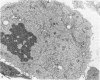
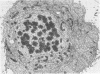
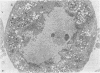
Abstract
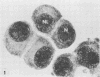
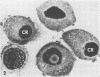
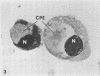
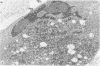
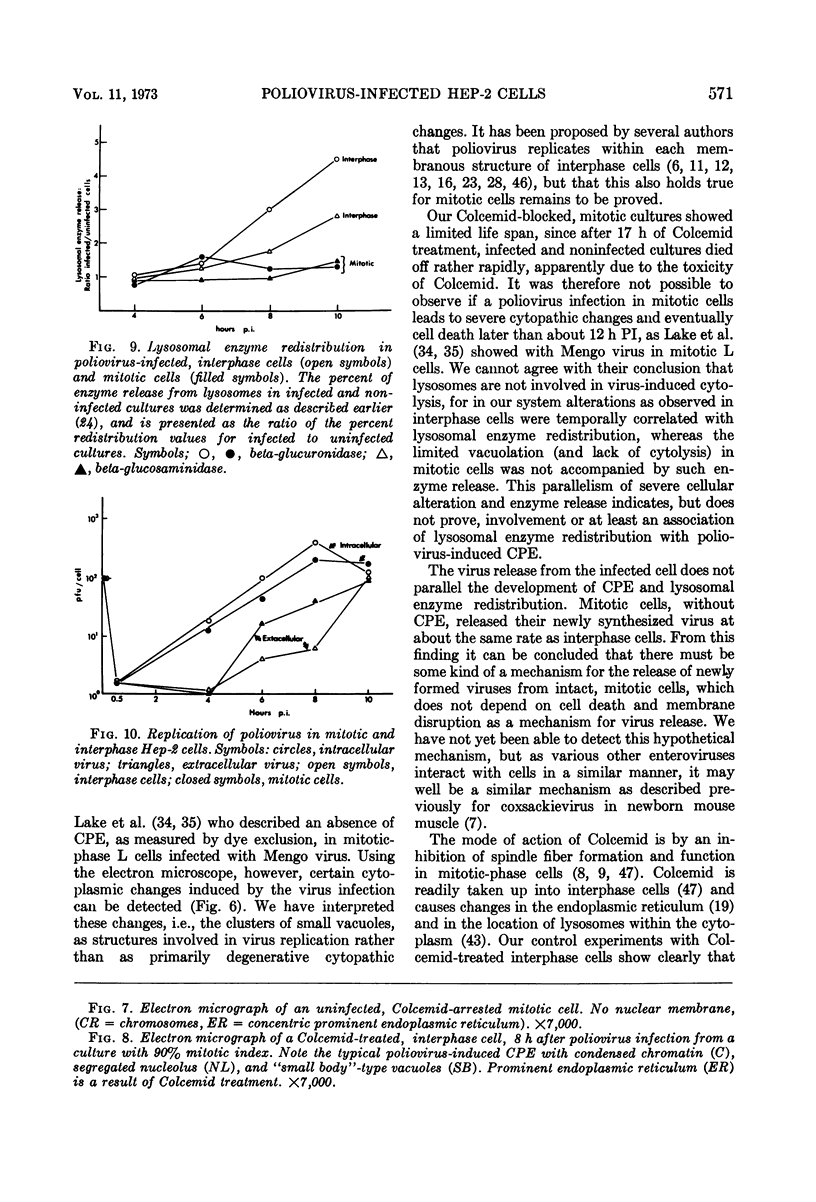
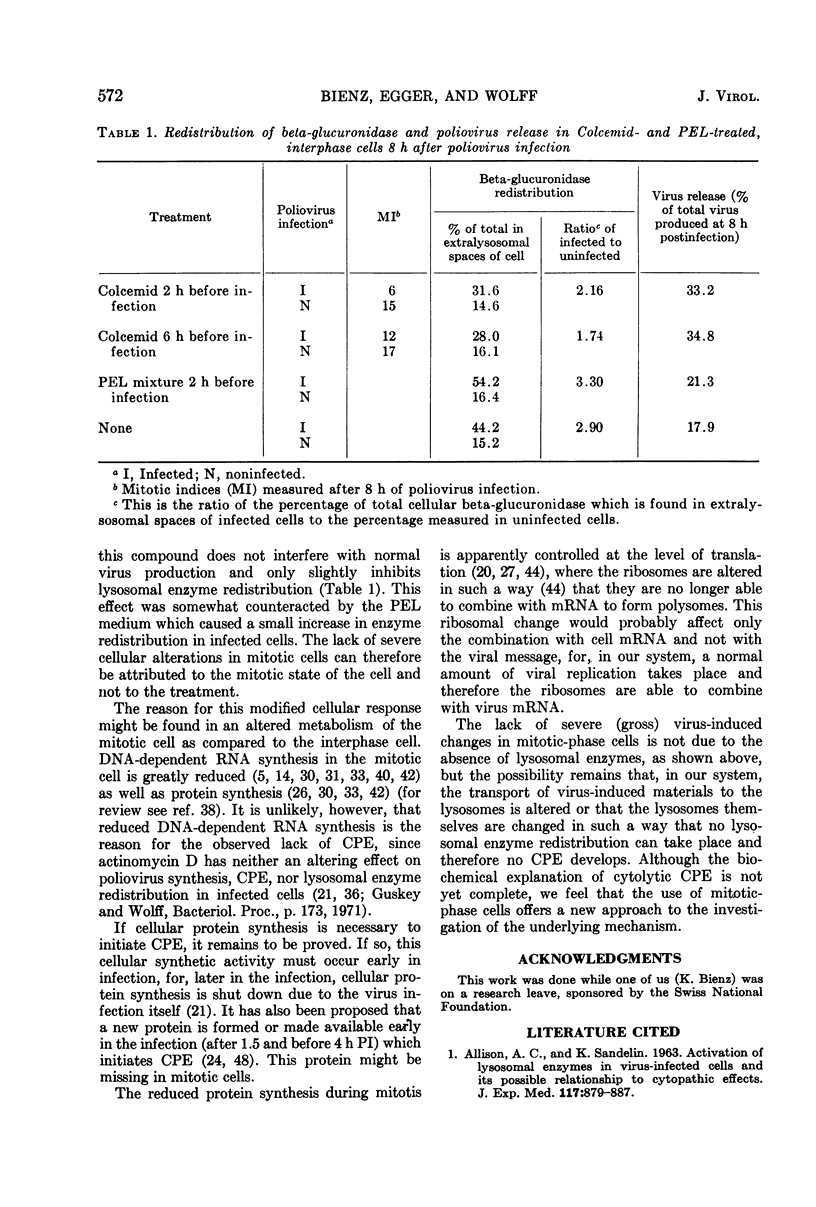
Mitotic Hep-2 cells, selected by the PEL (colloidal silica) density gradient method and held in mitosis with Colcemid, are readily infected by poliovirus type I (Mahoney). They produce and release the same amount of virus as interphase, random-growing cells. In contrast to interphase cells, mitotic cells show no detectable virus-induced cytopathic effect at the light microscopy level and only slight alterations, consisting of small clusters of vacuoles, at the electron microscopy level. Mitotic cells contain the same total amount of lysosomal enzymes per cell as interphase cells, but they display no redistribution of lysosomal enzymes during the virus infection as interphase cells do. This supports the view that lysosomal enzyme redistribution is associated with the cytopathic effect in poliovirus infection but shows that virus synthesis and release is not dependent on either the cytopathic effect or lysosomal enzyme release. The possible reasons for the lack of cytopathic effect in mitotic cells are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., MALLUCCI L. HISTOCHEMICAL STUDIES OF LYSOSOMES AND LYSOSOMAL ENZYMES IN VIRUS-INFECTED CELL CULTURES. J Exp Med. 1965 Mar 1;121:463–476. doi: 10.1084/jem.121.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON A. C., SANDELIN K. Activation of lysosomal enzymes in virus-infected cells and its possible relationship to cytopathic effects. J Exp Med. 1963 Jun 1;117:879–887. doi: 10.1084/jem.117.