Abstract
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is a devastating autosomal recessive disorder due to mutations in TYMP, which cause loss of function of thymidine phosphorylase (TP), nucleoside accumulation in plasma and tissues and mitochondrial dysfunction. The clinical picture includes progressive gastrointestinal dysmotility, cachexia, ptosis and ophthalmoparesis, peripheral neuropathy and diffuse leukoencephalopathy, which usually lead to death in early adulthood. Therapeutic options are currently available in clinical practice (allogeneic hematopoietic stem cell transplantation and carrier erythrocyte entrapped TP therapy) and newer, promising therapies are expected in the near future. However, successful treatment is strictly related to early diagnosis. We report on an incomplete MNGIE phenotype in a young man harboring the novel heterozygote c.199 C>T (Q67X) mutation in exon 2, and the previously reported c.866 A>C (E289A) mutation in exon 7 in TYMP. The correct diagnosis was achieved many years after the onset of symptoms and unfortunately, the patient died soon after diagnosis because of multiorgan failure due to severe malnutrition and cachexia before any therapeutic option could be tried. To date, early diagnosis is essential to ensure that patients have the opportunity to be treated. MNGIE should be suspected in all patients who present with both gastrointestinal and nervous system involvement, even if the classical complete phenotype is lacking.
Key words: Mitochondrial neurogastrointestinal encephalomyopathy, Thymidine phosphorylase, TYMP
Introduction
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is a rare, devastating disease that usually manifests in childhood and leads to death at a mean age of 35 years [1]. It is clinically characterized by progressive gastrointestinal dysfunction, cachexia, ptosis and ophthalmoparesis, peripheral neuropathy and diffuse leukoencephalopathy [1, 2]. However, patients with an atypical phenotype and variable onset, course and severity have also been described [3, 4, 5].
MNGIE is caused by mutations in the TYMP gene encoding thymidine phosphorylase (TP) [6]. Since TYMP was discovered as the causative gene of MNGIE, more than 60 different mutations associated with fewer than 250 cases have been reported [7]. Loss of TP activity determines marked elevation in thymidine and deoxyuridine levels in body fluids, nucleotide pool imbalance, subsequent instability of mitochondrial DNA (mtDNA) and impairment of the mitochondrial respiratory chain [1, 8].
We report the case of a young Caucasian patient with an incomplete MNGIE phenotype who harbored a novel disruptive TYMP mutation. His disease was diagnosed late, thus making any therapeutic option useless. Despite the current availability of effective treatment options such as allogeneic hematopoietic stem cell transplantation [9] and carrier erythrocyte entrapped TP therapy [10] as well as the promising future therapeutic approaches, e.g. gene therapy [11], it is essential to achieve early diagnosis to avoid the cumulative effects of imbalanced nucleosides on mtDNA and to prevent as much mitochondrial damage as possible [1, 8, 12].
Case Presentation
Since he was 7, our 24-year-old patient had complained of intermittent postprandial vomiting and gastrointestinal dysmotility with alternating episodes of diarrhea and constipation that led to progressive weight loss over the years. He presented with severe cachexia (BMI 11.15) and numbness in the extremities of all 4 limbs. Neurological examination showed absence of deep tendon reflexes, length-dependent decreased proprioception and vibratory sensation, mild generalized weakness and diffuse muscle atrophy. He did not have ophthalmoparesis or ptosis.
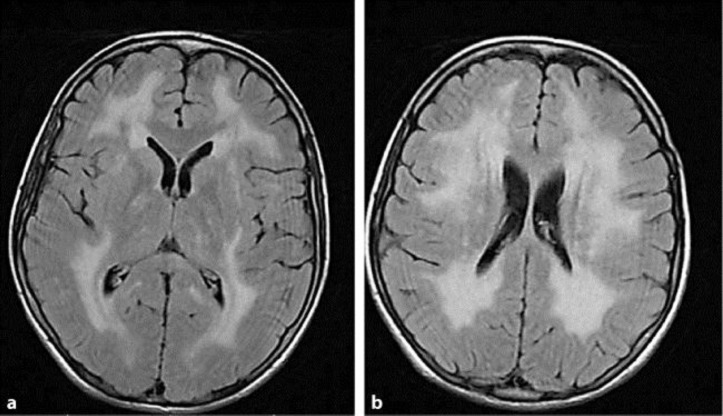
Electroencephalography was normal and a brain MRI showed diffuse leukoencephalopathy (fig. 1a, b). Electroneurography and electromyography were not performed. No cardiac, renal or hepatic involvement was reported.
