Abstract
Heart failure mortality is significantly increased in patients with baseline renal impairment and those with underlying heart failure who subsequently develop renal dysfunction. This accelerated progression occurs independent of the cause or grade of renal dysfunction and baseline risk factors. Recent large prospective databases have highlighted the depth of the current problem, while longitudinal population studies support an increasing disease burden. We have extensively reviewed the epidemiological and therapeutic data among these patients. The evidence points to a progression of heart failure early in renal impairment, even in the albuminuric stage. The data also support poor prescription of prognostic therapies. As renal function is the most important prognostic factor in heart failure, it is important to establish the current understanding of the disease burden and the therapeutic implications.
Key Words: Heart failure, Renal failure, Cardiorenal syndrome
Introduction
Comorbid heart failure (HF) and renal impairment (RI) is an evolving epidemic faced by an increasing number of medical practitioners. Coronary artery disease invigorated innovation and advancements in cardiac research and delivery of state-of-the-art pharmacological and device therapies. Without a doubt, these patients are enjoying a better standard of living and life expectancy. Since Lindner et al. [1] first described the association between cardiovascular diseases (CVD) in patients with end-stage renal disease (ESRD), we have come to realise this association is more complex. For example, the permutations relate to the primary organ involved, the severity of the organ involvement, the degree of organ cross talk and compensatory feedback, temporal factors and a host of other factors. Consequently, we often see these patients undertreated as physicians do not know where to start or fear that treating the worse organ may provoke the progression of the other. Quite strikingly, the number of patients at risk also continues to accelerate while there has been negligible progress in improving outcomes. The increased complexity of this syndrome may be a contributing factor in the slow progress. While we await novel diagnostics and therapeutics, the interim should focus on advancing the understanding of the cardiorenal pathophysiology and the interpretation of conventional biochemistry. We refer the reader to several recent reviews in the area that have addressed this issue [2,3,4,5,6,7]. This review focuses on updates on cardiorenal epidemiology and therapeutics, with special emphasis on both cardiorenal and renocardiac perspectives. Please note that in this article, we use chronic renal insufficiency (CRI) and reduced estimated glomerular filtration rate (eGFR) to imply renal functional limitations. We use chronic kidney disease (CKD), sparingly, to encompass the entire spectrum of renal diseases with or without functional limitations.
Epidemiological Association between CRI and HF
The last several years have shed significant insights into the cardiorenal epidemiology with data from three large registries [8,9,10,11,12]. On the therapeutic side, outcome data are still based on observational and post hoc analyses as most randomised HF trials excluded patients with high serum creatinine (SCr) levels. Unfortunately, it is this group that most likely benefit from prognostic medications, of which the dosage and clinical prescription are often dependent on the underlying renal function (RF) [13]. The present literature highlights 3 important viewpoints: the renal perspective, the cardiac perspective and, lastly, the issue of therapeutics. Table 1 summarises the relevant large studies with epidemiological data on congestive heart failure (CHF) and CRI.
Table 1.
Epidemiology of RI in HF
| First author [Ref] | Population | Participants | Age years | Female % | Black race % | Duration | RI prev/inc | Definition of CKD | Study design | ACM | CVM | Renal failure on CV endpoints |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sarnak [5] | General | 5.8 m | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Velavan [120] | Suspected HF admissions | 10,701 | 68 | 39 | NA | 90 days | 17 NA | SCr | PC | + | + |
|
| Patel [121] | ADHF | 15,560 | 76 | 50.1 | 17.7 | Length of hospitalization | 6.6–44 NA | MDRD | PC | + | + | Ischaemic HF prevalence with stages of RF: normal 8.5%, mild 26.8%, moderate 43.9%, severe 14.2%, and renal failure 6.6%, p < 0.0001; increased inpatient mortality with increasing severity of RI |
| Heywood [9, 15] | ADHF | 118,465 | 72.4 | 52 | 20 | Length of hospitalization | See figure 2 | MDRD | PC | + | + | See table 2 |
| Fonarow [11] | ADHF | 48,612 | 73.1 | 51.6 | 17.7 | 90 days | 19.6 20.4 | SCr | PC | + | + |
|
| Damman [33] | ADHF and CHF | 18,634 | 59–79 | 16–67 | NA | 30 days to 4.8 years | Variable NA | SCr, eGFR | M-A | + | + | Increased ACM with worsening grade AKI |
| Coca [122] | Variable HF presentations | 78,855 | NA | NA | NA | 30 days | 3.2–60 NA | SCr | M-A | + | + | Increasing risk of 30-day mortality with worsening grade AKI using percentage change and absolute change of SCr to define AKI |
| Smith [123] | ADHF and CHF | 80,098 | 52–79 | 9–100 | NA | NA | NA 63 | SCr | M-A | + | + |
|
| Smith [13] | ADHF | 53,640 | 79 | 58 | 11 | 1 year | NA 68 | SCr, MDRD | RC | + | + | An increase in creatinine by 0.5 mg/dl is associated with an increase in the 1-year death risk by 10% in blacks and by 15% in whites |
| Lewis [124] | AMI | 11,040 | 70 | 39.2 | 8.4 | NA | 6.3–8.5 10.3 | eGFR | RCT | + | + | Increased risk of HF or CVM HR 1.12 (95% CI 1.08 −1.16, p < 0.001) |
| Shlipak [125] | AMI | 130,099 | 77 | 46 | 7.7 | 1 year | 16–41 NA | SCr, C+G | RC | + | + |
|
| Stevens [126] | Elderly CKD | 27,017 | NA | 66.7 | 24.5 | NA | NA 55 | MDRD | KEEP registry | NA | NA | CHF 4.2 vs. 7.5 RR (p < 0.001) in patients with vs. without CKD |
| Bertoni [127] | DM | 151,738 | 75 | 60 | 12 | 5 years | 18.6 39.3 | NA | RC: America/Medicare program | + | + |
|
| Nichols [128] | DM | 17,076 | 63 | 48 | NA | 4.7 years | NA 50.1 | ACR, need for RRT | RC: Kaiser Permanente Northwest | + | + | micro HR 0.78 (95% CI 0.65–0.93, p = 0.006); macro HR 1.25 (95% CI 1.08–1.46, p = 0.004); ESRD HR 1.54 (95% CI 1.04–2.3, p = 0.032) |
| Go [19] | General: low risk | 1.12 m | 52 | 55 | 7.4 | 2.8 years | 2.1 NA | MDRD | PC: Kaiser Permanente renal registry | + | + | eGFR (in ml/min/1.73 m2): >60 (1.0%); 45–59 (5.2%); 30–44 (12.6%); 15–29 (20.8%); <15 (18.5%) |
This table only includes the major studies in CRS epidemiology. We included studies above 5,000 patients.
ACM = All-cause mortality; ACR = albumin:creatinine ratio; ADHF = acute decompensated heart failure; AKI = acute kidney injury; AMI = acute myocardial infarction; C+G = Cockroft and Gault; CHF = congestive heart failure; CKD = chronic kidney disease; CVM = cardiovascular mortality; DM = diabetes mellitus; ESRD = end-stage renal disease; HF = heart failure; prev/inc = prevalence/incidence as reported in the original study; LVEF = left ventricular ejection fraction; M-A = meta-analysis; macro = macro-albuminuria; MDRD = Modification of Diet in Renal Disease; micro = microalbuminuria; NA = not available; PC = prospective cohort study; RC = retrospective cohort study; RCT = randomised controlled trial; RF = renal function; RI = renal impairment; SCr = serum creatinine in μmol/l; WRF = worsening renal function.
(i) The Depth of the Problem
The cardiovascular system maintains a direct communication with other organ systems; however, the cardiorenal interaction appears to be the most substantial. This is supported by the position statement from the National Kidney Foundation Task Force on cardiovascular outcomes in CKD: ‘patients with CKD be considered in the “highest risk group” for subsequent cardiovascular events and that treatment recommendations based on cardiovascular risk stratification should take into account the highest-risk status of these patients’ [13,14].
HF is the most common admitting diagnosis in the United States for patients aged >65 years [15]. CKD is a worldwide public health problem with a rising incidence and a prevalence associated with poor outcomes and high cost. The US health system requirements for dialysis and transplantation exceeded 320,000 people in 1998 and surpassed 650,000 people within a decade. Iatrogenic renal replacement therapies are associated with a high mortality; in 45- to 54-year-olds, the cardiovascular mortality rate is 65 times higher than in the general population, and a more dramatic 500 times among younger cohorts [16]. A higher prevalence is noted for the earlier stages [5]. Although most of the traditional risk factors are prevalent in patients with CKD, studies have shown that the Framingham risk equation is insufficient to capture the extent of CVD, an important contributor to CHF, in patients with CKD. While this may result from the influence of non-traditional risk factors, it also highlights a unique entity with the potential for devastating and unpredictable consequences [15]. In patients with diagnosed CHF, significant RI is also common. Underlying RI contributes to poorer in-hospital outcomes and a grim prognosis [15,17]. Worsening renal function (WRF) in the absence of primary renal disease is also a major determinant of outcomes in HF [15,18].