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzai T., Ozaki Y. Intranuclear crystal formation of poliovirus: electron microscopic observations. Exp Mol Pathol. 1969 Apr;10(2):176–185. doi: 10.1016/0014-4800(69)90038-0. [DOI] [PubMed] [Google Scholar]
- BARSKI G., KINHIDA T. Continuous phase contrast observation of cells infected with Mengo-EMC viruses. Virology. 1961 Jun;14:299–301. doi: 10.1016/0042-6822(61)90211-2. [DOI] [PubMed] [Google Scholar]
- BASERGA R. THE RELATIONSHIP OF THE CELL CYCLE TO TUMOR GROWTH AND CONTROL OF CELL DIVISION: A REVIEW. Cancer Res. 1965 Jun;25:581–595. [PubMed] [Google Scholar]
- BECKER Y., PENMAN S., DARNELL J. E. A CYTOPLASMIC PARTICULATE INVOLVED IN POLIOVIRUS SYNTHESIS. Virology. 1963 Oct;21:274–276. doi: 10.1016/0042-6822(63)90271-x. [DOI] [PubMed] [Google Scholar]
- Bienz K., Bienz-Isler G., Egger D., Weiss M., Loeffler H. Coxsackievirus infection in skeletal muscles of mice. An electron microscopic study. II. Appearance and fate of virus progeny. Arch Gesamte Virusforsch. 1970;31(3):257–265. doi: 10.1007/BF01253760. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967 Aug;34(2):525–533. doi: 10.1083/jcb.34.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Colchicine binding to sea urchin eggs and the mitotic apparatus. J Cell Biol. 1967 Aug;34(2):535–548. doi: 10.1083/jcb.34.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers W. E., Finkenstaedt J. T., de Duve C. Lysosomes in lymphoid tissue. I. The measurement of hydrolytic activities in whole homogenates. J Cell Biol. 1967 Feb;32(2):325–337. doi: 10.1083/jcb.32.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Characterization of poliovirus-specific structures associated with cytoplasmic membranes. Virology. 1970 Sep;42(1):112–122. doi: 10.1016/0042-6822(70)90243-6. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Membranous structures associated with translation and transcription of poliovirus RNA. Science. 1969 Nov 14;166(3907):885–886. doi: 10.1126/science.166.3907.885. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. The role of cytoplasmic membranes in poliovirus biosynthesis. Virology. 1970 Sep;42(1):100–111. doi: 10.1016/0042-6822(70)90242-4. [DOI] [PubMed] [Google Scholar]
- Crippa M. The rate of ribonucleic acid synthesis during the cell cycle. Exp Cell Res. 1966 May;42(2):371–375. doi: 10.1016/0014-4827(66)90301-6. [DOI] [PubMed] [Google Scholar]
- DALES S., EGGERS H. J., TAMM I., PALADE G. E. ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF POLIOVIRUS. Virology. 1965 Jul;26:379–389. doi: 10.1016/0042-6822(65)90001-2. [DOI] [PubMed] [Google Scholar]
- DALES S., FRANKLIN R. M. A comparison of the changes in fine structure of L cells during single cycles of viral multiplication, following their infection with the viruses of Mengo and encephalomyocarditis. J Cell Biol. 1962 Aug;14:281–302. doi: 10.1083/jcb.14.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremenko T., Benedetto A., Volpe P. Poliovirus replication during HeLa cell life cycle. Nat New Biol. 1972 May 24;237(73):114–116. doi: 10.1038/newbio237114a0. [DOI] [PubMed] [Google Scholar]
- Eremenko T., Benedetto A., Volpe P. Virus infection as a function of the host cell life cycle: replication of poliovirus RNA. J Gen Virol. 1972 Jul;16(1):61–68. doi: 10.1099/0022-1317-16-1-61. [DOI] [PubMed] [Google Scholar]
- Erlandson R. A., de Harven E. The ultrastructure of synchronized HeLa cells. J Cell Sci. 1971 Mar;8(2):353–397. doi: 10.1242/jcs.8.2.353. [DOI] [PubMed] [Google Scholar]