Fig. 1.
Brain MRIs with fluid attenuated inversion recovery. The images show sparing of the basal ganglia and corpus callosum (a) as well as diffuse leukoencephalopathy in the semioval centers (b).
Despite the lack of eye movement involvement, MNGIE was suspected and confirmed by measuring plasma thymidine and deoxyuridine [6 μmol/l (normal values <0.05) and 17 μmol/l (normal values <0.05), respectively] as well as TP activity in platelets [4 nmol/h/mg protein (normal values 377–1,320)].
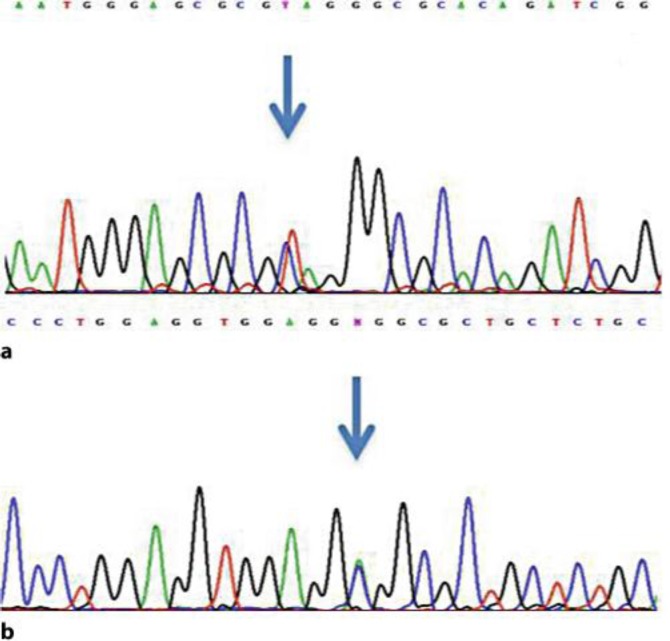
TYMP sequence analysis showed the novel heterozygote c.199 C>T (Q67X) mutation in exon 2 (fig. 2a) and the previously reported c.866 A>C (E289A) mutation in exon 7 (fig. 2b) (GenBank reference sequence NM_001113755.1), thus genetically proving the diagnosis of MNGIE. The novel mutation leads to a change from glutamine to stop codon and was not detected in 200 ethnically matched control chromosomes by direct gene sequencing.
Fig. 2.
Electropherogram showing the two mutations in TYMP, namely a the heterozygote c.199 C>T mutation (arrow) and b the heterozygote c.866 A>C mutation (arrow).
Unfortunately, despite parenteral nutrition, the patient died of multiorgan failure due to severe malnutrition and cachexia a few weeks after diagnosis before any therapeutic option could be tried. A family history was not available.
Discussion
MNGIE is a multisystem autosomal recessive disorder mainly characterized by gastrointestinal and central/peripheral nervous system involvement [1]. Gastrointestinal dysmotility, abdominal pain and distension, diarrhea, progressive weight loss and severe cachexia are the prominent features of the disease, associated with ptosis and ophthalmoparesis, peripheral neuropathy and diffuse leukoencephalopathy [1, 2]. Patients usually die of complications resulting from their gastrointestinal disorders and inadequate nutritional status [1]. Diagnosis is achieved by demonstrating high plasma, urine thymidine and deoxyuridine concentrations, reduced TP enzyme activity and pathogenic mutations in TYMP [8]. Disease-related TYMP mutations determine loss of TP function, subsequent mitochondrial nucleotide pool imbalance, mtDNA instability and impairment of the mitochondrial genome replication [8]. A relationship between the clinical phenotype of MNGIE and TP activity is generally acknowledged: <10% of the normal activity causes typical MNGIE, 10–20% residual activity produces less severe late-onset phenotypes and >30% activity does not cause overt disease [13, 14]. However, there are relevant exceptions to this rule. Late-onset or less severe phenotypes in patients with greatly reduced or virtually absent TP activity have been reported [3, 5]. Intrafamilial phenotypical variability, ranging from extremely severe to very mild clinical pictures, has also been described, thus adding further difficulties in predicting disease evolution and prognosis [5, 7].
