Abstract
Aim/Goal
To recommend a set of neuropsychological and physical exercise tests for researchers to assess cognition and physical fitness in clinical trials with older patients with dementia; to create consensus, decrease heterogeneity, and improve research quality.
Methods
A literature search (2005–2011) yielded 89 randomized controlled trials. To provide information on test recommendations the frequency of test use, effect size of the test outcome, study quality, and psychometric properties of tests were analyzed.
Results
Fifty-nine neuropsychological tests (cognitive domains: global cognition, executive functioning, memory, and attention) and 10 exercise tests (physical domains: endurance capacity, muscle strength, balance, and mobility) were found.
Conclusion
The Severe Impairment Battery, Mini Mental State Examination, and Alzheimer Disease Assessment Scale – cognitive subscale were recommended to measure global cognition. The Verbal Fluency Test Category/Letters, Clock Drawing Test, and Trail Making Test-B were recommended to measure executive functioning. No specific memory test could be recommended. The Digit Span Forward, Digit Span Backward, and Trail Making Test-A were recommended to measure attention. As physical exercise tests, the Timed Up and Go and Six Meter Walk for mobility, the Six Minute Walk Distance for endurance capacity, and the Tinetti Balance Scale were recommended.
Key Words: Dementia, Neuropsychological tests, Exercise tests, Tool use, Outcome measures, Systematic review
Introduction
With the worldwide trend of an aging population, the number of patients with dementia will increase dramatically in the coming decades [1]. Dementia is characterized by a loss of neurons and atrophy of brain tissue [2,3,4,5]. Eventually, this leads to limitations in cognitive performance of executive functioning, memory, and attention [6,7]. The neurodegenerative processes in the brain go hand in hand with limitations in physical performance of endurance capacity, muscle strength, balance, and mobility [6,8]. Eventually, decline in cognitive performance and physical performance results in problems in activities of daily living and behavior, leading to institutionalization and a decreased quality of life [3,9,10]. Therefore, prevention of decline and preferably an improvement in both cognitive and physical performance in patients with dementia are of utmost importance.
With the growing impact of dementia on today's society, new treatments need to be developed that effectively reduce the limitations caused by a decline of cognitive and physical performance in patients with dementia [11]. Meta-analysis and systematic reviews reported that pharmacological (e.g. medication) and non-pharmacological (e.g. exercise) interventions may have a positive effect on cognition and physical functioning [12,13,14,15,16,17,18]. However, the individual studies in these reviews showed ambiguous results and the tests that measured cognitive and physical functioning appeared to show large heterogeneity. Consequently, the comparability of the outcomes of clinical trials is hampered [19]. Therefore, future intervention studies that aim to improve cognitive functioning, physical functioning, or a combination of both should strive to use a limited number of generally accepted, feasible, reliable, and valid tests that adequately cover the domains of cognitive and physical functioning in patients with dementia. This study is intended to contribute to this goal.
Recommendations on cognitive assessment tests for the purpose of diagnosing dementia were recently provided by Chaves et al. [20] and Young et al. [21]. Information regarding the tests on cognition that researchers should use for measuring treatment effects, however, is not available yet. To the authors’ knowledge, recommendations regarding the use of exercise tests evaluating physical functioning in clinical trials are fully lacking.
The aim of this systematic review is to give up-to-date recommendations of both neuropsychological and physical exercise tests for researchers who have the aim to investigate treatment effects on cognition and physical functioning in older patients with dementia. Firstly, a comprehensive overview of tests on the basis of randomized controlled trials (RCTs) is presented. Frequently used neuropsychological and physical exercise tests are evaluated in relation with study quality of RCTs, nature of the interventions in RCTs, type of dementia that was studied, and sensitivity to change of the tests. Secondly, the reliability and validity of frequently used tests was reviewed.
Methods
Data Sources
Between August 2010 and August 2011, computer databases PubMed, EMBASE, Biological abstracts, Web of Science (ISI), PsycINFO, CINAHL, and Cochrane Library were searched for relevant studies published between 2005 and 2011. Limits for the searches in the computer databases were set on: clinical trial, humans, and age ≥65 years. Keywords in the search included terms from Medical Subject Headings (MESH) and EMBASE thesaurus (EMTREE). The following terms were used in the MESH database and EMTREE thesaurus: dementia, Alzheimer disease, vascular dementia, frontotemporal dementia, Lewy body disease, neuropsychological tests, and exercise tests. Keywords for dementia (dementia OR Alzheimer disease OR vascular dementia OR frontotemporal dementia OR Lewy body disease) were combined (with ‘AND’) with terms that expressed the use of neuropsychological or exercise tests (neuropsychological tests OR exercise tests). In addition, reference lists of reviews regarding the subject were thoroughly hand searched for additional studies.
Inclusion Criteria
Studies were included if they met the following criteria: (1) the design was a RCT; (2) the participants had a diagnosis of dementia; (3) the participants were on average older than 65 years; (4) neuropsychological tests and/or exercise tests were used to measure the effects of an intervention, and (5) the study was written in English, German, French or Dutch.
Selection Process
After the literature search, a first selection of studies was made according to their titles, followed by a selection after reading the abstracts. Two reviewers (WB and MvH) independently performed both steps to identify those studies that met the inclusion criteria (agreement 94%, disagreement 6%). Disagreement was solved with full-text screening. Full-text analysis to check the inclusion criteria was performed for the studies identified in the preceding steps. Subsequently, reviews were hand searched for clinical trials that were not already found in the literature search. Finally, full-text analysis of and data extraction from the selected studies was performed.
Data Extraction
From the selected RCTs the following data were extracted: neuropsychological tests, physical exercise tests, type of dementia, sample size, and data regarding the intervention description (e.g. pharmacological, exercise). For each neuropsychological or physical exercise test the overall means and standard deviations were calculated from all RCTs that used a given test. Further, on the basis of the selected RCTs the overall means and standard deviations were calculated for age, baseline scores, and posttest scores.
Effect Size
In order to express the sensitivity to change for each neuropsychological or physical exercise test, Cohen's d effect sizes (ESs) for a test were calculated on the basis of the selected RCTs [22,23]. If the mean and standard deviation of pretest and posttest were presented in the RCT, the following formula was used:
d = [(postexp – preexp) – (postcont – precont)]/Sqrt[([s2 preexp (nexp) + s2 precont (ncont)]/[nexp + ncont]) + ([s2 postexp (nexp) + s2 postcont (ncont)]/[nexp + ncont])/2] [24]
If the means and standard deviations were not presented in the RCT, the F statistic was used with the following formula:
d = Sqrt[F([(nexp + ncont)/(nexp · ncont)] · [(nexp + ncont)/(nexp + ncont − 2)])] [24]
The overall ES was calculated as the mean of individual ESs weighted for the sample size. Cohen's benchmarks were used to indicate small (d = 0.20), medium (d = 0.50), and large (d = 0.80) ESs [22].
