Abstract
Adalimumab (ADA) is applied to induce remission in patients with Crohn's disease (CD) naïve to chimeric anti-tumor necrosis factor-α (anti-TNF-α), infliximab or patients with loss of response to scheduled maintenance infliximab. Adsorptive granulocyte and monocyte apheresis (GMA) depletes elevated/activated myeloid lineage leucocytes as sources of inflammatory cytokines and has been used to treat patients with CD. This study was to investigate the efficacy of intensive GMA in combination with ADA as remission induction therapy in cases of CD refractory to medications including anti-TNF-α therapies. Between December 2010 and February 2012, 5 consecutive cases with refractory CD were treated with intensive GMA (2 sessions per week) plus ADA to induce remission. CD activity index (CDAI), C-reactive protein (CRP), and endoscopic findings based on the simple endoscopic score for CD (SES-CD) at baseline and 10 weeks post 5 ADA injections were applied to determine treatment efficacy outcomes. At week 10 post ADA treatment, clinical remission together with normal CRP levels were achieved in all 5 cases, while SES-CD scores reflected marked improvement in 3 cases and partial improvement in 2 cases who had extensive deep longitudinal CD lesions. The CDAI and CRP values at baseline were 324 ± 118 and 4.9 ± 3.3 mg/dl, respectively. The corresponding values after treatment were 100 ± 28 (p = 0.024) and 0.2 ± 0.2 mg/dl (p = 0.038). In these 5 cases with medication-refractory CD, combination therapy with intensive GMA followed by 5 ADA shots appeared to be an effective and safe intervention for inducing clinical remission.
Key words: Refractory Crohn's disease, Adalimumab, Intensive adsorptive granulocyte and monocyte apheresis, Clinical remission, Simple endoscopic score for Crohn's disease
Introduction
Crohn's disease (CD) is a chronic and progressive inflammatory bowel disease (IBD) which afflicts millions of individuals throughout the world with symptoms that impair function and quality of life. With the knowledge that tumor necrosis factor-α (TNF-α) has a major role in the immunopathogenesis of IBD, treatment with anti-TNF-α antibodies like infliximab (IFX) and adalimumab (ADA) has been applied to induce remission in patients with CD. Further, ADA with a reduced immunogenicity has been considered as an alternative anti-TNF-α biologic for CD patients with loss of response to IFX [1, 2, 3]. However, for remission induction therapy in CD, the efficacy rate for ADA in randomized trials has been around 50% at week 10 among the patients who responded at 4 weeks [3]. This is considered to be a serious limitation of this biologic when long-term remission is in mind.
Adsorptive granulocyte and monocyte apheresis (GMA) with an Adacolumn can selectively deplete myeloid lineage leucocytes, which in patients with IBD are elevated with activation behavior and are thought to be significant sources of TNF-α [4]. Accordingly, in Japan and the European Union, GMA is another safe and non-drug therapeutic option for patients with IBD refractory to pharmacological therapy [5, 6, 7], in spite of inconsistent efficacy outcome in severe, refractory ulcerative colitis with long-term exposure to conventional medications [8]. Additionally, intensive GMA, involving 2 sessions per week, has recently been shown to be superior to the routine weekly GMA, both with respect to remission rate and time to remission in patients with refractory ulcerative colitis [7]. Likewise, a recent report demonstrated that combination therapy with GMA plus ADA induced rapid remission in a CD case with loss of response to scheduled anti-TNF-α biologics maintenance therapy [9].
With the above background in mind, we here report on combination therapy with intensive GMA plus ADA in 5 CD cases who were found to be refractory to conventional medication including scheduled maintenance anti-TNF-α biologics (2 of 5 cases).