- FRANKLIN R. M., BALTIMORE D. Patterns of macromolecular synthesis in normal and virus-infected mammalian cells. Cold Spring Harb Symp Quant Biol. 1962;27:175–198. doi: 10.1101/sqb.1962.027.001.019. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Flanagan J. F. Hydrolytic enzymes in KB cells infected with poliovirus and herpes simplex virus. J Bacteriol. 1966 Feb;91(2):789–797. doi: 10.1128/jb.91.2.789-797.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskey L. E., Smith P. C., Wolff D. A. Patterns of cytopathology and lysosomal enzyme release in poliovirus-infected HEp-2 cells treated with either 2-(alpha-hydroxybenzyl)-benzimidazole or guanidine HCl. J Gen Virol. 1970 Jan;6(1):151–161. doi: 10.1099/0022-1317-6-1-151. [DOI] [PubMed] [Google Scholar]
- HINZ R. W., BARSKI G., BERNHARD W. An electron microscopic study of the development of the encephalomyocarditis (EMC) virus propagated in vitro. Exp Cell Res. 1962 Mar;26:571–586. doi: 10.1016/0014-4827(62)90163-5. [DOI] [PubMed] [Google Scholar]
- Hodge L. D., Robbins E., Scharff M. D. Persistence of messenger RNA through mitosis in HeLa cells. J Cell Biol. 1969 Feb;40(2):497–507. doi: 10.1083/jcb.40.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. C., Holland J. J. Ribonucleic acid and protein synthesis in mitotic HeLa cells. J Cell Biol. 1965 Dec;27(3):565–574. doi: 10.1083/jcb.27.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jézéquel A. M., Steiner J. W. Some ultrastructural and histochemical aspects of Coxsackie virus-cell interactions. Lab Invest. 1966 Jun;15(6):1055–1083. [PubMed] [Google Scholar]
- KONRAD C. G. PROTEIN SYNTHESIS AND RNA SYNTHESIS DURING MITOSIS IN ANIMAL CELLS. J Cell Biol. 1963 Nov;19:267–277. doi: 10.1083/jcb.19.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten F. H., Strasser F. F. Nucleic acid synthetic patterns in synchronized mammalian cells. Nature. 