Study Quality
Study quality of each RCT that used a given test was assessed with the Physiotherapy Evidence Database (PEDro) [25]. According to the PEDro scoring system, a score of 9–10 was considered as excellent, a score of 6–8 as good, a score of 4–5 as moderate, and a score of 0–3 as poor [25]. For further analysis of neuropsychological and physical exercise tests in this review, the study quality of at least 5 RCTs must be good or excellent.
Reliability and Validity of Frequently Used Tests
After identifying the tests that were used in ≥5 good or excellent quality RCTs, a second search in PubMed was conducted through September 2011 to select the studies aimed at reporting the reliability and validity of these tests as evidenced in a population with dementia. Searches were performed by combining the terms ‘reliability’ OR ‘validity’ OR ‘reproducibility of results’ in combination with (with ‘AND’) keywords for dementia and the selected neuropsychological and physical exercise tests. By means of references, additional reliability and validity studies were searched.
Results
Study Characteristics
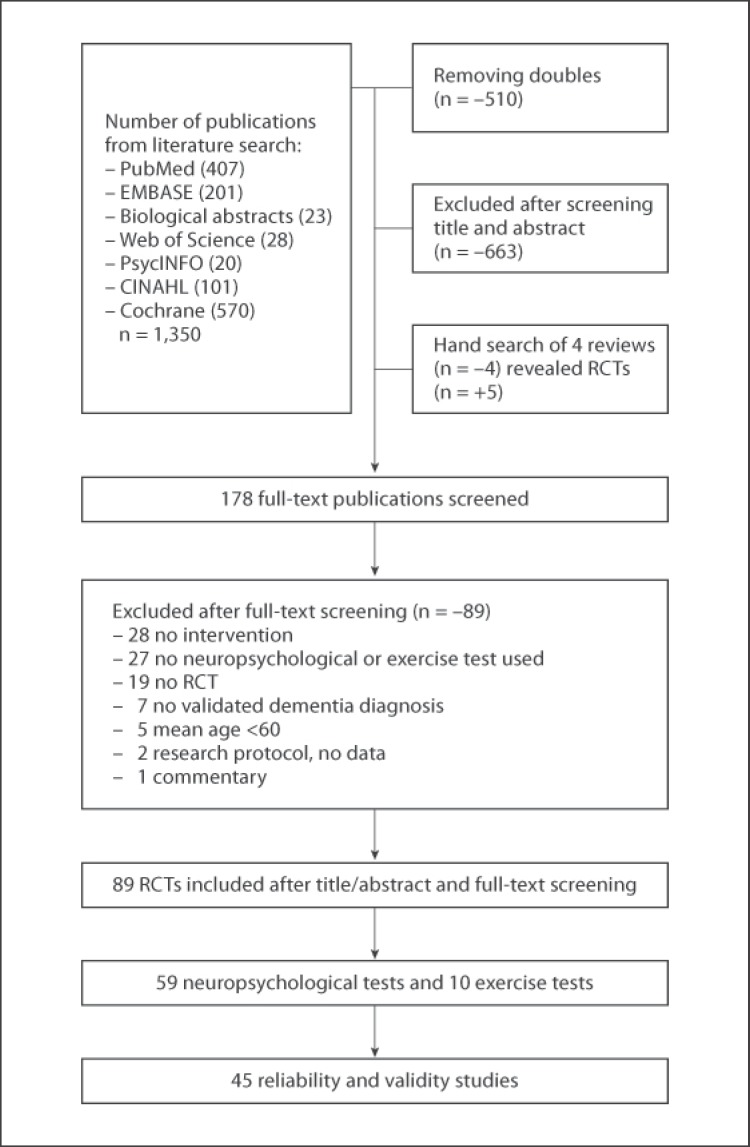
The literature searches for RCTs yielded a total of 840 potentially relevant publications. Eventually, 178 publications were full text screened of which 89 were excluded. A flowchart of the process is presented in figure 1. The results are described in two separate sections: (1) neuropsychological tests and (2) exercise tests. These sections describe the test use in RCTs (number of RCTs), test use related to intervention type, test use related to dementia type, ESs measured with the tests, and study quality of RCTs (PEDro). Table 1 describes 59 neuropsychological tests that covered the cognitive domains global cognition, executive functioning, memory, and attention. Thereafter, table 2 describes the psychometric data of the neuropsychological tests that were most often used. Finally, 10 exercise tests that covered the physical domains endurance capacity, muscle strength, balance, and mobility are presented in table 3.
Fig. 1.
Flow chart of literature search and study selection.
Table 1.
Frequency of use of 59 neuropsychological tests (cognitive domain), descriptive statistics of the populations and RCTs in which these tests were used, the overall Cohen's d ES (small/medium/large) for the tests in these RCTs, and range of the study quality (PEDro) of RCTs (n = 63 in total) that used a given test
| Neuropsychological test (test domain) | Studies, n | Participants, n | Intervention type (n of RCTs) | Mean agea ± SD years | Gender % ♀ | Dementia type (%) | Mean baselinea ± SD | ESa | PEDro |
|---|---|---|---|---|---|---|---|---|---|
| MMSE (global functioning) [26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79] | 54 | 7,606 | pharmacological (39), cognitive (9), exercise (5), acupuncture (1) | 75.8 ± 6.7 | 58 | AD (80), VaD (18), LB (2) | 8.0 ± 4.0 | small | E (6), G (45), Mo (3), P (0) |
| ADAS-cog (global functioning) [26, 27, 28, 31, 32, 35, 39, 41, 43, 47, 48, 51, 54, 55, 58, 61, 63, 64, 67, 68, 70, 71, 72, 75, 77, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97] | 43 | 10,133 | pharmacological (37), cognitive (5), exercise (1) | 74.4 ± 6.7 | 59 | AD (81), VaD (17), LB (2) | 24.4 ± 11.0 | small | E (12), G (28), Mo (3), P (0) |
| Verbal Fluency Test Category (EF) [31, 36, 46, 47, 56, 98, 99, 100, 101, 102, 103, 104] | 12 | 726 | pharmacological (5), cognitive (2), exercise (1), hand movement (1), airway stimuli (1), CES (1), nerve stimuli (1) | 74.2 ± 7.1 | 56 | AD (47), VaD (29), n.r. (24) | 10.9 ± 9.2 | medium | E (4), G (6), Mo (1), P (1) |
| SIB (global functioning) [30, 37, 66, 73, 74, 105, 106, 107] | 8 | 2,134 | pharmacological (8) | 76.6 ± 7.7 | 67 | AD (100) | 69.3 ± 19.2 | small | E (3), G (5), Mo (0), P (0) |
| Digit Span Forward (attention) [31, 33, 49, 99, 101, 102, 103, 104] | 8 | 342 | pharmacological (1), cognitive (2), exercise (1), hand movement (1), airway stimuli (1), CES (1), nerve stimuli (1) | 82.6 ± 6.7 | 55 | AD (76), n.r. (24) | 5.4 ± 2.3 | small | E (3), G (4), Mo (0), P (1) |
| Digit Span Backward (attention) [31, 49, 99, 101, 102, 103, 104] | 7 | 280 | cognitive (2), exercise (1), hand movement (1), airway stimuli (1), CES (1), nerve stimuli (1) | 82.2 ± 6.5 | 50 | AD (44), n.r. (56) | 3.9 ± 1.5 | small | E (3), G (3), Mo (0), P (1) |
| Clock Drawing Test (EF) [42, 50, 56, 95, 100, 108] | 6 | 1,674 | pharmacological (5), exercise (1) | 72.1 ± 7.7 | 68 | AD (82), VaD (17), LB (1) | 4.5 ± 3.2 | small | E (3), G (2), M (0), P (1) |