Case Series
Between December 2010 and February 2012, 5 consecutive cases were selected for this GMA plus ADA combination therapy. The baseline demographic variables of the 5 cases are presented in table 1. All 5 patients, 1 female and 4 male, were registered at our hospital, the Nagoya City University Hospital. Patients had moderately to severely active ileocolic or colonic type CD at baseline according to CD activity index (CDAI) score. Among the 5 patients, 4 had previously diagnosed CD that was found to be refractory to medications including anti-TNF-α biologics, and 1 patient had recently been diagnosed to have CD. Further, of the 5 cases, the first 2 received intensive GMA [7] involving 2 sessions/week for 5 consecutive weeks immediately followed by ADA (160/80/40 mg, every other week) as remission induction therapy to strengthen the effect of intensive GMA. The other 3 patients received ADA injections during the course of intensive GMA (Adacolumn; JIMRO, Takasaki, Japan) [4, 5, 6, 7, 8, 9]. Concurrent therapies included stable dosages of 5-aminosalicylic acid (or sulfasalazine), corticosteroids and azathioprine, all started ≥4 weeks prior to the combination therapy with GMA and ADA.
Table 1.
Baseline demographic variables of the 5 cases with CD refractory to medications including anti-TNF-α therapies who were selected for this combination therapy with ADA plus intensive GMA
| Demography | Number of patients |
|---|---|
| Male/female | 4/1 |
| Age, years | 36 ± 17 |
| Mean disease duration, years | 8.5 ± 5.1 |
| Involved intestinal segment | |
| Ileocolitis | 1 |
| Colitis | 4 |
| Previous bowel resection | 1 |
| Perianal fistula | 1 |
| Concurrent medication | |
| Corticosteroids | 3 |
| Mesalamine/sulfasalazine | 4 |
| Metronidazole | 1 |
| Azathioprine | 1 |
| Infliximab | 1 |
| Adalimumab | 1 |
| Baseline CDAI | 324 ± 118 |
| Baseline CRP, mg/dl | 4.9 ± 3.3 |
CDAI scores, C-reactive protein (CRP) levels and colonoscopy findings were recorded at baseline and again 10 weeks following 5 shots of ADA (post ADA therapy). Endoscopic assessment of mucosal improvement was based on the simple endoscopic score for CD (SES-CD) [10]. These measurements were applied to assess treatment efficacy outcomes. Among the concurrent conventional medications, the corticosteroid dosage was to be tapered in line with improvements of patients’ CD. Any adverse event, including date of onset, severity, outcome and relationship of events to the interventions was recorded. The data are presented as the mean ± SD values, and comparisons were made by using the t test. A significance level of 0.05 was set for all statistical tests, and two-tailed tests were applied when appropriate.
The demographic variables listed in table 1 show the mean age of the 5 cases was 36 ± 17 years (range 22–62 years), and the mean duration of CD was 8.5 ± 5.1 years (range 14 years to 6 months). The location of CD was ileocolic in one case and colonic in 4 cases. Perianal fistulae were observed in 2 cases. Concurrent medications, including aminosalicylates, corticosteroids, metronidazole, azathioprine and anti-TNF-α biologics, are shown in table 2. However none of the 5 cases had received new medications or an increase in the dosage of a concurrent medication ≥4 weeks prior to the initiation of the present interventions (GMA and ADA combination therapy). Further, of the 5 cases, the first 2 had CD refractory to corticosteroids (case 1) or to scheduled maintenance IFX (case 2) and received intensive GMA (2 sessions/week) for 5 consecutive weeks followed by ADA as remission induction therapy. The mean CDAI scores and CRP levels at baseline and when intensive GMA was completed showed 250 points and 2.82 mg/dl, and 189 points and 2.75 mg/dl, respectively (table 2). At an additional 10 weeks following the last of the 5 ADA injections, the mean CDAI scores and CRP levels were 106 points and 0.37 mg/dl, respectively. The remaining 3 cases who were refractory to scheduled maintenance ADA (case 3) or had extensive longitudinal ulcers with perianal fistulae (case 4) or disease onset with severe mucosal inflammation together with extensive longitudinal ulcers (case 5) received concurrent intensive GMA and ADA injections. The evolution of the mean CDAI scores and CRP levels at baseline and 10 weeks post 5 ADA shots showed 373 ± 138 points and 6.3 ± 5.1 mg/dl, and 96 ± 49 points and 0.1 ± 0.1 mg/dl, respectively (table 2, fig. 1).
Table 2.
