Abstract
Acute confusional migraine (ACM) is recognized as a rare, but highly disabling migraine equivalent, mostly reported in children and adolescents. Herein we describe the case of a 12-year-old girl admitted to hospital for an acute confusional state and severe psychomotor agitation, associated with a pulsating headache and nausea, which turned out to be a manifestation of ACM. The girl was discharged on topiramate prophylaxis, titrated up to 75 mg/die; no recurrence of confusional and/or headache episodes has been reported over the last 14 months to date. Due to the rarity of this clinical entity, only anecdotal reports about acute and prophylactic treatment of ACM are available in the literature. The case reported herein suggests that topiramate seems to be effective in ACM prophylaxis, although a longer observation period in our patient and more cases are needed to confirm any long-term clinical benefit.
Key words: Acute confusional migraine, Migraine, Migraine prophylaxis, Topiramate
Introduction
Acute confusional migraine (ACM) is recognized as a rare, but highly disabling migraine equivalent, mostly reported in children and adolescents. It was first described by Gascon and Barlow (1970) [1] as an acute confusional state during an attack of migraine in 4 pediatric patients. While neuroradiological examinations and routine laboratory tests are usually unremarkable, ictal EEG is characterized by marked, diffuse slowing and, at times, by a FIRDA pattern [2]. Based mainly on functional neuroimaging studies, cortical spreading depression is currently hypothesized as the pivotal pathophysiological mechanism [3]. ACM is reported in a low percentage (0.45) of migraineurs referred to pediatric neurology outpatient divisions [4]. The confusional state may manifest with a wide range of cortical dysfunctions, such as speech difficulties, increased alertness, agitation and amnesia, and it may be triggered by mild head trauma in half of the cases [5]. Even if ACM is far from common, it is most likely underdiagnosed as a cause of confusional state and agitation in the young population, where a prompt differential diagnosis is mandatory so as to exclude a variety of disorders, such as toxic-metabolic psychosis, encephalitis and, mainly, epileptic seizures, which share the symptoms of mental confusion [5].
Herein we describe the case of a 12-year-old girl admitted to hospital for acute confusional state and severe psychomotor agitation, associated with a pulsating headache and nausea, which turned out to be a manifestation of ACM.
Case Study
A 12-year-old adolescent was admitted to the Emergency Department of our hospital for sudden onset of acute confusional state and severe psychomotor agitation, associated with headache, nausea and vomiting whilst at school. The teacher and the other bystanders saw her as agitated and confused, screaming nonsense. Indeed, she was described as being similar to someone who was ‘possessed by the devil’. The emergency service responsible for her transport to the Emergency Department was obliged to sedate her with intravenous midazolam 15 mg. At admission, she was pale, somnolent, in a confusional state, restless with purposeless movements, not oriented to place or time and cried on and off for no apparent reason, complaining of a pulsating, bilateral frontal headache. All but her mental status was unremarkable, namely her physical and neurological examination, vital signs, laboratory tests and temperature. She was right-handed and her past medical history showed nothing of relevance, such as head trauma, except for 2 previous similar episodes, 4 and 2 weeks before her admission, respectively, characterized by less marked agitation and confusion. Both episodes were accompanied by frontal headache, nausea and vomiting, and lasted about 6–7 h. The girl's mother reported she suffered from migraine without aura at anamnesis.