| Trail Making Test-A (attention) [31, 42, 83, 101, 102, 103] | 6 | 1,502 | pharmacological (3), cognitive (1), airway stimuli (1), CES (1) | 73.8 ± 8.0 | 67 | AD (98), FTD (2) | 161 ± 82.5 | small | E (1), G (4), Mo (0), P (1) |
| Verbal Fluency Test Letters (EF) [36, 56, 99, 101, 102, 103] | 6 | 319 | pharmacological (1), cognitive (1), exercise (1), airway stimuli (1), CES (1), nerve stimuli (1) | 78.9 ± 5.6 | 44 | AD (56), PD (44) | 16.3 ± 4.6 | small | E (2), G (3), Mo (0), P (1) |
| Trail Making Test-B (EF) [27, 31, 47, 98, 101] | 5 | 214 | pharmacological (3), cognitive (1), airway stimuli (1) | 73.9 ± 6.2 | 58 | AD (100) | 242.8 ± 91.3 | small | E (2), G (3), Mo (0), P (0) |
| Logical Memory Test – immediate recall (memory) [28, 49, 69, 70] | 4 | 277 | pharmacological (2), cognitive (2) | 75.6 ± 6.8 | 69 | AD (100) | 11.5 ± 4.7 | small | E (2), G (2), Mo (0), P (0) |
| Logical Memory Test – delayed recognition (memory) [28, 49, 69, 70] | 4 | 277 | pharmacological (2), cognitive (2) | 75.6 ± 6.8 | 69 | AD (100) | 2.8 ± 2.9 | small | E (2), G (2), Mo (0), P (0) |
| Logical Memory Test – delayed recall (memory) [28, 49, 69, 70] | 4 | 277 | pharmacological (2), cognitive (2) | 75.6 ± 6.8 | 69 | AD (100) | 0.9 ± 1.6 | small | E (2), G (2), Mo (0), P (0) |
| Eight Word Test – immediate recall (memory) [99, 102, 103, 104] | 4 | 196 | exercise (1), hand movement (1), CES (1), nerve stimuli (1) | 84.7 ± 6.1 | 62 | AD (38), n.r. (62) | 17.6 ± 8.1 | small | E (2), G (1), Mo (0), P (1) |
| Eight Word Test – delayed recall (memory) [99, 102, 103, 104] | 4 | 196 | exercise (1), hand movement (1), CES (1), nerve stimuli (1) | 84.7 ± 6.1 | 62 | AD (38), n.r. (62) | 0.35 ± 0.94 | small | E (2), G (1), Mo (0), P (1) |
| Eight Word Test – delayed recognition (memory) [99, 102, 103, 104] | 4 | 196 | exercise (1), hand movement (1), CES (1), nerve stimuli (1) | 84.7 ± 6.1 | 62 | AD (38), n.r. (62) | 10.7 ± 3.9 | small | E (2), G (1), Mo (0), P (1) |
| Rivermead Behavioral Memory Test – face recognition (memory) [99, 102, 103, 104] | 4 | 196 | exercise (1), hand movement (1), CES (1), nerve stimuli (1) | 84.6 ± 6.1 | 59 | AD (54), n.r. (46) | 6.6 ± 3.5 | small | E (2), G (1), Mo (0), P (1) |
| Rivermead Behavioral Memory Test – picture recognition (memory) [99, 102, 103, 104] | 4 | 196 | exercise (1), hand movement (1), CES (1), nerve stimuli (1) | 84.6 ± 6.1 | 59 | AD (54), n.r. (46) | 12.2 ± 6.3 | small | E (2), G (1), Mo (0), P (1) |
| Mattis Dementia Rating Scale (global functioning) [46, 50, 101] | 3 | 105 | pharmacological (2), airway stimuli (1) | 77.1 ± 7.3 | 67 | AD (78), LB (22) | 108.8 ± 15.7 | small | E (1), G (2), Mo (0), P (0) |
| Modified Boston Naming Test (language) [31, 49, 69] | 3 | 62 | pharmacological (1), cognitive (2) | 77.4 ± 7.8 | 76 | AD (100) | 10.4 ± 4.6 | small | E (1), G (2), Mo (0), P (0) |
| Syndrome Kurtz Test (attention/memory) [52, 100] | 2 | 290 | pharmacological (2) | 65.2 ± 7.5 | 64 | AD (38), VaD (62) | 16.0 ± 3.7 | large | E (2), G (0), Mo (0), P (0) |
| Digit Symbol Test (attention) [42, 101] | 2 | 273 | pharmacological (1), airway stimuli (1) | 73.8 ± 8.0 | 72 | AD (100) | 11.7 ± 5.8 | small | E (1), G (1), Mo (0), P (0) |
| STROOP color-word interference (EF) [98, 101] | 2 | 67 | pharmacological (1), airway stimuli (1) | 77.7 ± 6.6 | 54 | AD (100) | 65.5 ± 41.2 | small | E (1), G (1), Mo (0), P (0) |
| Cambridge Neuropsychological Test Battery (global functioning) [78, 109] | 2 | 50 | pharmacological (1), exercise stimuli (1) | 71.2 ± 8.1 | 56 | AD (65), FTD (35) | – | – | E (0), G (1), Mo (1), P (0) |
| Visual Memory Span (memory) [102, 103] | 2 | 38 | CES (1), nerve stimuli (1) | 84.4 ± 6.3 | 59 | AD (100) | 9.0 ± 3.8 | small | E (0), G (1), Mo (0), P (1) |
| Selective Reminding Test (memory) [28, 49] | 2 | 35 | cognitive (2) | 72.9 ± 7.1 | 89 | AD (100) | 13.3 ± 12.4 | small | E (2), G (0), Mo (0), P (0) |
| Block Design Test (EF) [84, 98] | 2 | 31 | pharmacological (2) | 72.8 ± 6.8 | 0 | AD (100) | 45.5 ± 14.9 | small | E (2), G (0), Mo (0), P (0) |
| The Executive Interview (EF) [81] | 1 | 363 | pharmacological (1) | 72.3 ± 9.0 | 38 | VaD (100) | 18.3 ± 7.0 | small | E (0), G (1), Mo (0), P (0) |
| Cambridge Cognitive Examination (global functioning) [38] | 1 | 179 | pharmacological (1) | 87.4 ± 6.0 | 57 | AD (100) | 69.0 ± 13.0 | small | E (0), G (1), Mo (0), P (0) |
| Age-Adjusted Concentration Task (attention) [65] | 1 | 65 | pharmacological (1) | 77.8 ± 5.6 | 58 | AD (66), VaD (11) | – | small | E (0), G (1), Mo (0), P (0) |
| Auditory Verbal Learning Test – Chinese version (memory) [33] | 1 | 62 | pharmacological (1) | 83.9 ± 78.6 | 80 | AD (100) | 2.6 ± 1.5 | small | E (0), G (0), Mo (1), P (0) |