1966 Jul 9;211(5045):135–140. doi: 10.1038/211135a0. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Perez A. G. Ribonucleic acid synthesis in synchronously dividing populations of HeLa cells. Nature. 1965 Aug 28;207(5000):974–975. doi: 10.1038/207974a0. [DOI] [PubMed] [Google Scholar]
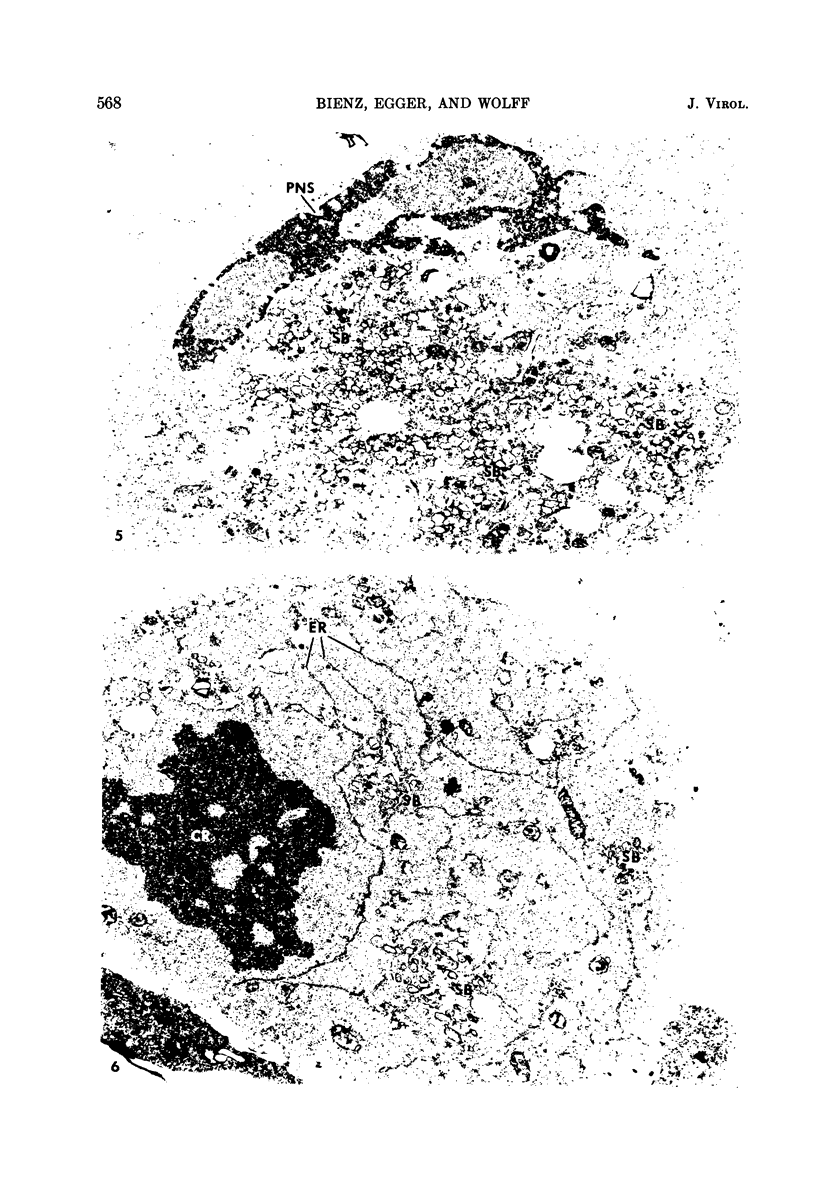
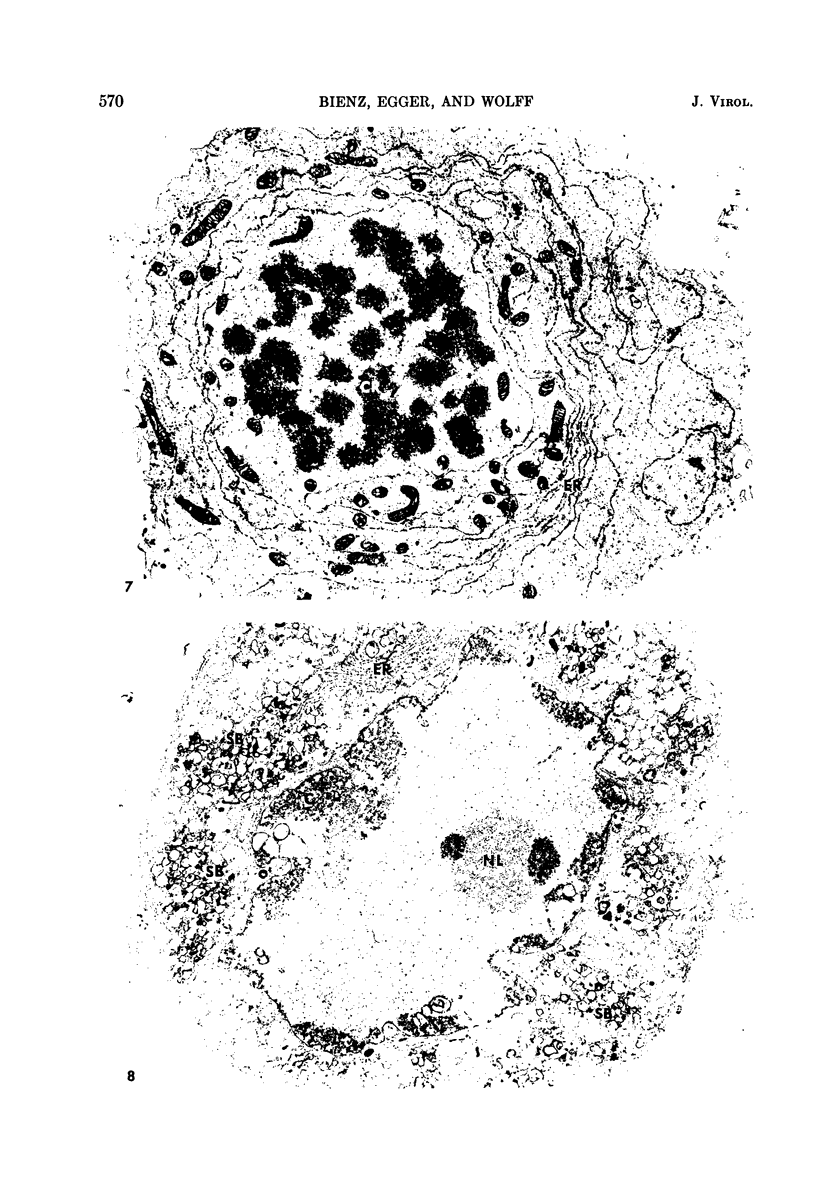
- Lake R. S., Ludwig E. H. Cellular changes attending mengovirus-induced cytolysis of mouse L-cells. Biochim Biophys Acta. 1971 Aug 19;244(2):466–477. doi: 10.1016/0304-4165(71)90251-0. [DOI] [PubMed] [Google Scholar]
- Lake R. S., Winkler D. C., Ludwig E. H. Delay of mengovirus-induced cytopathology in mitotic L-cells. J Virol. 1970 Feb;5(2):262–263. doi: 10.1128/jvi.5.2.262-263.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCUS P. I., ROBBINS E. VIRAL INHIBITION IN THE METAPHASE-ARREST CELL. Proc Natl Acad Sci U S A. 1963 Dec;50:1156–1164. doi: 10.1073/pnas.50.6.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern C. F., Daniel W. A. Replication of poliovirus in HeLa cells: electron microscopic observations. Virology. 1965 Aug;26(4):646–663. doi: 10.1016/0042-6822(65)90328-4. [DOI] [PubMed] [Google Scholar]
- Nias A. H., Fox M. Synchronization of mammalian cells with respect to the mitotic cycle. Cell Tissue Kinet. 1971 Jul;4(4):375–398. doi: 10.1111/j.1365-2184.1971.tb01547.x. [DOI] [PubMed] [Google Scholar]
- PHILLIPS H. J., TERRYBERRY J. E. Counting actively metabolizing tissue cultured cells. Exp Cell Res. 1957 Oct;13(2):341–347. doi: 10.1016/0014-4827(57)90013-7. [DOI] [PubMed] [Google Scholar]