(ii) Prevalence of HF with Varying Stages of CKD
When looking outside the broad classification of CVD, the prevalence of CHF among the different stages of CRI is actually not well established. Broadly extrapolating CVD data from CKD databases highlights mortality rates 10–30 times higher in dialysis patients and 5-fold higher after stratification for age [15]. While data are lacking in patients with CKD stages 1–4 (kidney transplant recipients) and CKD stages 3–4 (diabetic and non-diabetic kidney diseases), data from the Dialysis Morbidity and Mortality Study (Wave 2), United States Renal Data System Annual Data Report 1997, suggest a CHF prevalence of 40%. Proteinuria and microalbuminuria which act as surrogates for early non-diabetic CKD and early diabetic CKD are also associated with adverse CHF outcomes [5,19], but actual rates are also still less well defined.
(iii) Prevalence of CKD in Patients with HF
Existing data support 4 important perspectives. Firstly, a high prevalence of CRI in CHF. Secondly, worse CHF outcomes when baseline RF is abnormal. Thirdly, admitted patients with WRF carry a significantly poorer prognosis. Fourthly, CRI is underdetected among HF patients who are at a higher risk of progression to end-stage renal insufficiency (ESRI).
Baseline RF and HF Outcomes
The ADHERE database, which was set up in October 2001, recorded data from 175,000 admissions across 280 participating centres from August 2005. From this database, Heywood et al. [15] were able to determine the prevalence and severity of RI at the time of hospital admission in patients with acute decompensated HF (ADHF), and to relate the degree of RI to treatments and in-hospital outcomes. It has been noted that previous large outpatient CHF studies excluded patients with RI; furthermore, the diagnosis was made solely on the basis of an elevated SCr level. The National Kidney Foundation has advocated eGFR using the Modification of Diet in Renal Disease (MDRD) formula for all patients, and thus the degree and impact of RI in HF based on SCr are likely underestimated [15,20]. Hence, for the first time we have an accurate estimate of the prevalence and impact of the cardiorenal syndrome (CRS) on clinical outcomes. Table 2 summarises the major findings from the ADHERE database. The findings suggest a significant burden of CRI, worse outcomes and underprescription of disease-modifying therapeutics. Several smaller studies using CRI diagnosed based on eGFR highlighted RF as the most powerful prognostic indicator, exceeding functional status and ejection fraction [13,15,17,21,22,23,24,25,26,27].
Table 2.
Major findings from the ADHERE database (modified from [14])
| Baseline demographics | In-hospital clinical outcomes |
|---|---|
| 1 Only 10,660 (9%) of the study population were classified as stage 1 (normal RF), while 63.6% were classified as either moderate or severe (stage V renal failure). | 1 In-hospital outcomes worsened with grade of RI, inclusive of mechanical ventilation, ICU admission, cardiopulmonary resuscitation and new-onset dialysis. |
| 2 Although 59.3% of men and 67.6% of women had at least moderate renal dysfunction at admission, only 33.4% of men and 27.3% of women were reported as having renal insufficiency in the database. Only less than 10% of patients with moderate renal dysfunction (stage III) had a baseline SCr level >2.0 mg/dl. | 2 Length of hospital stay correlated with grade of baseline RI, except for stage V, which correlated with moderate RI. |
| 3 MDRD-based eGFR predicts frequency of common risk factors, e.g. hypertension, diabetes and clinical atherosclerosis as manifested by coronary artery disease or peripheral vascular disease. | 3 In-hospital mortality increased with severity of baseline RI. GFR remained an independent predictor of mortality (OR with 10 ml/min/1.73 m2 decrease in GFR was 1.23; 95% CI 1.21–1.25). |
| 4 The mean systolic ejection fraction was similar (37.3–37.8%) for all stages of kidney function except for stage V, where it was 40.3% (p < 0.0001). | 4 Worsening of RF during hospitalization was also associated with increasingly unfavourable outcomes. |
The ADHERE database, which was set up in October 2001, recorded data from 175,000 admissions across 280 participating centres from August 2005.
WRF and Outcomes
The ADHERE database has provided resounding evidence that even small to moderate decreases in RF are associated with significant morbidity and mortality, supporting multiple smaller studies [13,15,20,28,29]. Among 1,002 patients, Gottlieb et al. [27] showed that the majority had some increase in SCr, while 30% had a 20% increase. While any increase in SCr level was significant, an increase of 0.3 mg/dl had a sensitivity of 81% and a specificity of 62% in predicting either death or a length of stay of 10 days or more [20]. Systematic meta-analyses and smaller series have shown that a 0.2-mg/dl increase predicted a worse outcome, and an increase of 25% was a very specific marker of poor prognosis [30]; at discharge, 12% of patients had a 25% decrease in eGFR [31], and WRF developed in 27%, usually within 3 days of admission [32]. The severity of WRF was also associated with greater mortality [18,33,34,35,36]. In addition, it has been shown that in children, death is more likely than progression to ESRI [37,38].
Surprisingly, WRF occurs even in the absence of clear precipitants such as hypotension, shock or a procedure that requires contrast or bypass surgery [29]. Krumholz et al. [39] looked at 1,681 discharges among patients aged ≥65 years who did not have clear precipitants for RI. WRF was associated with male gender, hypertension, basilar rales, pulse >100 bpm, systolic blood pressure >200 mm Hg and admission SCr >1.5 mg/dl. Based on the number of these factors, a patient's risk for developing WRF ranged between 16% (1 factor) and 53% (5 factors). After adjusting for confounding effects, WRF was associated with a significantly longer length of stay by 2.3 days, higher in-hospital cost by USD 1,758 and an increased risk of in-hospital mortality (OR 2.72; 95% CI 1.62–4.58) [39]. Predictive models based on the ADHERE database to provide clinicians with a validated, practical bedside tool for mortality risk stratification have been developed. While models like these predict risk, they do not allow us to tailor therapy towards the individual patients with exacting accuracy; thus, the patients with the highest risk may be denied therapy based on conjecture rather than sound physiology [40].
Progression to ESRD and Underestimation of CRI
Among randomly selected Medicare beneficiaries with a diagnosis of CHF or acute myocardial infarction, the prevalence of CRI, based on the estimated MDRD GFR of <60 ml/min/1.73 m2, was 60.4%. ESRI after discharge occurred in 32/640 patients (OR 34.5; 95% CI 4.23–429.43); only 1 patient had an eGFR >60 ml/min/1.73 m2. Many of those who progressed to ESRI did not have a diagnosis of CRI at discharge. Even at the most severe degrees (MDRD-estimated GFR of <30 ml/min/1.73 m2), fewer than half had a diagnosis of CRI. Among the 24 CHF patients who developed CRI, only 20% were discharged with that recorded diagnosis. These data suggest that there are substantial opportunities to improve the detection of CRI in those patients who are hospitalized with CHF [33,41].
Therapeutics in HF and CKD
The only proven therapies for CHF are pharmacological treatments as the first line, device therapy as the second line and cardiac transplantation when the others fail. Unfortunately, when therapy is omitted, these patients are relegated to the placebo arm of HF trials, with ongoing exposure to the detrimental independent effects of impaired RF; in other words, a ‘double whammy’. Most major randomised controlled CHF trials, except for CHARM, excluded patients with moderate to advanced CRI and infrequently performed subgroup analysis. Furthermore, these trials predominately used SCr to determine RI. The Cardiovascular Health Study highlighted the inability to identify CRI in the elderly as SCr is not an effective measure of RF [42], which may raise doubts on the conclusions drawn from subgroup analysis. Table 3 summarises the medications and outcomes from the major HF trials. There is a clear consensus that all patients who have systolic dysfunction should be on an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB) if ACE-I is contraindicated, on hydralazine and isosorbide mononitrate if the latter are contraindicated, or on beta-blockers. For symptomatic patients and patients with more severe forms of CHF, addition of aldosterone blockade has additional prognostic benefit, as does device therapy in carefully selected individuals [38]. The fear of iatrogenic side effects (WRF, hyperkalaemia) has consequently led to lower prescription rates in this vulnerable group [43]. These factors are, however, determined by estimating the underlying eGFR accurately. For the purposes of this review, we have focused the discussion specifically on CRS types 2 and 4. Treating the aetiology is the common denominator in the management of all types of the CRS. Prognostic HF therapy is also indicated in all types; the severity and chronology of comorbid illness as well as the physicians’ confidence may influence individual prescribing practices [7].
Table 3.