| Stop Signal Reaction Time (attention) [104] | 1 | 61 | hand movement (1) | 84.7 ± 5.1 | 58 | n.r. | – | – | E (0), G (0), Mo (1), P (0) |
| Attention Network Task (attention) [104] | 1 | 61 | hand movement (1) | 84.7 ± 5.1 | 58 | n.r. | – | – | E (0), G (0), Mo (1), P (0) |
| Hasegawa's Evaluation of Cognitive Functioning (global functioning) [76] | 1 | 60 | acupuncture (1) | 66.7 ± 10.5 | 34 | VaD (100) | 11.3 ± 4.5 | small | E (0), G (0), Mo (1), P (0) |
| Cognitive Abilities Screening Instrument (global functioning) [40] | 1 | 60 | cognitive (1) | 82.3 ± 5.9 | 67 | VaD (100) | 54.6 ± 15.3 | small | E (1), G (0), Mo (0), P (0) |
| Digit Cancellation Task (attention) [101] | 1 | 52 | airway stimuli (1) | 78.2 ± 7.2 | 69 | AD (100) | – | – | E (0), G (0), Mo (1), P (0) |
| Hopkins Verbal Learning Test – revised (memory) [101] | 1 | 52 | airway stimuli (1) | 78.2 ± 7.2 | 69 | AD (100) | 3.3 ± 1.5 | small | E (0), G (1), Mo (0), P (0) |
| Wisconsin Card Sorting Test (EF) [101] | 1 | 52 | airway stimuli (1) | 78.2 ± 7.2 | 69 | AD (100) | – | – | E (0), G (1), Mo (0), P (0) |
| The Executive Clock Drawing Task 1 (EF) [110] | 1 | 51 | pharmacological (1) | 77.9 ± 7.0 | 55 | AD (100) | 6.2 ± 3.7 | small | E (0), G (1), Mo (0), P (0) |
| The Executive Clock Drawing Task 2 (EF) [110] | 1 | 51 | pharmacological (1) | 77.9 ± 7.0 | 55 | AD (100) | 10.7 ± 3.0 | small | E (0), G (1), Mo (0), P (0) |
| Rey-Osterrieth Complex Figure Test – Copy (EF) [36] | 1 | 32 | cognitive (1) | 73.0 ± 7.2 | 62 | AD (100) | 16.5 ± 14.6 | small | E (0), G (1), Mo (0), P (0) |
| Rey-Osterrieth Complex Figure Test – Recall (EF) [36] | 1 | 32 | cognitive (1) | 73.0 ± 7.2 | 62 | AD (100) | 1.2 ± 2.0 | small | E (0), G (1), Mo (0), P (0) |
| Three Dimensional Constructional Praxis (constructive abilities) [69] | 1 | 32 | cognitive (1) | 73.0 ± 7.2 | 62 | AD (100) | 11.9 ± 0.48 | – | E (0), G (1), Mo (0), P (0) |
| Extended Rivermead Behavioral Memory Test – profile (memory) [36] | 1 | 32 | cognitive (1) | 73.0 ± 7.2 | 62 | AD (100) | 1.2 ± 1.3 | small | E (0), G (1), Mo (0), P (0) |
| Attention Matrices Test (attention) [36] | 1 | 32 | cognitive (1) | 73.0 ± 7.2 | 62 | AD (100) | 32.4 ± 11.7 | small | E (0), G (1), Mo (0), P (0) |
| Visual Reproduction 1 (memory) [28] | 1 | 16 | cognitive (1) | 73.8 ± 4.8 | 82 | AD (100) | 13. ± 83.6 | large | E (1), G (0), Mo (0), P (0) |
| Rapid Evaluation of Cognitive Functioning (global functioning) [111] | 1 | 31 | exercise (1) | 81.8 ± 5.3 | 74 | AD (100) | 27.6 ± 6.8 | large | E (0), G (1), Mo (0), P (0) |
| Developmental Test of Visual Motor Integration (perception) [84] | 1 | 16 | pharmacological (1) | 69.8 ± 8.6 | 0 | AD (100) | 18.1 ± 2.7 | small | E (1), G (0), Mo (0), P (0) |
| Visual Reproduction 2 (memory) [28] | 1 | 16 | cognitive (1) | 73.8 ± 4.8 | 82 | AD (100) | 1.3 ± 2.8 | small | E (1), G (0), Mo (0), P (0) |
| Judgment of Line Orientation (constructive abilities) [84] | 1 | 16 | pharmacological (1) | 69.8 ± 8.6 | 0 | AD (100) | 18.2 ± 8.9 | small | E (1), G (0), Mo (0), P (0) |
| California Verbal Learning Test – delayed recall (memory) [84] | 1 | 16 | pharmacological (1) | 69.8 ± 8.6 | 0 | AD (100) | 1.6 ± 1.9 | small | E (1), G (0), Mo (0), P (0) |
| Recognition Memory Test – faces (memory) [28] | 1 | 16 | cognitive (1) | 73.8 ± 4.8 | 82 | AD (100) | 28.0 ± 5.9 | small | E (1), G (0), Mo (0), P (0) |
| Benton Visual Retention Test (memory) [49] | 1 | 19 | cognitive (1) | 72.1 ± 8.5 | 95 | AD (100) | 1.9 ± 1.8 | small | E (1), G (0), Mo (0), P (0) |
| Recognition Memory Test – words (memory) [28] | 1 | 16 | cognitive (1) | 73.8 ± 4.8 | 82 | AD (100) | 32.7 ± 8.9 | small | E (1), G (0), Mo (0), P (0) |
| Milan Overall Dementia Assessment (global functioning) [59] | 1 | 16 | cognitive (1) | 68.0 ± 6.5 | 48 | AD (100) | – | small | E (0), G (0), Mo (1), P (0) |
| Proactive Interference Test (memory) [98] | 1 | 15 | pharmacological (1) | 76.0 ± 4.0 | 0 | AD (100) | 7.7 ± 4.2 | small | E (1), G (0), Mo (0), P (0) |
| Route Test (EF) [98] | 1 | 15 | pharmacological (1) | 76.0 ± 4.0 | 0 | AD (100) | 15.1 ± 9.6 | small | E (1), G (0), Mo (0), P (0) |
| Story Recall Test (memory) [98] | 1 | 15 | pharmacological (1) | 76.0 ± 4.0 | 0 | AD (100) | 12.0 ± 13.2 | small | E (1), G (0), Mo (0), P (0) |
| Fuld Object and Memory Evaluation (memory) [31] | 1 | 13 | cognitive (1) | 73.3 ± 6.4 | 69 | AD (100) | 24.7 ± 11.1 | small | E (0), G (1), Mo (0), P (0) |
E = Excellent (9–10); G = good (6–8); Mo = moderate (4–5); P = poor (0–3); EF = executive functioning; CES = cranial electrostimulation; LB = Lewy body disease; PD = Pick's disease; FTD = frontotemporal dementia; n.r. = not reported.