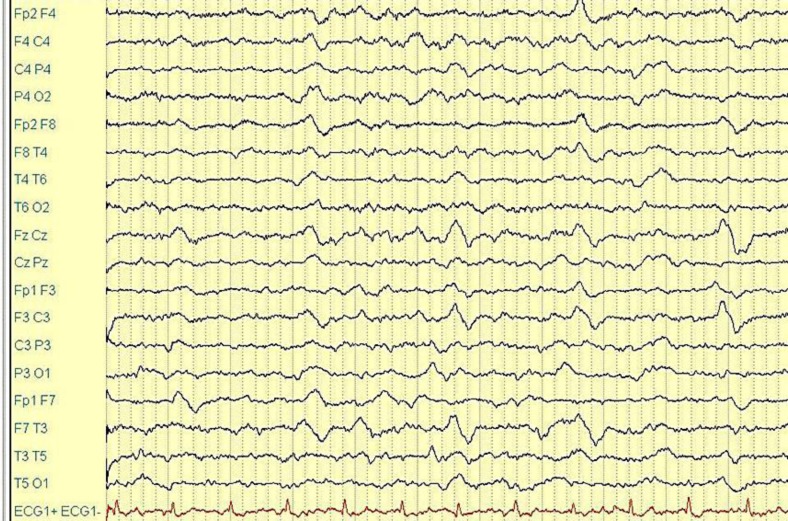
The patient's CT brain scan did not show any focal densitometric parenchymal alterations. Her mental status improved within 4 h: she appeared awake, cooperative and satisfactorily oriented, though she had complete amnesia of her previous state. She continued to complain of a frontal pulsating headache, which worsened if she moved, and of nausea. During the EEG recordings, performed 5 h after symptom onset with scalp electrodes according to the 10–20 international system, there was a widespread slowing, to a certain extent featuring a high-voltage, intermittent delta rhythmic activity in the frontotemporal bilateral regions (FIRDA), in the absence of epileptic discharges (fig. 1).
Fig. 1.
EEG recordings (5 h after symptom onset with scalp electrodes according to the 10–20 international system).
The headache and accompanying symptoms ceased spontaneously during nocturnal sleep. A control EEG, recorded about 20 h after the onset of symptoms, showed a persistent delta activity, although less marked than the previous one. A third EEG, carried out 4 days later, showed a complete recovery of the physiologic background rhythms. A brain MRI showed no parenchymal abnormalities. The girl was discharged on topiramate prophylaxis, gradually titrated up to 75 mg/die; no recurrence of confusional and/or headache episodes has been reported over the last 14 months to date.
Discussion
The clinical and EEG features of our patient were typical of ACM. On the basis of these findings, urgent invasive diagnostic procedures, such as lumbar puncture to exclude encephalitis and, above all, a pseudomigraine with temporary neurological deficits, were deemed unnecessary. The latter diagnosis was unlikely as there was no fever or neurological deficits other than the confusional state and amnesia, although the previous episodes of headache and mental alterations and the bilateral EEG slowing might have also been consistent with this rare form of headache [6], which, indeed, could have definitely been excluded by only a lumbar puncture. However, the ictal EEG abnormalities were typical of ACM and persisted after the disappearance of clinical symptoms, as previously reported [2]. The high-voltage, polymorphic delta activity was prevalent in the frontotemporal regions, in agreement with the clinical features of confusion, amnesia and agitation and the supposed transient dysfunction of temporobasal and frontal cortical structures, due to cortical spreading depression [2, 3, 4, 5, 6, 7].
As expected in cases of ACM, MRI did not detect any encephalopathy, which has been recently described in children showing alteration of consciousness, followed by agitation and aphasia quite similar to ACM, but in the context of familial hemiplegic migraine [6]. Since the clinical manifestations of ACM in our patient were highly disabling and the described episode had been preceded, in the previous 4 weeks, by 2 other similar episodes, even if less dramatic, she clearly required prophylactic treatment. However, due to the rarity of this clinical entity, only anecdotal reports about acute and prophylactic treatment of ACM are available in the literature. The efficacy of sodium valproate has recently been reported in a case of ACM in 10-year-old girl [4].
Topiramate has proven to be an effective and safe treatment for migraine prophylaxis in pediatric subjects aged 12–17 years in a randomized, double-blind, placebo-controlled study [9], although more evidence is required to better establish the efficacy of anticonvulsant agents in the prevention of migraine in children and adolescents [10]. The topiramate pharmacodynamic mechanisms include inhibitory effects on voltage-gated Na+ and Ca2+ channels and on glutamate-activated ion channels as well as variable modulatory effects on γ-aminobutyric acid-activated ion channels at AMPA glutamate receptors [11].