Therapeutics in HF and chronic renal failure
| Therapy | Number of studies including sub-studies | Number of participants | Number of trials with information on method of RF assessment |
Age years | Racea | Sexb | CKD as exclusion criterionc | Contraindication in CRF | AKI or ↑ K+ as adverse event | Post hoc analysis | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCr | BUN | MDRD | C-G | NA | ||||||||||
| Beta-blockers | 10 | 19,687 | 6 | 0 | 1 | 0 | 4 | 49–64 | 2 (5–23) | 17–27 | 7 (SCr >245.5 to >300) | No | No | 1 – SENIORS |
| ACE-I | 7 | 14,810 | 5 | 0 | 1 | 0 | 1 | 59–75 | 1 (9.5–15) | 18–57 | 7 (SCr >151 to >300) | Relative | Marginal | 2 – SOLVD, ATLAS, CONSENSUS |
| ATRA | 6 | 15,713 | 2 | 0 | 4 | 0 | 0 | 62–71 | 6 (1–7) | 20–60 | 6 (SCr >177 to >220) | Relative | Marginal | 2 – V-HEFT, CHARM |
| Aldosterone antagonist | 2 | 7,895 | 1 | 0 | 1 | 0 | 0 | 65 | 2 (1–13) | 27–30 | 2 (SCr >220) | Relative | Yes | 1 – RALES |
| A-HEFT | 1 | 1,050 | NA | 56 | 1 (100) | 59 | NA – prevalence RI 16–18 | No | No | No | ||||
| Inotropesd | 2 | 1,530 | 2 | 1 | 0 | 0 | 0 | 67 | 1 (<6) | 12–28 | 1 (SCr >450) | No | No | No |
| Device | 10 | 10,306 | 4 | 2 | 0 | 0 | 4 | 63–67 | 2 (8–23) | 8–27 | 1 (SCr >265) | No | No | 1 – MADIT |
The table is inclusive of those therapies that provide acute and chronic prognostic benefit. Other non-prognostic therapies including diuretics were not included. All data are expressed as number of studies or cumulative upper and lower values. AKI = Acute kidney injury; BUN = blood urea nitrogen in mmol/l; C-G = Cockcroft-Gault formula; CRF = chronic renal failure; K+ = serum potassium; NA = not available. SCr was measured in μmol/l.
Race: number of studies which included black race as participants, with cumulative lower and upper range as percentage of study population in parentheses.
Sex: cumulative lower and upper range of participants as percentage of study population who were female.
CKD: number of studies that excluded CKD patients and criterion provided, with cumulative method used to define CKD and lower and upper values used to define CKD in parentheses.
Levosimendan trials only.
Interventions Targeted at Prevention and Risk Factor Control
There are numerous other factors that secondarily affect outcomes. While treatments of each of these factors have not been proven to affect outcomes, they nonetheless reflect the severity of the underlying disease state and thus warrant treatment and improvement of the primary condition. One such factor is anaemia, which may reflect both worsening GFR and anaemia of chronic illness related to the severity of HF. This in itself is independently associated with poor outcomes. The fact that measures to secondarily improve the haemoglobin level have not affected outcomes is further evidence that pharmacological measures aimed at improving cardiac function and preventing deterioration of GFR are paramount [44,45,46,47,48].
Diabetes and the intensity of glycaemic control remain an area of significant controversy. Type 2 diabetes mellitus and insulin resistance are associated with an increased HF risk and risk of progression. A 1% increase in haemoglobin A1c may increase the HF risk by 8%; this association follows a U-curve, with excess mortality with lower haemoglobin A1c (<6.4%) as well. The risk of HF hospitalizations is, however, linear [49,50,51,52,53]. Poor glycaemic control patients have poor in-hospital and long-term outcomes. These patients are more likely to present in advanced New York Heart Association class, CHF or RI [54,55]. Several recent meta-analyses have added some confusion. The first, a meta-analysis of 7 studies with 34,144 participants, supported intensive glucose control with reduction on major cardiovascular events with no effects on all-cause mortality and HF at the expense of an increased hypoglycaemic risk [56]. A further meta-analysis of 13 studies including 34,533 patients showed limited benefit of intensive control on all-cause mortality and cardiovascular death, with a 2-fold increase in hypoglycaemia and a 47% increase in CHF risk [57]. With regard to therapy, metformin remains the only antiglycaemic agent with demonstrated prognostic benefit, but a HF-targeted prospective study is lacking. Thiazolidinediones are the only agents with proven adverse HF risk. The extent to which manipulation of glucose control effects the sympathetic or renin-angiotensin-aldosterone system (RAAS) activity in the CRS has not been explored. In temporal terms, glucose control is highly sensitive to therapy. The dependence of counterregulatory effectors in CRS haemodynamics would suggest caution and a gentle approach in regulating metabolic derangements [51,58].
Tonelli et al. [59] reviewed 1,711 participants in the CARE study followed up for a median of 58.9 months and noted that pravastatin was safe and effective for secondary prevention of cardiovascular events in persons with mild CRI (as defined by eGFR ≤75 ml/min/h; Cockcroft-Gault formula) but was underused in this setting. Various agents have reproduced these findings in microalbuminuric stages, CKD stages 2–3 and renal transplants. Observational data extend this benefit in ESRD. The only randomised study using atorvastatin did not reproduce these observations. An unexplained excess of stroke in the treatment arm and an overall mortality approaching 50%, where more than half were non-cardiac, highlight a vulnerable group with yet to be identified confounders in play. In addition, statins also reduce the rates of progression of CKD and offer protection from acute renal failure (ARF) during cardiac procedures. Despite the small (0.5–2%) risk of liver biochemical abnormalities and myalgia or myopathy, the benefits significantly outweigh the risks [60,61].
At the time of writing, the SHARP study conclusively showed benefit of lipid lowering in ESRI with simvastatin and ezetemibe [129]. While providing critical answers, it also raises questions on the class of agents with benefits, the role of ezetemibe, the discrepancies noted with combination therapies from earlier studies, and benefits with varying cause and stages of CKD. These are but a few questions in this advancing area.
Benefits of aspirin in vascular disease among CRI patients are unequivocal, although with potential to increase major bleeds and paradoxical thrombosis. Among hypertensive CKD patients, aspirin therapy was associated with greater absolute reductions in major cardiovascular events and mortality than with normal RF [62]. The physiological effect of aspirin on renal blood flow in HF is still contentious; as a single agent, it appears safe. In the CRS, if concomitant agents have negative effects on RF is unclear, but they seem unlikely to have a negative impact. Further studies should similarly demonstrate these positive effects. The availability of alternative oral antithrombotics adds encouragement for the overall safety in cardiorenal therapeutics [63,64,65]. The need for holistic care and secondary factors among CRS patients warrants consideration as well.
HF Medications
RAAS Blockade
Blockade of RAAS via ACE-I, all-trans retinoic acid (ATRA) and aldosterone is mainstay and alters prognosis positively. Foley et al. [66] noted that changes in echocardiographic parameters at inception compared to 1 year of dialysis for ESRD were associated with development of CHF, while regression of left ventricular abnormalities was associated with an improved cardiac outcome. In the CRS, WRF and hyperkalaemia are significant safety concerns for many physicians. Among 4,350 ADHF admissions, patients with the lowest eGFR levels were least likely to be prescribed ACE-I/ARBs or both ACE-I/ARB and beta-blockers [33]. The underprescription with worsening CRI has a clear mortality gradient. A small caveat was that, while most medications were associated with improved outcomes, patients with an eGFR 60 ml/min/1.73 m2 using ACE-I did not share the same benefit [43]. The authors speculated a possible interaction with aspirin in lowering renal perfusion, but also highlighted a degree of complexity with confounders. In the SOLVD study, patients assigned to enalapril had a 33% greater likelihood of decreased RF but were older or had diuretic therapy or diabetes.
Using a higher definition of WRF of ≥0.5 mg/dl, ARF was not significantly greater in patients on preadmission ACE-I/ARB or diuretic use, while the rate of renal recovery was not significantly different whether or not SCr increased. Many larger studies define ARF as an increase in SCr of only 0.3 mg/dl compared to admission level; this relatively small increase may be insufficiently specific within the range of expected laboratory variation [67]. Defining decreased RF as a rise in SCr of ≥0.5 mg/dl (44 μmol/l) from baseline, as mentioned above, undoubtedly detects deterioration in RF; it does not carefully implement a policy of selecting appropriate doses, timing of medications and detecting severity of renal injury in elderly patients in whom lower levels of SCr may be associated with RI. Rates of renal artery stenosis are more significant in this age group [68], which also requires consideration when prescribing medications for the elderly. Within a sicker cohort of 256 patients, in 89 patients on ACE-I circulatory-renal limitations of hypotension, progressive renal dysfunction or hyperkalaemia accounted for 60 cases (23%) and other adverse events, e.g. cough, accounted for 24 cases. Compared with patients on ACE-I, patients with circulatory-renal limitations were older, had a longer history of CHF, lower systolic blood pressure, lower sodium and higher SCr. Mortality was 57% in patients with circulatory-renal limitations and 22% in patients on ACE-I during a median follow-up period of 8.5 months (p = 0.0001); these data are supported by several other studies [69,70,71].