Pooled and weighted data as a function of the number of participants.
Table 2.
Reliability, validity, and summary of the psychometric properties of 10 selected neuropsychological tests (cognitive domain) that were used in ≥5 good- or high-quality RCTs
| Neuropsychological test (domain) | Reliability | Validity | Summary |
|---|---|---|---|
| MMSE (global functioning) |
|
concurrent validity with Wechsler adult intelligence scale verbal IQ (r = 0.78) and performance IQ (r = 0.66) [113] | reliable and valid test in dementia patients; there is a floor effect in severe dementia patients [119]; sensitivity to change over time is questionable because small changes could be due to measurement errors [125] |
| ADAS-cog (global functioning) |
|
concurrent validity with MMSE (r = −0.63) [135] | reliable and valid test in patients with mild to moderate dementia |
| Verbal Fluency Test Category (EF) | – | – | no information available about reliability and validity for dementia patients |
| SIB (global functioning) | concurrent validity with MMSE (r = 0.85) [140] | reliable and valid test in dementia patients [140]; this test is sensitive to changes in patients with moderate to severe dementia (MMSE 0–12) [140]; promising test for follow-up in therapeutic trials [138] | |
| Digit Span Forward (attention) | – | – | no information available about reliability and validity for dementia patients; Digit Span Test as a sub-test in the SIB was sensitive to change in dementia patients [141] |
| Verbal Fluency Test Letters (EF) | – | – | no information available about reliability and validity for dementia patients |
| Digit Span Backward (attention) | – | – | no information available about reliability and validity for dementia patients; Digit Span Test as a sub-test in the SIB was sensitive to change in dementia patients [141] |
| Clock Drawing Test (EF) | test-retest reliability (ICC): 0.70–0.78 [142] inter-rater reliability: ICC = 0.82 [143]; ICC = 0.92 [144]; ICC = 0.88 [145]; κ = 0.82–0.94 [146]; κ = 0.94 [147]; κ = 0.63–1.0 [148] internal consistency (α): 0.75 [142] | concurrent validity with MMSE (r = 0.13) [142] | reliable test in dementia patients |
| Trail Making Test-A (attention) | – | – | no information available about reliability and validity for dementia patients |
| Trail Making Test-B (EF) | – | – | no information available about reliability and validity for dementia patients |
EF = Executive functioning.
Table 3.
Frequency of use of 10 physical exercise tests (physical exercise domain), descriptive statistics of the populations and RCTs in which these tests were used, the overall Cohen's d ES (small/medium/large) for the tests in these RCTs, and range of the study quality (PEDro) of RCTs (n = 13 in total) that used a given test
| Physical exercise test (test domain) | Studies, n | Participants, n | Intervention type (n of RCTs) | Mean agea ± SD years | Gender % ♀ | Dementia type (%) | Mean baselinea ± SD | ESa | PEDro |
|---|---|---|---|---|---|---|---|---|---|
| Timed Up and Go Test (mobility) [45, 150, 151] | 3 | 179 | Exercise (3) | 81.9 ± 87.3 | 71 | AD (100) | 17.1 ± 7.5 | small | E (0), G (2), Mo (1), P (0) |
| Six Minute Walk Distance (endurance capacity) [45, 77] | 2 | 105 | Exercise (2) | 77.6 ± 86.6 | 65 | AD (39), VaD (16), LB (16), n.r. (29) | 221.0 ± 82.6 | medium | E (0), G (2), Mo (0), P (0) |
| Functional Reach Test (flexibility) [77, 150] | 2 | 94 | Exercise (2) | 76.6 ± 86.6 | 52 | AD (82), VaD (18) | 20.4 ± 8.1 | small | E (0), G (2), Mo (0), P (0) |
| Six Meter Walk (mobility) [151] | 1 | 134 | Exercise (1) | 83.0 ± 7.4 | 75 | AD (100) | 0.4 ± 0.2 | medium | E (0), G (1), Mo (0), P (0) |
| Abnormal One-Leg Balance (balance) [151] | 1 | 134 | Exercise (1) | 83.0 ± 7.4 | 75 | AD (100) | – | – | E (0), G (1), Mo (0), P (0) |
| Tinetti Balance Scale (balance) [56] | 1 | 116 | Pharmacological (1) | 73.4 ± 2.5 | 62 | AD (100) | 8.5 ± 1.2 | large | E (1), G (0), Mo (0), P (0) |
| Five Times Sit to Stand (leg strength) [150] | 1 | 29 | Exercise (1) | 76.9 ± 6.7 | 51 | AD (72), LB (28) | 18.9 ± 7.2 | small | E (0), G (1), Mo (0), P (0) |
| Berg Balance Scale (balance) [77] | 1 | 85 | Exercise (1) | 76.6 ± 6.5 | 52 | AD (61), VaD (20), LB (19) | 47.5 ± 16.9 | small | E (0), G (1), Mo (0), P (0) |
| 30 Second Chair Stand (leg strength) [152] | 1 | 16 | Exercise (1) | 74.5 ± − | 37 | AD (100) | – | – | E (0), G (0), Mo (1), P (0) |
| Two Minute Step Test (endurance capacity) [152] | 1 | 16 | Exercise (1) | 74.5 ± − | 37 | AD (100) | – | – | E (0), G (0), Mo (1), P (0) |
E = Excellent (9–10); G = good (6–8); Mo = moderate (4–5); P = poor (0–3); LB = Lewy body disease; n.r. = not reported.
Weighted data as a function of the number of participants.
Neuropsychological Tests
Frequency of Test Use
As is shown in table 1, global cognitive functioning was measured most often with the Mini Mental State Examination (MMSE) (n = 54), Alzheimer's Disease Assessment Scale – cognitive subscale (ADAS-cog) (n = 43), and the Severe Impairment Battery (SIB) (n = 8). Tests for global cognitive functioning were used more often in comparison with neuropsychological tests that covered a specific cognitive area.