The case reported herein suggests that topiramate seems to be effective in ACM prophylaxis, although a longer observation period in our patient and more cases are needed to confirm any long-term clinical benefit. Indeed, in a recent series of 8 adults and 2 adolescents suffering from migraine attacks associated with transient confusional states, only a minority of patients reported 3 or more such attacks, although the length of the follow-up was variable and limited to a few months in some cases [3].
However, even if the association between topiramate treatment and disappearance of attacks in the case reported remains only putative, it seems likely that topiramate as well as sodium valproate, may be effective in the prophylaxis of ACM due to their ability to affect neurotransmission and therefore the pathophysiological mechanisms related to cortical spreading depression, which are presumed to underlie ACM.
References
- 1.Gascon G, Barlow C. Juvenile migraine, presenting as an acute confusional state. Pediatrics. 1970;45:628–635. [PubMed] [Google Scholar]
- 2.Pietrini V, Terzano MG, D'Andrea G, Parrino L, Cananzi AR, Ferro-Milone F. Acute confusional migraine: clinical and electroencephalographic aspects. Cephalalgia. 1987;7:29–37. doi: 10.1046/j.1468-2982.1987.0701029.x. [DOI] [PubMed] [Google Scholar]
- 3.Gantenbein AR, Riederer F, Mathys J, Biethahn S, Gossrau G, Waldvogel D, Sándor PS. Confusional migraine is an adult as well as a childhood disease. Cephalalgia. 2011;31:206–212. doi: 10.1177/0333102410377361. [DOI] [PubMed] [Google Scholar]
- 4.Fujita M, Fujiwara J, Maki T, Shigeta M, Shibasaki K, Takahashi N, Takahashi M. The efficacy of sodium valproate and a MRA finding in confusional migraine. Brain Dev. 2007;29:178–181. doi: 10.1016/j.braindev.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Schipper S, Riederer F, Sándor PS, Gantenbein AR. Acute confusional migraine: our knowledge to date. Expert Rev Neurother. 2012;12:307–314. doi: 10.1586/ern.12.4. [DOI] [PubMed] [Google Scholar]
- 6.Gómez-Aranda F, Cañadillas F, Martí-Massó JF, Díez-Tejedor E, Serrano PJ, Leira R, Gracia M, Pascual J. Pseudomigraine with temporary neurological symptoms and lymphocytic pleocytosis. A report of 50 cases. Brain. 1997;120:1105–1113. doi: 10.1093/brain/120.7.1105. [DOI] [PubMed] [Google Scholar]
- 7.Sheth RD, Riggs JE, Bodensteiner JB. Acute confusional migraine: variant of transient global amnesia. Pediatr Neurol. 1995;12:129–131. doi: 10.1016/0887-8994(94)00154-t. [DOI] [PubMed] [Google Scholar]
- 8.Hart AR, Trinick R, Connolly DJ, Mordekar SR. Profound encephalopathy with complete recovery in three children with familial hemiplegic migraine. J Paediatr Child Health. 2009;45:154–157. doi: 10.1111/j.1440-1754.2009.01465.x. [DOI] [PubMed] [Google Scholar]
- 9.Lewis D, Winner P, Saper J, Ness S, Polverejan E, Wang S, Kurland CL, Nye J, Yuen E, Eerdekens M, Ford L. Randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of topiramate for migraine prevention in pediatric subjects 12 to 17 years of age. Pediatrics. 2009;123:924–934. doi: 10.1542/peds.2008-0642. [DOI] [PubMed] [Google Scholar]
- 10.Bakola E, Skapinakis P, Tzoufi M, Damigos D, Mavreas V. Anticonvulsant drugs for pediatric migraine prevention: an evidence-based review. Eur J Pain. 2009;13:893–901. doi: 10.1016/j.ejpain.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Shank RP, Maryanoff BE. Molecular pharmacodynamics, clinical therapeutics, and pharmacokinetics of topiramate. CNS Neurosci Ther. 2008;14:120–142. doi: 10.1111/j.1527-3458.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]