In short, the use of medical therapy appears to relate to the CRI stage. ACE-I or ATRA at discharge decreased markedly with increasing RI, using either SCr or eGFR. Our present understanding suggests a need for understanding the limitations of these tests and the inability of measured eGFR to provide a sound estimate of RF in the acute setting and SCr to diagnose CRI [7]. An editorial comment by Bart [72] suggests: ‘ACE-I continue to be the best tolerated and most effective agents for improving symptoms, decreasing hospitalizations and prolonging survival in patients with HF however their use ranges from 35–80%. While the reasons vary from truly intolerant patients, real or perceived contraindications, poor compliance and variability in physicians practice. As there are few absolute contraindications, of the 7,487 patients in SOLVD only 11 (0.15%) had azotemia and average increase in SCr was 0.02 mg/dl. Despite this 6–17.5% of HF patients remain untreated because of real or perceived contraindications. While WRF is highly dependent on volume status, degree of sodium depletion and concurrent medications (B-Blocker appears protective), a strategy of patient preparation, premedication, diuretic dose monitoring, and accurate assessment of baseline RF and early detection of renal injury would seem a new direction in the use of these medications before they are relegated to a lifetime of absolute contraindication.’
Presently, recommendations to withdraw ACE-I should only occur when the rise in SCr exceeds 30% above baseline level within the first 2 months of initiation; in addition, a rise in SCr which gradually improves is a normal haemodynamic response to improvements in left ventricular function [73,74]. As for cardiovascular mortality, all-cause mortality and HF hospitalization, the risk reduction was significantly smaller in those without RI than in those with RI. Thus, the frequent practice of withholding ACE-I in patients with CRI is unwarranted [75,76,77]. New data from the HOPE study further suggest that hyperkalaemia (<6.5 mmol/l) does not increase the risk of cardiovascular events, whereas hypokalaemia (<3.5 mmol/l), which is mitigated by ACE-I, is harmful [77]. These issues reinforce the need for careful monitoring and improvements in the learning curve [78,79,80,81,82].
Aldosterone blockade has proven to be beneficial in symptomatic CHF or CHF after acute myocardial infarction and has been shown to delay progression of CKD. Anecdotal evidence supports improvement in left ventricular end diastolic parameters in early CKD and elderly patients with more severe RI. Direct evidence is lacking as patients with SCr >221 μmol/l were excluded from randomised trials. Safety concerns, particularly hyperkalaemia, still plague its use in severe RI. Among the major CHF trials, hyperkalaemia (potassium >6 mEq/l) was 2–5.9% treatment versus 1–3.9%. Risk factors included eGFR <45 ml/min/ 1.73 m2, older age, use of NSAID, potassium-promoting drugs or supplements and fluid balance not controlled for. RAAS blockade, beta-blockers and digoxin all contribute to hyperkalaemia in 5–10% of cases by suppressing aldosterone, although in most cases it is mild (0.1–0.3 mEq/l). Careful monitoring of these agents, serum digoxin levels, avoiding potassium supplements and NSAID, low-potassium diets, addition of potassium-wasting diuretics and increased vigilance in the lower GFR ranges and when serum potassium is >5.5 mEq/l will make a difference. Physicians should also consider halving doses or stop administration when levels are above 6 mEq/l. Another important consideration is the potential for GFR to decline further in ADHF. However, as there are no data for patients with eGFR <30 ml/min/1.73 m2, the use of aldosterone blockade in the lower GFR ranges should be made at an individual patient level until diagnostic advances are available [83,84,85,86,87,88]. In ADHF when eGFR levels may decline, clinical judgement should be used. No trial will provide evidence in this regard.
Direct renin inhibitors have also shown promise in HF therapeutics and may have some relevance. Firstly, much is still unknown about the source of sympathetic nervous system activity (SNSA) in CRI. Whether the juxtaglomerular apparatus is the source or effector of renal sympathetic nerve activity remains unclear; thus, consideration of renin inhibition at its source may warrant exploration. Secondly, pathophysiological observation of aldosterone ‘escape’, non-ACE generation of angiotensin II and a compensatory rise in renin and downstream RAAS effectors that may overwhelm ACE-I-blocking effects. As to which of these agents is used primarily or in combination to block the rate-limiting step in RAAS requires further exploration [89,90,91].
Beta-Adrenoceptor Blockade
Beta-blockers are the mainstay therapy for CHF, but debate still continues as to the principal class of drug as first choice in the CRS. The principles of prescribing remain similar; however, several additional factors require consideration [92,93,94,95,96,97,98,99,100,101]. Many CRS patients have an autonomous and chronically elevated SNSA, which affects remodelling of beta-adrenergic receptors and contributes to disease progression and poor outcome [102]. Anecdotal evidence suggests first-generation and non-selective beta-blockers may actually decrease GFR and renal blood flow, the effects of unopposed α1ARs, while blocking A2ARs-induced vasodilatation causes reflex sympathetic nerve activity and raises SVR/RVR leading to this reduction [103]; among second- and third-generation agents, carvedilol remains the strongest contender as agent of first choice from several small studies [104,105,106]. Of interest is nebivolol, a novel third-generation agent with NO-potentiating properties. A post hoc analysis from the SENIORS study revealed encouraging results; however, larger prospective studies are required [107,108]. In a recent meta-analysis, Badve et al. [109] showed that beta-blockers significantly improved all-cause mortality. While this result is encouraging within the spectrum of the CRS, consideration for renal physiology and more severe RI in a well-designed prospective study is needed [44,109]. The role of beta blockade in the CRS is evolving as we further understand the pathophysiology and deleterious impact of SNSA. The availability of direct sinus node inhibitors to control the chronotropic effects of SNSA without significant effects on haemodynamics, mood, fatigue, sexual function and RF (>15 ml/min/1.73 m2) offers considerable promise for future studies [45].
Revascularization
The optimal mode of revascularization in ischaemic cardiomyopathy is still evolving. Early revascularization with functional evidence of ischaemia and viability from observational data may be associated with improved survival. There are, however, no randomised trials to support this, and significant confounders still plague the findings [110,111]. Interestingly, among diabetics with a median eGFR 70 ml/min/1.73 m2, medical therapy and percutaneous revascularization were equally beneficial. Rates from major cardiovascular events were, however, significantly lower in the surgical stratum. HF as an adverse complication occurred with equal frequency, 20% in both groups [112]. In addition when trials like CRUSADE are further scrutinized, at discharge the invasive groups are more likely to receive standard therapy, secondary preventive measures and support to modify risk factors [113]. In entirety, an individual's risk of renal deterioration with surgery and the lack of evidence for percutaneous revascularization highlight the need for meticulous planning and optimizing medical care in these patients. The decision on revascularization, mode and urgency needs to be made on a case-by-case basis with a high-risk mind-set [114,115,116,117].
Agents for Symptoms
Sodium and fluid balances with diuretics are the mainstay for symptomatic HF and RF. They offer no prognostic benefit. The advantages of potassium depletion have to be balanced with the risk of further impairing RF and physiological adaptations such as diuretic resistance, thiazide (GFR <40 ml/min/1.73 m2) and loop (GFR <30 ml/min/1.73 m2). Digoxin improves symptoms and reduces CHF hospitalizations. It is 85% renally excreted; as such, higher levels are maintained with GFR <50 ml/min/1.73 m2 and levels >1.2 ng/ml are associated with worse outcomes. These factors have to be considered when digoxin is used simultaneously with other prognostic medications with care and consideration for dosing, drug levels and serum biochemistry monitoring, diet modifications and multidisciplinary involvement are essential [83,92].
Prescribing Practices for CRS Patients
As highlighted, undertreating patients does not lead to improved outcomes where the baseline risk is dismal to start with. Poor judgment in the timing of introducing an agent, starting dose, interval in increasing the dose and combination with other agents can also contribute to poorer outcomes. Are there simple strategies we can observe? The American Society of Nephrology and other national bodies have published recommendations for pharmacological prescriptions in RI. These guidelines should form the basis for most practices. We are keen to highlight several additional pointers that may be considered in patients with varying forms of the CRS:
Prognostic Therapeutics. There is never a good time to withhold or start a therapeutic agent with prognostic benefit. Most of the studies (studies on inotropes excluded) enrolled class 1 or 2, and stable class 3 or 4 patients. The study population was not confounded by comorbidities and electrolyte instability as is common in RI. The eventual benefit of these agents comes from the long-term use and dosing regimen that alter systemic physiology. Several agents such as RAAS and aldosterone blockade may have early symptom benefit. We recommend that if an agent is considered for the former purpose, it be withheld until a degree of clinical stability is guaranteed.