Thirty-two domain-specific neuropsychological tests were used in 63 RCTs, of which 7 tests were used in ≥5 RCTs. Executive functioning was measured with the Verbal Fluency Test Category (n = 12), Clock Drawing Test (n = 6), Verbal Fluency Test Letters (n = 6), and the Trail Making Test-B (n = 5). Attention was measured with the Digit Span Forward (n = 8), Digit Span Backward (n = 7), and Trail Making Test-A (n = 6).
In summary, global cognitive tests were used more often than neuropsychological tests that covered a specific cognitive area. Frequently used neuropsychological tests that were used in ≥5 RCTs covered the cognitive domains executive functioning and attention. Tests that were used in >5 RCTs which measured the cognitive domain memory were not found.
Dementia Type
A majority of the participants were diagnosed with Alzheimer's disease (AD; 84%) or vascular dementia (VaD; 7%) (table 1). Neuropsychological tests that were only administered in RCTs with AD patients were the SIB (global cognitive functioning), Verbal Fluency Test Letters (executive functioning), Trail Making Test-A, Digit Span Forward, and Digit Span Backward (attention). Tests used in RCTs with AD or VaD patients were the MMSE and ADAS-cog (global cognitive functioning), Verbal Fluency Test Category, Clock Drawing Test, and Trail Making Test-B (executive functioning). Tests that were used in RCTs with only VaD, Lewy body disease, Pick's disease, and frontotemporal dementia patients were not found.
Effect Size
Pooled ESs ranged from small (d = −0.16) to large (d = 1.58). The global cognitive test Rapid Evaluation of Cognitive Functioning measured a large ES (d = 1.12). The global cognitive tests SIB (d = 0.34), ADAS-cog (d = 0.19), and MMSE (d = 0.09) showed small overall ESs. Overall ESs were small for both pharmacological and non-pharmacological RCTs. Furthermore, two neuropsychological tests that measured memory revealed large pooled ESs with the Visual Reproduction Test (d = 1.58) and the Syndrome Kurtz Test (d = 0.82). The Verbal Fluency Test Category that measures executive functioning measured a medium pooled ES (d = 0.61).
Study Quality
According to the PEDro scale, the study quality of RCTs that used neuropsychological tests ranged from 2 (poor) to 10 (excellent). Three RCTs with poor study quality used the Verbal Fluency Test Category, Digit Span Forward, Digit Span Backward, Clock Drawing Test, Trail Making Test-A, and Verbal Fluency Test Letters. Because these tests were also found in RCTs with excellent and good study quality, this had no effect on the selection process of these neuropsychological tests.
Reliability and Validity
Table 2 presents the reliability and validity of 10 neuropsychological tests that were used in ≥5 good or excellent RCTs. The global cognitive tests MMSE, ADAS-cog, and SIB were found to be reliable and valid tools for dementia patients. The Clock Drawing Test was reliable but showed an unsatisfactory concurrent validity with other tests that measured executive functioning [149]. No reliability or validity studies with dementia patients were found for the Verbal Fluency Test Category, Verbal Fluency Test Letters, Trail Making Test-B, Digit Span Forward, Digit Span Backward, and Trail Making Test-A.
Exercise Tests
Frequency of Test Use
Ten different exercise tests were used in 13 RCTs (table 3). These tests measured the physical exercise domains endurance capacity with the Six Minute Walk Distance and Two Minute Step Test; muscle strength with the Five Times Sit To Stand and 30 Second Chair Stand; balance with the Tinetti Balance Scale, Abnormal One-Leg Balance, and Berg Balance Scale; mobility with the Timed Up and Go and Six Meter Walk, and flexibility with the Functional Reach Test. All physical exercise tests were used in non-pharmacological RCTs, except for the Tinetti Balance Scale that was also used in 1 pharmacological RCT.
Dementia Type
A majority of the participants were diagnosed with AD (84%) or VaD (6%). Six exercise tests were used only in AD patients and covered the physical exercise domains endurance capacity (Two Minute Step Test), muscle strength (30 Second Chair Stand), balance (Tinetti Balance Scale, Abnormal One-Leg Balance), and mobility (Timed Up and Go, Six Meter Walk). Physical exercise tests were not used in RCTs only including VaD patients. In RCTs that included both AD and VaD patients, 3 physical exercise tests measured the physical exercise domains endurance capacity (Six Minute Walk Distance), flexibility (Functional Reach Test), and balance (Berg Balance Scale).
Study Quality
The study quality of RCTs ranged from 5 (moderate) to 9 (excellent). Only the Tinetti Balance Scale was used in a RCT with excellent study quality (PEDro 9).
Effect Size
Pooled ESs of RCTs ranged from small (d = 0.02) to large (d = 0.87). A large ES was found with the Tinetti Balance Scale (d = 0.87). Medium ESs were found with the Six Meter Walk (d = 0.58) and the Six Minute Walk Distance (d = 0.51).
Reliability and Validity
The Timed Up and Go [intraclass correlation (ICC) = 0.985–0.988], Six Minute Walk Distance (ICC = 0.982–0.987), and Six Meter Walk (ICC = 0.973–0.977) showed excellent test-retest values for older patients with dementia [153]. For the remaining physical exercise tests that are presented in table 3, no psychometric studies for the reliability and validity with dementia patients were found.
Discussion
To improve the study quality and increase comparability of clinical trials and observational studies, researchers should strive to use a limited number of generally accepted, feasible, reliable, and valid tests that cover the domains of cognitive and physical functioning. Following previous studies that recommended neuropsychological tests for the diagnoses of dementia [20,21] and studies that stated the importance of physical exercise to attenuate cognitive impairment in older patients with dementia [154], the aim of the current review was to give up-to-date recommendations of both neuropsychological and physical exercise tests for high-quality experimental research with older patients with dementia.
Neuropsychological Tests
This study revealed 59 different neuropsychological tests that were used in 63 RCTs. This confirms the assumption that there is a large heterogeneity in neuropsychological test use in RCTs with older patients with dementia. The results showed that global cognitive tests were used more often in comparison with neuropsychological tests that measured one specific cognitive domain.
In particular, the global cognitive tests MMSE, ADAS-cog, and the SIB were standing out because of their excellent reliability and validity (table 2), and were often used in high-quality RCTs which suggest that they are feasible. However, for all 3 tests the sensitivity to change was low. In line with this, the sensitivity to change of the MMSE and ADAS-cog was also challenged in other studies [140], because changes in performance measured with these tests can easily be caused by small measurement errors [125]. For the SIB, research [140] showed that this test is sensitive to change in patients with severe dementia. Altogether, on the basis of feasibility, sensitivity, reliability, and validity we recommend the use of the SIB in RCTs to measure global cognitive treatment effects.
Memory tests could not be selected in this review due to the large heterogeneity that was found in memory test use. Earlier work on memory tests for diagnosing dementia showed that verbal memory, visual memory, and non-verbal memory can be assessed with several tests [20]. Most of these tests were only used once in RCTs between 2005 and 2011, and 1 test (Word List of the Consortium to Establish a Registry for Alzheimer's disease) was not used at all in RCTs over that period. Additionally, because these tests were specifically recommended for diagnosis of dementia, we suggest that it is not feasible to measure effects over time with these tests. Furthermore, studies that investigated the psychometric properties of these memory tests are lacking.