- PRESCOTT D. M., BENDER M. A. Synthesis of RNA and protein during mitosis in mammalian tissue culture cells. Exp Cell Res. 1962 Mar;26:260–268. doi: 10.1016/0014-4827(62)90176-3. [DOI] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. HISTOCHEMICAL AND ULTRASTRUCTURAL STUDIES ON HELA CELL CULTURES EXPOSED TO SPINDLE INHIBITORS WITH SPECIAL REFERENCE TO THE INTERPHASE CELL. J Histochem Cytochem. 1964 Sep;12:704–711. doi: 10.1177/12.9.704. [DOI] [PubMed] [Google Scholar]
- Salb J. M., Marcus P. I. Translational inhibition in mitotic HeLa cells. Proc Natl Acad Sci U S A. 1965 Nov;54(5):1353–1358. doi: 10.1073/pnas.54.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler R., Ramseier L., Schaer J. C., Grieder A. Studies on the division cycle of mammalian cells. 3. Preparation of synchronously dividing cell populations by isotonic sucrose gradient centrifugation. Exp Cell Res. 1970 Jan;59(1):90–96. doi: 10.1016/0014-4827(70)90627-0. [DOI] [PubMed] [Google Scholar]
- TAYLOR E. W. THE MECHANISM OF COLCHICINE INHIBITION OF MITOSIS. I. KINETICS OF INHIBITION AND THE BINDING OF H3-COLCHICINE. J Cell Biol. 1965 Apr;25:SUPPL–SUPPL:160. doi: 10.1083/jcb.25.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacore H., Wolff D. A. Activation of isolated lysosomes by poliovirus-infected cell extracts. Nature. 1968 Jun 15;218(5146):1063–1064. doi: 10.1038/2181063a0. [DOI] [PubMed] [Google Scholar]
- WOLFF D. A., BUBEL H. C. THE DISPOSITION OF LYSOSOMAL ENZYMES AS RELATED TO SPECIFIC VIRAL CYTOPATHIC EFFECTS. Virology. 1964 Nov;24:502–505. doi: 10.1016/0042-6822(64)90196-5. [DOI] [PubMed] [Google Scholar]
- Whitmore G. F. Natural and induced synchronous cultures. In Vitro. 1971 Jan-Feb;6(4):276–285. doi: 10.1007/BF02625942. [DOI] [PubMed] [Google Scholar]
- Wolff D. A., Pertoft H. Separation of HeLa cells by colloidal silica density gradient centrifugation. I. Separation and partial synchrony of mitotic cells. J Cell Biol. 1972 Dec;55(3):579–585. doi: 10.1083/jcb.55.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]