Relevant demographic variables and treatment outcomes 10 weeks post ADA in 5 cases with CD refractory to medications including anti-TNF-α therapies
| No. | Age, years | Sex | Disease duration, years | Type | Concurrent medications | Background | Baseline |
Treatment |
10 weeks post 5 ADA shots |
Clinical remission | Adverse event | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CDAI | CRP | intensive GMA | ADA | CDAI | CRP | |||||||||
| 1 | 62 | F | 11 | colitis | PSL/SSAL | corticosteroid-refractory | 259 | 3.89 | yes | yes | 91 | 0.5 | yes | none |
| 2 | 35 | M | 14 | ileocolitis | IFX/AZA | IFX failure | 241 | 1.75 | yes | yes | 121 | 0.23 | yes | none |
| 3* | 21 | M | 7 | colitis | 5-ASA/PSL, ADA | ADA failure | 483 | 6.68 | yes | yes | 83 | 0.17 | yes | none |
| 4 | 41 | M | 10 | colitis | SSAL/PSL | corticosteroid-refractory | 220 | 2.39 | yes | yes | 137 | 0.21 | yes | none |
| 5 | 22 | M | 0.5 | colitis | 5-ASA | disease onset | 415 | 9.7 | yes | yes | 68 | 0.03 | yes | none |
5-ASA = 5-aminosalicylic acid; AZA = azathioprine; PSL = prednisolone; SSAL = sulfasalazine.
From reference 9.
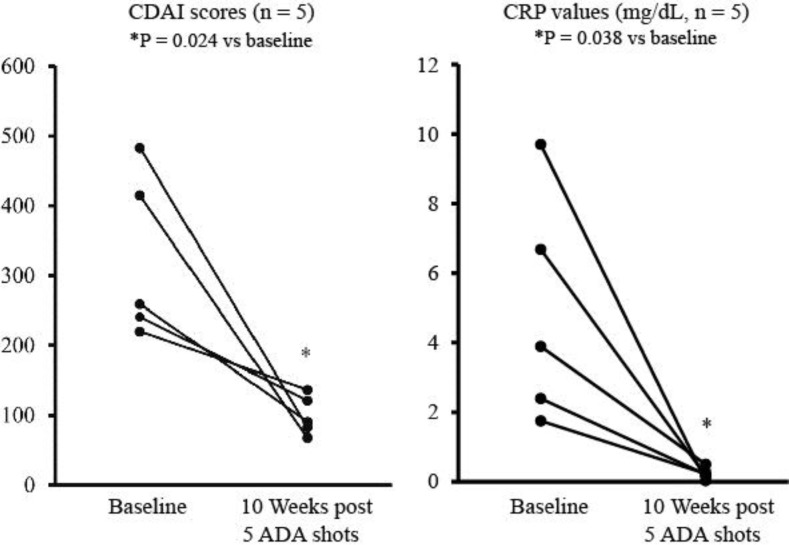
Fig. 1.
Overall changes in CDAI scores (a) and CRP levels (b) at baseline and 10 weeks after patients received 5 ADA shots for all 5 cases of this study. The comparisons were made by using the t test.
Figure 1 shows the overall changes in the CDAI scores and CRP levels for all 5 cases. The measurements showed a CDAI score of 324 ± 118 and a CRP level of 4.9 ± 3.3 mg/dl at baseline. At 10 weeks post 5 ADA shots, these measurements were significantly decreased down to 100 ± 28 points (p = 0.024) and 0.2 ± 0.2 mg/dl (p = 0.038), respectively. Clinical remission (CDAI < 150) and normal CRP levels were obtained at 10 weeks post ADA in all 5 cases (table 2). Marked decreases in endoscopic scores were obtained in 3 cases, including 1 case with complete mucosal healing (cases 1, 2 and 3), and partial improvement was achieved in 2 cases (cases 4 and 5) (table 3). During intensive GMA and ADA therapy, no adverse event was observed.
Table 3.