The mutations reported herein, including the novel Q67X nonsense mutation, cause a profound deficiency in TP activity (4 nmol/h/mg protein). This may explain the severe phenotype in our patient. Interestingly, ophthalmoplegia, which is a fundamental element in suspecting the disease, was absent in this patient and may have contributed to the delay in diagnosis. Although most patients present with the classical phenotype and report the first symptoms already in childhood, the correct diagnosis is usually established years later [5]. A late diagnosis of the disease greatly reduces the chances of a positive outcome after therapy because patients start treatment when they are already in poor health and therefore have limited capacities to tolerate therapy and combat disease-related complications [5, 15]. The available therapeutic procedures aim to eliminate the toxic levels of thymidine and deoxyuridine by restoring at least partial TP activity. The earlier patients are treated, the less organ damage there is and the better the outcome will be [1, 9, 14]. In our patient, the correct diagnosis was achieved too late when multisystem involvement was so severe that it led to his death within a few weeks.
Conclusion
Despite the enormous efforts in optimizing therapies for MNGIE, one must not ignore the need to achieve a correct diagnosis as soon as possible. MNGIE should be suspected and TP activity and nucleoside levels measured in all patients who present with both gastrointestinal and nervous system involvement, even if the classical complete phenotype is lacking. Early identification of patients can save lives.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgements
We kindly thank Drs. Moreno Ferrarini, Federica Taioli and Silvia Testi from the Department of Neurological, Neuropsychological, Morphological and Movement Sciences, University of Verona, Italy, for technical support.
References
- 1.Garone C, Tadesse S, Hirano M. Clinical and genetic spectrum of mitochondrial neurogastrointestinal encephalomyopathy. Brain. 2011;134:3326–3332. doi: 10.1093/brain/awr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishino I, Spinazzola A, Papadimitriou A, Hammans S, Steiner I, Hahn CD, Connolly AM, Verloes A, Guimarães J, Maillard I, Hamano H, Donati MA, Semrad CE, Russell JA, Andreu AL, Hadjigeorgiou GM, Vu TH, Tadesse S, Nygaard TG, Nonaka I, Hirano I, Bonilla E, Rowland LP, DiMauro S, Hirano M. Mitochondrial neurogastrointestinal encephalomyopathy: an autosomal recessive disorder due to thymidine phosphorylase mutations. Ann Neurol. 2000;47:792–800. [PubMed] [Google Scholar]
- 3.Martín MA, Blázquez A, Martí R, Bautista J, Lara MC, Cabello A, Campos Y, Belda O, Andreu AL, Arenas J. Lack of gastrointestinal symptoms in a 60-year-old patient with MNGIE. Neurology. 2004;63:1536–1537. doi: 10.1212/01.wnl.0000141857.37073.97. [DOI] [PubMed] [Google Scholar]
- 4.Massa R, Tessa A, Margollicci M, Micheli V, Romigi A, Tozzi G, Terracciano C, Piemonte F, Bernardi G, Santorelli FM. Late-onset MNGIE without peripheral neuropathy due to incomplete loss of thymidine phosphorylase activity. Neuromuscul Disord. 2009;19:837–840. doi: 10.1016/j.nmd.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Filosto M, Scarpelli M, Tonin P, Testi S, Cotelli MS, Rossi M, Salvi A, Grottolo A, Vielmi V, Todeschini A, Fabrizi GM, Padovani A, Tomelleri G. Pitfalls in diagnosing mitochondrial neurogastrointestinal encephalomyopathy. J Inherit Metab Dis. 2011;34:1199–1203. doi: 10.1007/s10545-011-9332-6. [DOI] [PubMed] [Google Scholar]