Physiological Considerations. If a chronic patient is haemodynamically brittle, admission to optimize therapeutics is an option. We recommend starting with a short-acting RAAS blockade, e.g. captopril, or lowest-dose aldosterone blockade, e.g. spironolactone 12.5 mg daily or alternate day. Absolute contraindications are >30% deterioration of RF, change in functional status or urine output, blood pressure <80/50, heart rate <50 bpm or serum potassium >6.0 mmol/l. We have stated these parameters at the lower or upper end of convention as these are often the clinical scenarios faced by physicians. A contraindication in these situations should not stay a contraindication indefinitely. The intervals at which doses are increased should be individualized.
Clinical Goal Setting. This is an important consideration. It should be considered early in the patient's consultation. The complexity of the problem should be highlighted to patients, and they should be active partners in the decision-making and follow-up. In all cases, prognostic medications at the maximum tolerated doses should be the goal. When there is deviation from this, the reasons should be highlighted to the patient and physicians sharing the care with the client. If the goal is proscription or suboptimal therapy, reasonable intervals should be stipulated to reassess the goals. Far too often, CRS patients are provided a goal that may be suboptimal or could contribute to future complications, which remain indefinitely.
Choice of Agent. The underlying aetiology of the organ primarily impaired, comorbidities, e.g. diabetes, baseline haemodynamics, e.g. heart rate, blood pressure, and serum potassium can determine the first agent to be introduced. Whether it is better to introduce one agent at the highest tolerated dose or several at low doses is unclear. Targeting clinical goals such as heart rates of 60–70 bpm in CHF or measures to reduce intraglomerular pressures and proteinuria may help clinicians moving forward.
Device Therapy
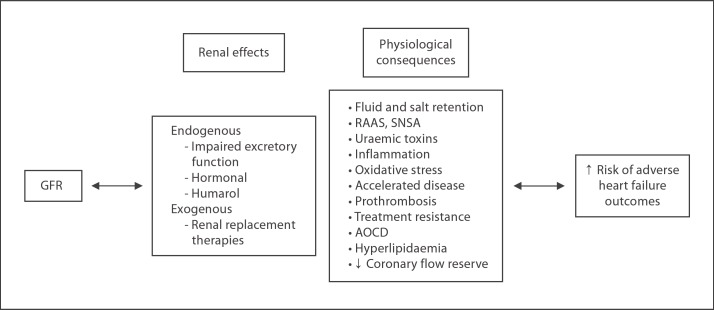
A subgroup analysis from the MADIT-2 study showed that the risk of sudden cardiac death increases with declining RF; however, the benefits of ICD therapy appears to be attenuated in patients with advanced renal disease [118,119]. While this conclusion may hold true, the group with more advanced renal disease was statistically significantly older (>65%), more likely to be NYHA class >2, hypertensive, diabetic, had previous coronary artery vein bypass graft, QRS duration >0.12 s, lower ejection fraction (<25%), higher heart rates of <80 bpm and were less likely to be on ACEI, beta-blockers, lipid-lowering therapies and more likely to be on diuretics. Each of these characteristics alone point toward a higher-risk group with lower rates of pharmacological treatments [118], thus the need for prevention of sudden cardiac death requires equal baseline therapeutics in patients with eGFR in all ranges. It would appear that device therapy is not an alternative to conventional pharmacotherapy but complements optimal medical care. The inability to provide baseline pharmacotherapy should raise ‘alarm bells’ among physicians. Presently, an approach of considering these patients in the high category while providing closer observation and creative prescribing methods through a multidisciplinary approach may seem reasonable, at least until novel diagnostic and therapeutic therapies are clinically available. Figure 1 summarises the factors contributing to poor HF outcomes with RI.
Fig. 1.
Contributors of adverse outcomes in RI. As GFR progressively declines, there is a diminishing capacity to maintain excretory and endocrine function. Through the imbalance of the immune-neuro-hormonal axis, retained uraemic toxins and later renal replacement therapies, HF risk develops or is propagated at an accelerated rate.
Conclusion
Although we have known of the existence of the CRS, only recently have we understood the extent of the clinical significance backed by prospective evidence. This raises the issue of approach for these patients. Firstly, it is clear that we have underestimated the extent of the disease burden. Secondly, the tools we use are subject to multiple variables, and thus accuracy becomes a factor. Thirdly, clinical decisions are initially made on SCr and currently on eGFR; the consequences of the decisions have significant prognostic implications for the patients. While there are no actual guidelines or models we can use to predict with certainty patients likely to develop WRF, the evidence supports significantly worse outcomes when treatments are denied. Clinical tools are required that can detect the onset of renal injury and failure earlier in the clinical course to support timely clinical decisions. The two issues raised above call for a unique approach to the CRS. This would entail improving the temporal profile of diagnosing AKI and increased accuracy in estimating baseline RI. We are currently awaiting the outcomes of several prospective diagnostic and therapeutic studies. These should tell us where to direct future research in the area. We would also encourage future studies to be more inclusive of patients with more advanced RI. Finally, it is important that treating physicians be aware of the increasing burden of such patients, the associated risks and the importance of pharmacotherapy in improving outcomes.
Disclosure Statement
All authors have individually received funding from the government and industry for studies in the CRS, but none pose a conflict of interest for this short review.
References
- 1.Lindner A, Charra B, Sherrard DJ, Scribner BH. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Eng J Med. 1974;290:697–701. doi: 10.1056/NEJM197403282901301. [DOI] [PubMed] [Google Scholar]
- 2.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 3.Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 4.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease – effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfray P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 6.Solomon R, Segal A. Defining acute kidney injury: what is the most appropriate metric? Nat Clin Pract Nephrol. 2008;4:208–215. doi: 10.1038/ncpneph0746. [DOI] [PubMed] [Google Scholar]
- 7.Iyngkaran P, Schneider H, Devarajan P, Anavekar N, Krum H, Ronco C. Cardio-renal syndrome: new perspective in diagnostics. Semin Nephrol. 2012;32:3–17. doi: 10.1016/j.semnephrol.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed A, Campbell RC. Epidemiology of chronic kidney disease in heart failure. Heart Fail Clin. 2008;4:387–399. doi: 10.1016/j.hfc.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heywood JT. The cardiorenal syndrome: lessons from the ADHERE database and treatment options. Heart Fail Rev. 2004;9:195–201. doi: 10.1007/s10741-005-6129-4. [DOI] [PubMed] [Google Scholar]
- 10.Adams KF, Fonarrow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz Galvao M, Horton DP, ADHERE Scientific Advisory Committee and Investigators Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Fonarow GC, Abraham WT, Albert NM, Gattis Stough W, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy CW, Young JB, OPTIMIZE-HF Investigators and Hospitals Influence of a performance-improvement initiative on quality of care for hospitalized patients with heart failure: results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) Arch Intern Med. 2007;167:1493–1502. doi: 10.1001/archinte.167.14.1493. [DOI] [PubMed] [Google Scholar]
- 12.Cleland JG, Swedberg K, Follath F, Komajda Cohen-Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, van Gilst WH, Widimsky J, Freemantle N, Eastaugh J, Mason J, Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology The EuroHeart Failure survey programme – a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24:442–463. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 13.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: systematic review and meta-analyisis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Coresh J, Balk E, Kausz AT, Levin S, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G, National Kidney Foundation National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 15.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Zocalli C. Cardiorenal risk as a new frontier of nephrology: research needs and areas for intervention. Nephrol Dial Transplant. 2002;17:50–54. doi: 10.1093/ndt/17.suppl_11.50. [DOI] [PubMed] [Google Scholar]
- 17.Parfrey PS, Foley R. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. 1999;10:1606–1615. doi: 10.1681/ASN.V1071606. [DOI] [PubMed] [Google Scholar]