Endoscopic assessment outcomes based on the SES-CD in all 5 cases
| Case No. | Baseline | 10 weeks post 5 ADA shots |
|---|---|---|
| 1 | 13 | 0 |
| 2 | 5 | 2 |
| 3 | 13 | 4 |
| 4 | 14 | 10 |
| 5 | 23 | 14 |
Discussion
Here we report on the therapeutic outcomes in 5 cases with CD refractory to conventional medications, including 2 cases who were refractory to scheduled maintenance anti-TNF-α biologics. All 5 cases responded well to the combination therapy with intensive GMA plus ADA, achieving clinical remission, normal serum CRP levels and a marked healing of the CD lesions. In clinical settings, ADA is expected to induce sustained clinical remission in patients with moderate to severe active luminal CD. However, the rates of clinical remission for biologic-naïve patients at best have been 36% and about 50% at 4 and 10 weeks, respectively [1, 3], which is not very impressive. Likewise, its efficacy for patients with loss of response or intolerant to IFX was 21% at 4 weeks [11]. Therefore, the efficacy outcomes for ADA monotherapy in patients with active CD have been limited. This suggests that in order to achieve sustained clinical remission in the majority of CD patients, biologic-naïve or with loss of response to TNF-α antagonists, new treatment strategies are desirable. Based on the outcomes in 5 cases with medication-refractory CD, intensive GMA combined with ADA looks promising with no safety concerns. However, our view is that the therapeutic outcomes of the present case studies need to be supported by additional controlled studies in large cohorts of patients.
GMA is available in Europe and Japan for the treatment of patients with active IBD that may or may not be refractory to standard drug-based medication, including TNF-α blockers. GMA depletes elevated and activated myeloid lineage leucocytes and has been associated with a marked downregulation of inflammatory cytokines including interleukin-1β (IL-1β), IL-6, IL-8 and TNF-α, which are released by myeloid leucocytes and lymphocytes, most likely via an upstream mechanism that involves adsorption of cytokine-producing cells [4, 12, 13]. This suggests that GMA, which suppresses the immune profile in patients with CD, has a strong and favorable impact on the efficacy of ADA (or any anti-TNF biologic). Again, this assertion needs to be supported by additional reports in this clinical setting. The clinical outcomes in another case report [14] also showed that as a non-drug-based intervention, weekly GMA when added to scheduled IFX maintenance therapy was effective in a CD case with active disease while under IFX therapy. Further, it should be stated that intensive GMA involving 2 sessions per week has been found to be better than conventional weekly GMA for refractory IBD [7]. GMA as a non-drug therapeutic intervention is not likely to cause refractoriness often seen with drugs. Similarly, serious adverse side effects have been very rare in patients receiving GMA [4, 5, 6, 7, 8]. There are no apparent differences in efficacy in cases where GMA is performed simultaneously with ADA and in cases where GMA is preceded by ADA. Any time may be acceptable to add GMA therapy to ADA.
Deep and widespread longitudinal ulcers in the gut and a history of stricture and fistulae are considered to be features associated with intractable CD during therapeutic interventions [15]. Accordingly, in the present study, during our observation time, combination therapy with intensive GMA plus ADA could not achieve complete mucosal healing in all 5 cases, notably patients with deep and widespread longitudinal ulcers in the ileum and the colon, perhaps suggesting that a longer treatment course or follow-up time should have been considered.
In conclusion, in the present 5 cases with medication-refractory CD, combination therapy with intensive GMA plus ADA was very effective and induced clinical remission in all 5 cases together with marked healing of CD lesions and normalized serum CRP levels. Our impression is that GMA by depleting activated leucocytes as additional sources of inflammatory cytokines including TNF-α has a favorable effect on the efficacy of anti-TNF-α biologics. An additional study in a large cohort of patients is warranted to support the finding of this study in 5 cases with refractory CD.