- 6.Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science. 1999;283:689–692. doi: 10.1126/science.283.5402.689. [DOI] [PubMed] [Google Scholar]
- 7.Libernini L, Lupis C, Mastrangelo M, Carrozzo R, Santorelli FM, Inghilleri M, Leuzzi V. Mitochondrial Neurogastrointestinal Encephalomyopathy: novel pathogenic mutations in thymidine phosphorylase gene in two Italian brothers. Neuropediatrics. 2012;43:201–218. doi: 10.1055/s-0032-1315431. [DOI] [PubMed] [Google Scholar]
- 8.Martí R, Spinazzola A, Tadesse S, Nishino I, Nishigaki Y, Hirano M. Definitive diagnosis of mitochondrial neurogastrointestinal encephalomyopathy by biochemical assays. Clin Chem. 2004;50:120–124. doi: 10.1373/clinchem.2003.026179. [DOI] [PubMed] [Google Scholar]
- 9.Halter J, Schüpbach WM, Casali C, Elhasid R, Fay K, Hammans S, Illa I, Kappeler L, Krähenbühl S, Lehmann T, Mandel H, Marti R, Mattle H, Orchard K, Savage D, Sue CM, Valcarcel D, Gratwohl A, Hirano M. Allogeneic hematopoietic SCT as treatment option for patients with mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): a consensus conference proposal for a standardized approach. Bone Marrow Transplant. 2011;46:330–337. doi: 10.1038/bmt.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran NF, Bain MD, Muquit MM, Bax BE. Carrier erythrocyte entrapped thymidine phosphorylase therapy for MNGIE. Neurology. 2008;71:686–688. doi: 10.1212/01.wnl.0000324602.97205.ab. [DOI] [PubMed] [Google Scholar]
- 11.Torres-Torronteras J, Gómez A, Eixarch H, Palenzuela L, Pizzorno G, Hirano M, Andreu AL, Barquinero J, Martí R. Hematopoietic gene therapy restores thymidine phosphorylase activity in a cell culture and a murine model of MNGIE. Gene Ther. 2011;18:795–806. doi: 10.1038/gt.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scarpelli M, Cotelli MS, Mancuso M, Tomelleri G, Tonin P, Baronchelli C, Vielmi V, Gregorelli V, Todeschini A, Padovani A, Filosto M. Current options in the treatment of mitochondrial diseases. Recent Pat CNS Drug Discov. 2010;5:203–209. doi: 10.2174/157488910793362412. [DOI] [PubMed] [Google Scholar]
- 13.Martí R, Verschuuren J, Buchman A, Hirano I, Tadesse S, van Kuilenburg AB, van Gennip AH, Poorthuis BJ, Hirano M. Late-onset MNGIE due to partial loss of thymidine phosphorylase activity. Ann Neurol. 2005;58:649–652. doi: 10.1002/ana.20615. [DOI] [PubMed] [Google Scholar]
- 14.Hirano M, Garone C, Quinzii C. CoQ(10) deficiencies and MNGIE: two treatable mitochondrial disorders. Biochim Biophys Acta. 2012;1820:625–631. doi: 10.1016/j.bbagen.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filosto M, Scarpelli M, Tonin P, Lucchini G, Pavan F, Santus F, Parini R, Donati MA, Cotelli MS, Vielmi V, Todeschini A, Canonico F, Tomelleri G, Padovani A, Rovelli A. Course and management of allogeneic stem cell transplantation in patients with mitochondrial neurogastrointestinal encephalomyopathy. J Neurol. 2012;259:2699–2706. doi: 10.1007/s00415-012-6572-9. [DOI] [PubMed] [Google Scholar]