- 18.Ruilope LM, van Veldhuisen DJ, Ritz E, Luscher TF. Renal function: the Cinderella of cardiovascular risk profile. J Am Coll Cardiol. 2001;38:1782–1787. doi: 10.1016/s0735-1097(01)01627-8. [DOI] [PubMed] [Google Scholar]
- 19.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 20.Kearney MT, Fox KA, Lee AJ, et al. Predicting death due to progressive heart failure in patients with mild to moderate heart failure. J Am Coll Cardiol. 2002;40:1801–1808. doi: 10.1016/s0735-1097(02)02490-7. [DOI] [PubMed] [Google Scholar]
- 21.Hilleage HL, Girbes ARJ, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- 22.Miyagishima K, Hiramitsu S, Kimura H, Mori K, Ueda T, Kato S, Kato Y, Ishikawa S, Iwase M, Morimoto Shin-ichiro, Hishida H, Ozaki Y. Long term prognosis of chronic heart failure: reduced versus preserved left ventricular ejection fraction. Circ J. 2009;73:92–99. doi: 10.1253/circj.cj-07-1016. [DOI] [PubMed] [Google Scholar]
- 23.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 24.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 25.McAlister FA, Ezekowitz J, Tonelli MR, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 26.Mahon NG, Blackstone EH, Francis GS, Starling RC, Young JB, Lauer MS. The prognostic value of estimated creatinine clearance alongside functional capacity in ambulatory patients with mild to moderate chronic heart failure. J Am Coll Cardiol. 2002;40:1106–1113. doi: 10.1016/s0735-1097(02)02125-3. [DOI] [PubMed] [Google Scholar]
- 27.Gottlieb SS, Abraham W, Butler J, et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002;8:136–141. doi: 10.1054/jcaf.2002.125289. [DOI] [PubMed] [Google Scholar]
- 28.Hilleage HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergen J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ, Candersartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators: Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 29.Schrier RW. Role of diminished renal function in cardiovascular mortality: marker or pathogenetic factor? J Am Coll Cardiol. 2006;47:1–8. doi: 10.1016/j.jacc.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 30.Smith GL, Vaccarino V, Kosiborod M, et al. Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure? J Card Fail. 2003;9:13–25. doi: 10.1054/jcaf.2003.3. [DOI] [PubMed] [Google Scholar]
- 31.Klein L, Massie BM, Leimberger JD, O'Connor CM, Pina Il, Adams KF, Califf RM, Gheorgide M, OPTIME-CHF Investigators Admission or changes in renal function during hospitalizations for worsening heart failure predict postdischarge survival: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Circ Heart Fail. 2008;1:25–33. doi: 10.1161/CIRCHEARTFAILURE.107.746933. [DOI] [PubMed] [Google Scholar]
- 32.Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 33.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Henry RMA, Kostense PJ, Bos G, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Mild renal insufficiency is associated with increased cardiovascular mortality: The Hoorn Study. Kidney Int. 2002;62:1402–1407. doi: 10.1111/j.1523-1755.2002.kid571.x. [DOI] [PubMed] [Google Scholar]
- 35.Ritz E, McClellan WM. Overview: increased risk in patients with minor renal dysfunction: an emerging issue with far reaching consequences. J Am Soc Nephrol. 2004;15:513–516. doi: 10.1097/01.asn.0000115398.92270.30. [DOI] [PubMed] [Google Scholar]
- 36.Ritz E. Minor renal dysfunction: an emerging independent cardiovascular risk factor. Heart. 2003;89:963–964. doi: 10.1136/heart.89.9.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ismailov RM, Goldberg RJ, Lessard D, Spencer FA. Decompensated heart failure in the setting of kidney dysfunction: a community-wide perspective. Nephron Clin Pract. 2007;107:c147–c155. doi: 10.1159/000110035. [DOI] [PubMed] [Google Scholar]
- 38.Wilson AC, Mitsnefes MM. Cardiovascular disease in CKD in children: update on risk factors, risk assessment and management. Am J Kidney Dis. 2009;54:3345–3360. doi: 10.1053/j.ajkd.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krumholz HM, Chen YT, Vaccarino V, Wang Y, Radford MJ, Bradford WD, Horwitz RI. Correlates and impact of worsening renal function in patients ≥65 years of age with heart failure. Am J Cardiol. 2000;85:1110–1113. doi: 10.1016/s0002-9149(00)00705-0. [DOI] [PubMed] [Google Scholar]
- 40.Fonarrow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 41.McClellan WM, Langston RD, Presley R. Medicare patients with cardiovascular disease have a high prevalence of chronic kidney disease and a high rate of progression to end-stage renal disease. J Am Soc Nephrol. 2004;15:1912–1919. doi: 10.1097/01.asn.0000129982.10611.4c. [DOI] [PubMed] [Google Scholar]
- 42.Shlipak MG, Fried LF, Stehman-Breen C, Siscovick D, Newman AB. Chronic renal insufficiency and cardiovascular events in the elderly: findings from the Cardiovascular Health Study. Am J Geriatr Cardiol. 2004;13:81–90. doi: 10.1111/j.1076-7460.2004.02125.x. [DOI] [PubMed] [Google Scholar]
- 43.Ezekowitz J, McAlister FA, Humphries KH, Norris CM, Tonelli M, Ghali WA, Knudston ML, APPRAOCH Investigators The association among renal insufficiency, pharmacotherapy, and outcomes in 6,247 patients with heart failure and coronary artery disease. J Am Coll Cardiol. 2004;44:1587–1592. doi: 10.1016/j.jacc.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 44.Chang TI, Chertow GM. Chronic kidney disease and cardiovascular therapeutics: time to close the gaps. J Am Coll Cardiol. 2011;58:1162–1163. doi: 10.1016/j.jacc.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tardiff JC. Slowing heart rate with ivabradine: new treatment options. Eur Heart J. 2011;13(suppl C):C19–C24. [Google Scholar]
- 46.Al-Ahmad A, Rand WM, Manjunath G, Konstam MA, Salem DN, Levey AS, Sarnak MJ. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;38:955–962. doi: 10.1016/s0735-1097(01)01470-x. [DOI] [PubMed] [Google Scholar]
- 47.McClellan WM, Flanders WD, Langston RD, Jurkovitz C, Presley R. Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: a population based study. J Am Soc Nephrol. 2002;13:1928–1936. doi: 10.1097/01.asn.0000018409.45834.fa. [DOI] [PubMed] [Google Scholar]
- 48.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113:2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 49.Gaddam KK, Ventura HO, Lavie CJ. Metabolic syndrome and heart failure – the risk, paradox, and treatment. Curr Hypertens Rep. 2011;13:142–148. doi: 10.1007/s11906-011-0179-x. [DOI] [PubMed] [Google Scholar]
- 50.van Melle JP, Bot M, de Jonge P, de Boer RA, van Veldhuisen DJ, Whooley MA. Diabetes, glycemic control, and new-onset heart failure in patients with stable coronary artery disease: data from the heart and soul study. Diabetes Care. 2010;33:2084–2089. doi: 10.2337/dc10-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Opie LH, Yellon DM, Gersh BJ. Controversies in the cardiovascular management of type 2 diabetes. Heart. 2011;97:6–14. doi: 10.1136/hrt.2010.214031. [DOI] [PubMed] [Google Scholar]
- 52.Aguilar D, Bozkurt B, Ramasubbu K, Deswal A. Relationship of hemoglobin A1C and mortality in heart failure patients with diabetes. J Am Coll Cardiol. 2009;54:422–428. doi: 10.1016/j.jacc.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eshaghian S, Horwich TB, Fonarrow GC. An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure. Am Heart J. 2006;151(91):e1–91.e6. doi: 10.1016/j.ahj.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 54.Ascione R, Rogers CA, Rajakurana C, Angelini GD. Inadequate blood glucose is associated with in-hospital mortality and morbidity in diabetic and non-diabetic patients undergoing cardiac surgery. Circulation. 2008;118:113–123. doi: 10.1161/CIRCULATIONAHA.107.706416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kosiborod M, Inzucchi SE, Spertus JA, Wang Y, Masoudi FA, Havranek EP, et al. Elevated admission glucose and mortality in elderly patients hospitalized with heart failure. Circulation. 2009;119:1899–1907. doi: 10.1161/CIRCULATIONAHA.108.821843. [DOI] [PubMed] [Google Scholar]
- 56.Zhang CY, Sun AJ, Zhang SN, Wu CN, Fu MQ, Xia G, et al. Effects of intensive glucose control on incidence of cardiovascular events in patients with type 2 diabetes: A meta-analysis. Ann Med. 2010;42:305–315. doi: 10.3109/07853891003796752. [DOI] [PubMed] [Google Scholar]
- 57.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassai B, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ. 2011;343:d4169. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodarzi MO, Psaty BM. Glucose lowering to control macrovascular disease in type 2 diabetes: treating the wrong surrogate end point? JAMA. 2008;300:2051–2053. doi: 10.1001/jama.2008.510. [DOI] [PubMed] [Google Scholar]