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333. doi: 10.1053/j.gastro.2005.11.030. quiz 591. [DOI] [PubMed] [Google Scholar]
- 2.Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, Panaccione R, Wolf D, Kent JD, Bittle B, Li J, Pollack PF. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, Pollack PF. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Saniabadi AR, Hanai H, Fukunaga K, Sawada K, Shima C, Bjarnason I, Lofberg R. Therapeutic leukocytapheresis for inflammatory bowel disease. Transfus Apher Sci. 2007;37:191–200. doi: 10.1016/j.transci.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda Y, Matsui T, Suzuki Y, Kanke K, Matsumoto T, Takazoe M, Motoya S, Honma T, Sawada K, Yao T, Shimoyama T, Hibi T. Adsorptive granulocyte and monocyte apheresis for refractory Crohn's disease: an open multicenter prospective study. J Gastroenterol. 2004;39:1158–1164. doi: 10.1007/s00535-004-1465-z. [DOI] [PubMed] [Google Scholar]
- 6.Ljung T, Thomsen OO, Vatn M, Karlen P, Karlsen LN, Tysk C, Nilsson SU, Kilander A, Gillberg R, Grip O, Lindgren S, Befrits R, Lofberg R. Granulocyte, monocyte/macrophage apheresis for inflammatory bowel disease: the first 100 patients treated in Scandinavia. Scand J Gastroenterol. 2007;42:221–227. doi: 10.1080/00365520600979369. [DOI] [PubMed] [Google Scholar]
- 7.Sakuraba A, Motoya S, Watanabe K, Nishishita M, Kanke K, Matsui T, Suzuki Y, Oshima T, Kunisaki R, Matsumoto T, Hanai H, Fukunaga K, Yoshimura N, Chiba T, Funakoshi S, Aoyama N, Andoh A, Nakase H, Mizuta Y, Suzuki R, Akamatsu T, Iizuka M, Ashida T, Hibi T. An open-label prospective randomized multicenter study shows very rapid remission of ulcerative colitis by intensive granulocyte and monocyte adsorptive apheresis as compared with routine weekly treatment. Am J Gastroenterol. 2009;104:2990–2995. doi: 10.1038/ajg.2009.453. [DOI] [PubMed] [Google Scholar]
- 8.Sands BE, Sandborn WJ, Feagan B, Lofberg R, Hibi T, Wang T, Gustofson LM, Wong CJ, Vandervoort MK, Hanauer S. A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology. 2008;135:400–409. doi: 10.1053/j.gastro.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Ozeki K, Tanida S, Mizushima T, Mizoshita T, Tsukamoto H, Hirata Y, Murakami K, Shimura T, Kataoka H, Kamiya T, Joh T. Combination therapy with adalimumab plus intensive granulocyte and monocyte adsorptive apheresis induced clinical remission in a Crohn's disease patient with the loss of response to scheduled adalimumab maintenance therapy: a case report. Intern Med. 2012;51:595–599. doi: 10.2169/internalmedicine.51.6801. [DOI] [PubMed] [Google Scholar]
- 10.Daperno M, D'Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, Sostegni R, Rocca R, Pera A, Gevers A, Mary JY, Colombel JF, Rutgeerts P. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 11.Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Colombel JF, Panaccione R, D'Haens G, Li J, Rosenfeld MR, Kent JD, Pollack PF. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838. doi: 10.7326/0003-4819-146-12-200706190-00159. [DOI] [PubMed] [Google Scholar]
- 12.Kashiwagi N, Hirata I, Kasukawa R. A role for granulocyte and monocyte apheresis in the treatment of rheumatoid arthritis. Ther Apher. 1998;2:134–141. doi: 10.1111/j.1744-9987.1998.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 13.Saniabadi AR, Hanai H, Takeuchi K, Umemura K, Nakashima M, Adachi T, Shima C, Bjarnason I, Lofberg R. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. 2003;7:48–59. doi: 10.1046/j.1526-0968.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez Carro P, Perez Roldan F, Roncero Garcia Escribano O, Lafuente R, Legaz Huidobro ML, Amigo Echenagusia A. Case report: Combination therapy with granulocyte apheresis and infliximab for refractory Crohn's disease. J Clin Apher. 2006;21:249–251. doi: 10.1002/jca.20093. [DOI] [PubMed] [Google Scholar]
- 15.Allez M, Lemann M, Bonnet J, Cattan P, Jian R, Modigliani R. Long term outcome of patients with active Crohn's disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol. 2002;97(16):947–953. doi: 10.1111/j.1572-0241.2002.05614.x. [DOI] [PubMed] [Google Scholar]