To measure executive functioning, we recommend the use of the Verbal Fluency Test Category, Clock Drawing Test, Verbal Fluency Test Letters, and the Trail Making Test-B because they were frequently used in good- or excellent-quality RCTs. However, we found that only the Verbal Fluency Test Category was able to detect change, and only the Clock Drawing Test was found to be reliable for the population of older dementia patients (table 2). Since information and psychometric quality is in many cases still insufficient, the recommended selection should be used with care and further evaluation of these tests is needed.
For attention we recommend the Digit Span Forward, Digit Span Backward, and Trail Making Test-A because of their frequent use in high-quality studies. However, no studies were found that investigated the psychometric properties of these tests. Furthermore, the results showed that the sensitivity to change was small.
Table 4 sums up the best currently available tests used in international intervention studies with older persons with dementia. Although they are widely applied, it was shown that the recommended neuropsychological tests lack psychometric studies. Therefore, future research into the psychometric quality of the tests found in this review is essential. The recommended selection of currently optimal cognitive tests should be used with care. Researchers are advised to select the recommended tests that most closely fit their study objectives.
Table 4.
Recommendations of global and specific neuropsychological tests ordered on the basis of frequency of test use, overall ES, study quality, reliability, and validity for global cognitive functioning, executive functioning, memory, and attention
| Global functioning | Executive functioning | Memory | Attention | |
|---|---|---|---|---|
| 1 | SIBa, b, c, d | Verbal Fluency Test Categorya, b, c | Visual Reproduction Test*, b, c | Digit Span Forwarda, c |
| 2 | MMSEa, c, d | Clock Drawing Testa, c, d | Eight Word Test*, c | Digit Span Backwarda, c |
| 3 | ADAS-coga, c, d | Verbal Fluency Test Lettersa, c | Logical Memory Test*, c | Trail Making Test-Aa, c |
| 4 | Rapid Evaluation of Cognitive Functioning*, b, c, d | Trail Making Test-Ba, c |
Frequently used in RCTs (feasibility).
Able to measure an effect (sensitivity to change).
Test was used in excellent/good quality RCTs (PEDro).
Reliable/valid in dementia patients.
More research is needed to recommend these tests.
Physical Exercise Tests
This review found 10 different exercise tests that covered the domains endurance capacity, muscle strength, balance, and mobility. However, there is a large heterogeneity in tests used and none of the tests were used frequently enough in RCTs to recommend them. Preliminary recommendations based on the results of this review may be a first step for the selection of exercise tests.
For endurance capacity, the results showed that the Six Minute Walk Distance is reliable [153] and sensitive to change. Muscle strength was measured with the Five Times Sit to Stand and the 30 Second Chair Stand. However, no studies were available that investigated the feasibility and psychometric properties for these tests. For balance, results showed that the Tinetti Balance Scale was sensitive to change, but again no studies were available that investigated the feasibility and psychometric properties. Mobility was measured with the Six Meter Walk and the Timed Up and Go. The results showed that both tests are reliable [153]. However, only the Six Meter Walk was sensitive to change. Based on the limited information at hand, the best exercise tests available so far are summed up in table 5.
Table 5.
Domain-specific physical exercise tests, ordered on the basis of frequency of tests use, ES, study quality, reliability, and validity for the physical exercise domains endurance capacity, muscle strength, balance, and mobility
| Endurance capacity | Muscle strength* | Balance | Mobility | |
|---|---|---|---|---|
| 1 | Six Minute Walk Distance*, a, b, c | Five Times Sit to Stand* | Tinetti Balance Scale*, a, b | Timed Up and Go*, b, c |
| 2 | 30 Second Chair Stand* | Six Meter Walk*, a, b, c |
Able to measure an effect (sensitivity to change).
Test was used in excellent/good-quality RCTs (PEDro).
Reliable/valid in dementia patients.
More research is needed to recommend these tests.
Because of the importance of physical functioning in the disease process of dementia [154], it is essential that future research obtains more information on the feasibility, sensitivity to change, reliability, and validity of physical exercise tests that were found in this review. Since this information is in many cases still insufficient, the recommendation of optimal physical tests should be used with care.
Conclusion
This review mapped the large heterogeneity in cognitive and physical functioning tests used in international intervention studies with older persons with dementia. The provided neuropsychological (table 4) and exercise (table 5) test recommendations from this systematic analysis may lead to a more evidence-based choice of tests that better fit the research questions of future studies. Since information on psychometric quality is in many cases still insufficient, the recommended selection of currently optimal cognitive and physical tests should be used with care. Researchers are advised to select those recommended tests that most closely fit their study objectives.
Disclosure Statement
Open access costs were funded by Fonds NutsOhra. All authors declare that there are no conflicts of interest.