- 59.Tonelli M, Moye L, Sacks FM, Kiberd B, Curhan G, CARE Trial Investigators Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med. 2003;138:98–104. doi: 10.7326/0003-4819-138-2-200301210-00010. [DOI] [PubMed] [Google Scholar]
- 60.McCullough PA, Rocher LR. Statin therapy in renal disease: harmful or protective? Curr Atheroscler Rep. 2007;9:18–24. doi: 10.1007/BF02693936. [DOI] [PubMed] [Google Scholar]
- 61.Baber U, Toto RD, de Lemos JA. Statin and cardiovascular risk reductions in patients with chronic kidney disease and end-stage renal failure. Am Heart J. 2007;153:471–477. doi: 10.1016/j.ahj.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 62.Jardine MJ, Ninomiya T, Perkovic V, Cass A, Turnbull F, Gallagher MP, et al. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol. 2010;56:956–965. doi: 10.1016/j.jacc.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 63.Evans M, Fored CM, Bellocco R, Fitzmaurice G, Fryzek JP, McLaughlin JK, Nyren O, Elinder CG. Acetominophen, aspirin and progression of advanced chronic kidney disease. Nephrol Dial Transplant. 2009;24:1908–1918. doi: 10.1093/ndt/gfn745. [DOI] [PubMed] [Google Scholar]
- 64.Wali RK. Aspirin and the prevention of cardiovascular disease in chronic kidney disease: time to move forward? J Am Coll Cardiol. 2010;56:966–968. doi: 10.1016/j.jacc.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 65.Chade AR, Brosh D, Higano ST, Lennon RJ, Lerman LO, Lerman A. Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease. Kidney Int. 2006;69:266–271. doi: 10.1038/sj.ki.5000031. [DOI] [PubMed] [Google Scholar]
- 66.Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE. Serial change in echocardiographic parameters and cardiac failure in end-stage renal disease. J Am Soc Nephrol. 2000;11:912–916. doi: 10.1681/ASN.V115912. [DOI] [PubMed] [Google Scholar]
- 67.Chittineni H, Miyawaki N, Gulipelli S, Fishbane S. Risk for acute renal failure in patients hospitalized for decompensated congestive heart failure. Am J Nephrol. 2007;27:55–62. doi: 10.1159/000099012. [DOI] [PubMed] [Google Scholar]
- 68.Knight EL, Glynn RJ, McIntyre KM, Mogun H, Avorn J, Mass B. Predictors of decreased renal function in patients with heart failure during angiotensin-converting enzyme inhibitor therapy: results from the studies of left ventricular dysfunction (SOLVD) Am Heart J. 1999;138:849–855. doi: 10.1016/s0002-8703(99)70009-8. [DOI] [PubMed] [Google Scholar]
- 69.Kittleson M, Hurwitz S, Shah MR, Nohria A, Lewis E, Givertz M, Fang J, Jarcho J, Mudge G, Stevenson LW. Development of circulatory-renal limitations to angiotensin converting enzyme inhibitors identifies patients with severe heart failure and early mortality. J Am Coll Cardiol. 2003;41:2029–2035. doi: 10.1016/s0735-1097(03)00417-0. [DOI] [PubMed] [Google Scholar]
- 70.Gotsman I, Rubonivich S, Azaz-Livshits T. Use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with congestive heart failure: an observational study of treatment rates and clinical outcomes. Isr Med Assoc J. 2008;10:214–218. [PubMed] [Google Scholar]
- 71.Glowinska I, Grochowski J, Malyszko J. Cardiovascular complication in patients with diabetic nephropathy receiving pharmacological versus renal replacement therapy. Pol Arch Medy Wewn. 2008;118:404–411. [PubMed] [Google Scholar]
- 72.Bart BA. Concern for azotemia with angiotensin-converting enzyme inhibitors: public health implications and clinical relevance. Am Heart J. 1999;138:801–803. doi: 10.1016/s0002-8703(99)70001-3. [DOI] [PubMed] [Google Scholar]
- 73.de Silva R, Nikitin NP, Bhandari S, Nicholson A, Clark AL, Cleland JG. Atherosclerotic renovascular disease in chronic heart failure: should we intervene? Eur Heart J. 2005;26:1596–1605. doi: 10.1093/eurheartj/ehi304. [DOI] [PubMed] [Google Scholar]
- 74.Barkis GL, Weir MR. Angiotensin-converting enzyme inhibitor associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 75.Pinkau T, Hilgers KF, Veelken R, Mann JF. How does minor renal dysfunction influence cardiovascular risk and the management of cardiovascular disease? J Am Soc Nephrol. 2004;15:517–523. doi: 10.1097/01.asn.0000107565.17553.71. [DOI] [PubMed] [Google Scholar]
- 76.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 77.Mann JF, Gerstein HC, Dulau-Florea I, Lonn E. Cardiovascular risk in patients with mild renal insufficiency. Kidney Int Suppl. 2003;84:s192–s196. doi: 10.1046/j.1523-1755.63.s84.27.x. [DOI] [PubMed] [Google Scholar]
- 78.Mann JF, Yi QL, Sleight P, Dagenais GR, Probstfield J, Gerstein HC, Lonn EM, Bosch J, Yusuf S, HOPE investigators New trial data on prevention: potassium and CV risk in HOPE. Pacing Clin Electrophysiol. 2003;26:1565–1568. [Google Scholar]
- 79.Saltzman HE, Sharma K, Mather PJ, Rubin S, Adams S, Whellan DJ. Renal dysfunction in heart failure patients: what is the evidence? Heart Fail Rev. 2007;12:37–47. doi: 10.1007/s10741-007-9006-5. [DOI] [PubMed] [Google Scholar]
- 80.Lakhdar R, Al-Mallah MH, Lanfear DE. Safety and tolerability of angiotensin-converting enzyme inhibitor versus the combination of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker in patients with left ventricular dysfunction: a systematic review and metaanalysis of randomized controlled trials. J Card Fail. 2008;14:181–188. doi: 10.1016/j.cardfail.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 81.Ljungman S, Kjekshus J, Swedberg K. Renal function in severe congestive heart failure during treatment with enalapril (the Cooperative North Scandinavian Enalapril Survival Study [CONSENSUS] Trial) Am J Cardiol. 1992;70:479–487. doi: 10.1016/0002-9149(92)91194-9. [DOI] [PubMed] [Google Scholar]
- 82.Schoolwerth AC, Sica DA, Ballermann BJ, Wilcox CS. Renal consideration in angiotensin converting enzyme inhibitor therapy: a statement for healthcare professionals from the council on the Kidney in Cardiovascualr Disease and the council for High Blood Pressure Research of the American Heart Association. Circulation. 2001;104:1985–1991. doi: 10.1161/hc4101.096153. [DOI] [PubMed] [Google Scholar]
- 83.Abdo AS, Basu A, Geraci SA. Managing chronic heart failure patient in chronic kidney disease. Am J Med. 2011;124:26–28. doi: 10.1016/j.amjmed.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 84.Ruilope LM. Safety aspects of aldosterone-blocking drugs. Eur Heart J. 2011;13(suppl B):840–842. [Google Scholar]
- 85.Maron BA, Leopold JA. Aldosterone receptor antagonists: effective but often forgotten. Circulation. 2010;121:934–939. doi: 10.1161/CIRCULATIONAHA.109.895235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Edwards NC, Ferro CJ, Kirkwood H, Chue CD, Young AA, Stewart PM, Steeds RP, Townend JN. Effects of spironolactone on left ventricular systolic and diastolic function in patients with early chronic kidney disease. Am J Cardiol. 2010;106:1505–1511. doi: 10.1016/j.amjcard.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 87.Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli FM. Aldosterone antagonist for preventing the progression of chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:542–551. doi: 10.2215/CJN.04750908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malek F, Havrda M, Fruhaufova Z, Vranova J. Short-term effect of evidence-based medicine heart failure therapy on glomerular filtration rate in elderly patients with chronic cardiorenal syndrome. J Am Geriatr Soc. 2009;57:2385–2386. doi: 10.1111/j.1532-5415.2009.02599.x. [DOI] [PubMed] [Google Scholar]
- 89.Ludovit P, Unger T. Novel therapeutic targets for hypertension. Nat Rev Cardiol. 2010;7:431–441. doi: 10.1038/nrcardio.2010.85. [DOI] [PubMed] [Google Scholar]
- 90.McMurray JJ, Pitt B, Latini R, Maggioni AP, Solomon SD, Keefe DL, et al. Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure. Circ Heart Fail. 2008;1:17–24. doi: 10.1161/CIRCHEARTFAILURE.107.740704. [DOI] [PubMed] [Google Scholar]
- 91.Pitt B, Latini R, Maggioni AP, Solomon SD, Smith BA, Wright M, et al. Neurohumoral effects of aliskiren in patients with symptomatic heart failure receiving a mineralocorticoid receptor antagonist: the Aliskiren Observation of Heart Failure Treatment study. Eur J Heart Fail. 2011;13:755–764. doi: 10.1093/eurjhf/hfr034. [DOI] [PubMed] [Google Scholar]
- 92.Krum H, Iyngkaran P, Lekawanvijit S. Pharmacologic management of the cardiorenal syndrome in heart failure. Curr Heart Fail Rep. 2009;6:105–111. doi: 10.1007/s11897-009-0016-6. [DOI] [PubMed] [Google Scholar]
- 93.Boerrigter G, Burnett JC. Cardiorenal syndrome in decompensated heart failure: prognostic and therapeutic implications. Curr Heart Fail Rep. 2004;1:113–120. doi: 10.1007/s11897-004-0020-9. [DOI] [PubMed] [Google Scholar]
