ratio of prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:24–33. doi: 10.1016/j.ijrobp.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 15.Dutreix J, Cosset JM, Girinsky T. Biological equivalency of high single doses used in intraoperative irradiation. Bull Cancer Radiother. 1990;77:125–134. [PubMed] [Google Scholar]
- 16.Ritter M. Rationale, conduct, and outcome using hypofractionated radiotherapy in prostate cancer. Semin Radiat Oncol. 2008;18:249–256. doi: 10.1016/j.semradonc.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kestin LL, Martinez AA, Stromberg JS, et al. Matched-pair analysis of conformal high-dose-rate brachytherapy boost versus external-beam radiation therapy alone for locally advanced prostate cancer. J Clin Oncol. 2000;18:2869–2880. doi: 10.1200/JCO.2000.18.15.2869. [DOI] [PubMed] [Google Scholar]
- 18.Sathya JR, Davis IR, Julian JA, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol. 2005;23:1192–1199. doi: 10.1200/JCO.2005.06.154. [DOI] [PubMed] [Google Scholar]
- 19.Hoskin PJ, Motohashi K, Bownes P, et al. High dose rate brachytherapy in combination with external beam radiotherapy in the radical treatment of prostate cancer: initial results of a randomized phase three trial. Radiother Oncol. 2007;84:114–120. doi: 10.1016/j.radonc.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Pucar D, Hricak H, Shukla-Dave A, et al. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. 2007;69:62–69. doi: 10.1016/j.ijrobp.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 21.Poortmans P, Bartelink H, Horiot JC, et al. EORTC Radiotherapy and Breast Cancer Groups. The influence of the boost technique on local control in breast conserving treatment in the EORTC ‘boost versus no boost’ randomized trial. Radiother Oncol. 2004;72:25–33. doi: 10.1016/j.radonc.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Rosenstein BS, Lymberis SC, Formenti SC. Biologic comparison of partial breast irradiation protocols. Int J Radiat Oncol Biol Phys. 2004;60:1393–1404. doi: 10.1016/j.ijrobp.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 23.Antonucci JV, Wallace M, Goldstein NS, et al. Differences in Patterns of Failure in Patients Treated with Accelerated Partial Breast Irradiation Versus Whole-Breast Irradiation: A Matched-Pair Analysis with 10-Year Follow-Up. Int J Radiat Oncol Biol Phys. 2009;74:447–452. doi: 10.1016/j.ijrobp.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Jagsi R, Ben-David MA, Moran JM, et al. Unacceptable Cosmesis in a Protocol Investigating Intensity-Modulated Radiotherapy with Active Breathing Control for Accelerated Partial-Breast Irradiation. Int J Radiat Oncol Biol Phys. 2010;76:71–78. doi: 10.1016/j.ijrobp.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannoun-Levi JM, Ferre M, Ginot A, et al. High dose rate brachytherapy for cervix carcinoma using the Nice Gynecologic Applicator: A new procedure combining endocavitary and interstitial approach. Brachytherapy. 2009;8:129. [Google Scholar]
- 26.Resbeut MR, Alzieu C, Gonzague-Casabianca L, et al. Combined brachytherapy and surgery for early carcinoma of the uterine cervix: analysis of extent of surgery on outcome. Int J Radiat Oncol Biol Phys. 2001;50:873–881. doi: 10.1016/s0360-3016(01)01602-9. [DOI] [PubMed] [Google Scholar]