- 94.Petrie CJ, Mark PB, Weir RA. Broken pump or leaky filter? Renal dysfunction in heart failure a contemporary review. Int J Cardiol. 2008;128:154–165. doi: 10.1016/j.ijcard.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 95.Rastogi A, Fonarow GC. The cardiorenal connection in heart failure. Curr Cardiol Rep. 2008;10:190–197. doi: 10.1007/s11886-008-0033-1. [DOI] [PubMed] [Google Scholar]
- 96.Liu PP. Cardiorenal syndrome in heart failure: a cardiologist's perspective. Can J Cardiol. 2008;24(Suppl B):25B–29B. doi: 10.1016/s0828-282x(08)71027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shlipak MG. Pharmacotherapy for heart failure on patients with renal insufficiency. Ann Intern Med. 2003;138:917–924. doi: 10.7326/0003-4819-138-11-200306030-00013. [DOI] [PubMed] [Google Scholar]
- 98.Shlipak MG, Browner WS, Noguchi H, et al. Comparison of the effects of angiotensin converting-enzyme inhibitors and beta-blockers on survival in elderly patients with reduced left ventricular function after myocardial infarction. Am J Med. 2001;110:425–433. doi: 10.1016/s0002-9343(01)00652-0. [DOI] [PubMed] [Google Scholar]
- 99.Khan W, Deepak SM, Coppinger T, et al. Beta blocker treatment is associated with improvement in renal function and anaemia in patients with heart failure. Heart. 2006;92:1856–1857. doi: 10.1136/hrt.2005.083998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cice G, Ferrara L, D'Andrea A, et al. Carvedilol increases two-year survival in dialysis patients with dilated cardiomyopathy. J Am Coll Cardiol. 2003;41:1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 101.Sweileh WM, Sawalha AF, Jayousi HM, Zyoud SH, Al-Jabi SW. Predictors of ‘worsening renal function’ in patients hospitalized in internal medicine department. Curr Drug Saf. 2009;4:113–118. doi: 10.2174/157488609788173071. [DOI] [PubMed] [Google Scholar]
- 102.Triposkiadis T, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 103.Zech P, Pozet N, Labeeuw M, et al. Acute renal effects of beta-blockers. Am J Nephrol. 1986;6(suppl 2):15–19. doi: 10.1159/000167327. [DOI] [PubMed] [Google Scholar]
- 104.Cice G, Ferrara L, D'Andrea A, et al. Dilated cardiomyopathy in dialysis patients – beneficial effects of carvedilol: a double-blind, placebo-controlled trial. J Am Coll Cardiol. 2001;37:407–411. doi: 10.1016/s0735-1097(00)01158-x. [DOI] [PubMed] [Google Scholar]
- 105.Abraham WT, Tsvetkova T, Lowes BD, et al. Carvedilol improves renal hemodynamics in patients with chronic heart failure. Circulation. 1998;98:I-378–I-379. [Google Scholar]
- 106.Sanderson JE, Chan SKW, Yip G, Yeung LYC, Chan KW, Raymond K, Woo KS. Beta-blockade in heart failure: a comparison of carvedilol with metoprolol. J Am Coll Cardiol. 1999;34:1522–1528. doi: 10.1016/s0735-1097(99)00367-8. [DOI] [PubMed] [Google Scholar]
- 107.Cohen-Solal A, Kotecha D, van Veldhuisen DJ, Babalis D, Böhm M, Coats AJ, Roughton M, Poole-Wilson P, Tavazzi L, Flather M, SENIORS Investigators Efficacy and safety of nebivolol in elderly heart failure patients with impaired renal function: insights from the SENIORS trial. Eur J Heart Fail. 2009;11:872–880. doi: 10.1093/eurjhf/hfp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Munzel T, Gori T. Nebivilol: the somewhat-different beta-adrenergic receptor blocker. J Am Coll Cardiol. 2009;54:1491–1499. doi: 10.1016/j.jacc.2009.05.066. [DOI] [PubMed] [Google Scholar]
- 109.Badve SV, Roberts MA, Hawley CM, Cass A, Garg AX, Krum H, Tonkin A, Perkovic V. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:1152–1161. doi: 10.1016/j.jacc.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 110.Phillips HR, O'Connor CM, Rogers J. Revascularization for heart failure. Am Heart J. 2007;153:65–73. doi: 10.1016/j.ahj.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 111.Tarakji KG, Brunken R, McCarthy PM, Al-Chekakie MO, Abdel-Latif A, Pothier CE, et al. Myocardial viability and the effects of early intervention in patients with advanced left ventricular systolic dysfunction. Circulation. 2006;113:230–237. doi: 10.1161/CIRCULATIONAHA.105.541664. [DOI] [PubMed] [Google Scholar]
- 112.The BARI 2D Study Group A randomized trial of therapies for type 2 diabetics and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chan MY, Becker RC, Harrington RA, Peterson ED, Armstrong PW, White H, et al. Non-invasive, medical management for non-ST-elevation acute coronary syndromes. Am Heart J. 2008;155:397–407. doi: 10.1016/j.ahj.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 114.van Straten AH, Soliman Hamad MA, van Zundert AA, Martens EJ, Schonberger JP, de Wolf AM. Risk factors for deterioration of renal function after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;37:106–111. doi: 10.1016/j.ejcts.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 115.Hillis GS, Croal BL, Buchan KG, El-shafei H, Gibson G, Jeffrey RR, et al. Renal function and outcome from coronary artery bypass grafting. Circulation. 2006;113:1056–1062. doi: 10.1161/CIRCULATIONAHA.105.591990. [DOI] [PubMed] [Google Scholar]
- 116.Nishida H, Uchikawa S, Chikazawa G, Kurihara H, Kihara S, Uwabe K, et al. Coronary artery bypass grafting in 105 patients with hemodialysis-dependent renal failure. Artif Organs. 2001;25:268–272. [PubMed] [Google Scholar]
- 117.Hillis GS, Cuthbertson BH, Croal BL. Renal function, revascularization and risk. Eur Heart J. 2007;28:782–784. doi: 10.1093/eurheartj/ehm014. [DOI] [PubMed] [Google Scholar]
- 118.Goldenberg I, Moss AJ, McNitt S, Zareba W, Andrews ML, Hall WJ, Greenberg H, Case RB, Multicenter Automatic Defibrillator Implantation Trial-II Investigators Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2006;98:485–490. doi: 10.1016/j.amjcard.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 119.Choncol M, Goldenberg I, Moss AJ, McNitt S, Cheung AK. Risk factors for sudden cardiac death in patients with chronic renal insufficiency and left ventricular dysfunction. Am J Nephrol. 2007;27:7–14. doi: 10.1159/000098431. [DOI] [PubMed] [Google Scholar]
- 120.Velavan P, Khan NK, Goode K, Rigby AS, Loh PH, Komajda M, Follath F, Swedberg K, Madeira H, Cleland JG. Predictors of short term mortality in heart failure – insights from the Euro Heart Failure survey. Int J Cardiol. 2010;138:63–69. doi: 10.1016/j.ijcard.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 121.Patel UD, Hernandez AF, Liang L, Peterson ED, LaBresh KA, Yancy CW, Albert NM, Ellrodt G, Fonarow GC. Quality of care and outcomes among patients with heart failure and chronic kidney disease: A Get With the Guidelines – Heart Failure Program study. Am Heart J. 2008;156:674–681. doi: 10.1016/j.ahj.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Coca SG, Peixoto AJ, Garg AX, Krumholz HM, Parikh CR. The prognostic importance of a small acute decrement in kidney function in hospitalized patients: a systematic review and meta-analysis. Am J Kidney Dis. 2007;50:712–720. doi: 10.1053/j.ajkd.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 123.Smith GL, Shlipak MG, Havranek EP, et al. Race and renal impairment in heart failure: mortality in blacks versus whites. Circulation. 2005;111:1270–1277. doi: 10.1161/01.CIR.0000158131.78881.D5. [DOI] [PubMed] [Google Scholar]
- 124.Lewis EF, Velazquez EJ, Solomon SD, Hellkamp AS, McMurray JJ, Mathias J, Rouleau JL, Maggioni AP, Swedberg K, Kober L, White H, Dalby AJ, Francis GS, Zannad F, Califf RM, Pfeffer MA. Predictors of the first heart failure hospitalization in patients who are stable survivors of myocardial infarction complicated by pulmonary congestion and/or left ventricular dysfunction: a VALIANT study. Eur Heart J. 2008;29:748–756. doi: 10.1093/eurheartj/ehn062. [DOI] [PubMed] [Google Scholar]
- 125.Shlipak MG, Heidenreich PA, Naguchi H, Chertow GM, Browner WS, McClellan MB. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Int Med. 2002;137:555–562. doi: 10.7326/0003-4819-137-7-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 126.Stevens LA, Li S, Wang C, Huang C, Becker BN, Bomback AS, Brown WW, Burrows NR, Jurkovitz CT, McFarlane SI, Norris KC, Shlipak M, Whaley-Connell AT, Chen SC, Bakris GL, McCullough PA. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2010;55(3 suppl 2):S23–S33. doi: 10.1053/j.ajkd.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bertoni AG, Hundley WG, Massing MW, Bonds MW, Burke GL, Goff DC. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 128.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27:1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 129.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetemibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]