References
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M, Alzheimer's Disease International Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 3.Thomas VS, Hageman PA. Can neuromuscular strength and function in people with dementia be rehabilitated using resistance-exercise training? Results from a preliminary intervention study. J Gerontol A Biol Sci Med Sci. 2003;58:746–751. doi: 10.1093/gerona/58.8.m746. [DOI] [PubMed] [Google Scholar]
- 4.Clark CM, Forman MS. Frontotemporal lobar degeneration with motor neuron disease: a clinical and pathological spectrum. Arch Neurol. 2006;63:489–490. doi: 10.1001/archneur.63.4.489. [DOI] [PubMed] [Google Scholar]
- 5.Bird TD. Genetic aspects of Alzheimer disease. Genet Med. 2008;10:231–239. doi: 10.1097/GIM.0b013e31816b64dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barberger-Gateau P, Fabrigoule C, Amieva H, Helmer C, Dartigues JF. The disablement process: a conceptual framework for dementia-associated disability. Dement Geriatr Cogn Disord. 2002;13:60–66. doi: 10.1159/000048635. [DOI] [PubMed] [Google Scholar]
- 7.Gershon RC, Cella D, Fox NA, Havlik RJ, Hendrie HC, Wagster MV. Assessment of neurological and behavioural function: the NIH toolbox. Lancet Neurol. 2010;9:138–139. doi: 10.1016/S1474-4422(09)70335-7. [DOI] [PubMed] [Google Scholar]
- 8.Bruce B, Fries JF, Ambrosini D, Lingala B, Gandek B, Rose M, Ware JE., Jr Better assessment of physical function: item improvement is neglected but essential. Arthritis Res Ther. 2009;11:R191. doi: 10.1186/ar2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sauvaget C, Yamada M, Fujiwara S, Sasaki H, Mimori Y. Dementia as a predictor of functional disability: a four-year follow-up study. Gerontology. 2002;48:226–233. doi: 10.1159/000058355. [DOI] [PubMed] [Google Scholar]
- 10.Traykov L, Rigaud AS, Cesaro P, Boller F. Neuropsychological impairment in the early Alzheimer's disease. Encephale. 2007;33:310–316. doi: 10.1016/s0013-7006(07)92044-8. [DOI] [PubMed] [Google Scholar]
- 11.Haan MN, Wallace R. Can dementia be prevented? Brain aging in a population-based context. Annu Rev Public Health. 2004;25:1–24. doi: 10.1146/annurev.publhealth.25.101802.122951. [DOI] [PubMed] [Google Scholar]
- 12.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Eggermont L, Swaab D, Luiten P, Scherder E. Exercise, cognition and Alzheimer's disease: more is not necessarily better. Neurosci Biobehav Rev. 2006;30:562–575. doi: 10.1016/j.neubiorev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Yu F, Kolanowski AM, Strumpf NE, Eslinger PJ. Improving cognition and function through exercise intervention in Alzheimer's disease. J Nurs Scholarsh. 2006;38:358–365. doi: 10.1111/j.1547-5069.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 15.Netz Y, Axelrad S, Argov E. Group physical activity for demented older adults: feasibility and effectiveness. Clin Rehabil. 2007;21:977–986. doi: 10.1177/0269215507078318. [DOI] [PubMed] [Google Scholar]
- 16.Forbes D, Forbes S, Morgan DG, Markle-Reid M, Wood J, Culum I. Physical activity programs for persons with dementia. Cochrane Database Syst Rev. 2008;3:CD006489. doi: 10.1002/14651858.CD006489.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Liu-Ambrose T, Donaldson MG. Exercise and cognition in older adults: is there a role for resistance training programmes? Br J Sports Med. 2009;43:25–27. doi: 10.1136/bjsm.2008.055616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- 19.Maruta C, Guerreiro M, de Mendonca A, Hort J, Scheltens P. The use of neuropsychological tests across Europe: the need for a consensus in the use of assessment tools for dementia. Eur J Neurol. 2011;18:279–285. doi: 10.1111/j.1468-1331.2010.03134.x. [DOI] [PubMed] [Google Scholar]
- 20.Chaves MLF, Godinho CC, Porto CS, Mansur L, Carthery-Goulart MT, Yassuda MS, Beato R. Cognitive, functional and behavioral assessment: Alzheimer's disease. Dement Neuropsychol. 2011;5:153–166. doi: 10.1590/S1980-57642011DN05030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young J, Meagher D, Maclullich A. Cognitive assessment of older people. BMJ. 2011;343:d5042. doi: 10.1136/bmj.d5042. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. Statistical Power Analysis for the Behavioural Sciences. ed 2. Hillsdale: Erlbaum; 1988. [Google Scholar]
- 23.Blankevoort CG, van Heuvelen MJ, Boersma F, Luning H, de Jong J, Scherder EJ. Review of effects of physical activity on strength, balance, mobility and ADL performance in elderly subjects with dementia. Dement Geriatr Cogn Disord. 2010;30:392–402. doi: 10.1159/000321357. [DOI] [PubMed] [Google Scholar]
- 24.Thalheimer W, Cook S.How to calculate effect sizes from published research articles: a simplified methodology. 2002. http://worl-learning.com/effect_sizes.htm (accessed June 8, 2011)
- 25.De Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55:129–133. doi: 10.1016/s0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 26.Aisen PS, Saumier D, Briand R, Laurin J, Gervais F, Tremblay P, Garceau D. A phase II study targeting amyloid-beta with 3APS in mild-to-moderate Alzheimer disease. Neurology. 2006;67:1757–1763. doi: 10.1212/01.wnl.0000244346.08950.64. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez XA, Cacabelos R, Laredo M, Couceiro V, Sampedro C, Varela M, Corzo L, Fernandez-Novoa L, Vargas M, Aleixandre M, Linares C, Granizo E, Muresanu D, Moessler H. A 24-week, double-blind, placebo-controlled study of three dosages of cerebrolysin in patients with mild to moderate Alzheimer's disease. Eur J Neurol. 2006;13:43–54. doi: 10.1111/j.1468-1331.2006.01222.x. [DOI] [PubMed] [Google Scholar]
- 28.Avila R, Carvalho IA, Bottino CM, Miotto EC. Neuropsychological rehabilitation in mild and moderate Alzheimer's disease patients. Behav Neurol. 2007;18:225–233. doi: 10.1155/2007/915816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bentham P, Gray R, Sellwood E, Hills R, Crome P, Raftery J. Aspirin in Alzheimer's disease (AD2000): a randomised open-label trial. Lancet Neurol. 2008;7:41–49. doi: 10.1016/S1474-4422(07)70293-4. [DOI] [PubMed] [Google Scholar]
- 30.Black SE, Doody R, Li H, McRae T, Jambor KM, Xu Y, Sun Y, Perdomo CA, Richardson S. Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology. 2007;69:459–469. doi: 10.1212/01.wnl.0000266627.96040.5a. [DOI] [PubMed] [Google Scholar]
- 31.Bottino CMC, Carvalho IAM, Alvarez AMMA. Avila R, Zukauskas PR, Bustamante SEZ, Andrade FC, Hototian SR, Saffi F, Camargo CHP. Cognitive rehabilitation combined with drug treatment in Alzheimer's disease patients: a pilot study. Clin Rehabil. 2005;19:861–869. doi: 10.1191/0269215505cr911oa. [DOI] [PubMed] [Google Scholar]
- 32.Boxer AL, Lipton AM, Womack K, Merrilees J, Neuhaus J, Pavlic D, Gandhi A, Red D, Martin-Cook K, Svetlik D, Miller BL. An open-label study of memantine treatment in 3 subtypes of frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord. 2009;23:211–217. doi: 10.1097/WAD.0b013e318197852f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng ST, Chan AC, Yu EC. An exploratory study of the effect of mahjong on the cognitive functioning of persons with dementia. Int J Geriatr Psychiatry. 2006;21:611–617. doi: 10.1002/gps.1531. [DOI] [PubMed] [Google Scholar]
- 34.Choi AN, Lee MS, Cheong KJ, Lee JS. Effects of group music intervention on behavioral and psychological symptoms in patients with dementia: a pilot-controlled trial. Int J Neurosci. 2009;119:471–481. doi: 10.1080/00207450802328136. [DOI] [PubMed] [Google Scholar]
- 35.Cummings JL, Koumaras B, Chen M, Mirski D. Effects of rivastigmine treatment on the neuropsychiatric and behavioral disturbances of nursing home residents with moderate to severe probable Alzheimer's disease: a 26-week, multicenter, open-label study. Am J Geriatr Pharmacother. 2005;3:137–148. doi: 10.1016/s1543-5946(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 36.Farina E, Mantovani F, Fioravanti R, Pignatti R, Chiavari L, Imbornone E, Olivotto F, Alberoni M, Mariani C, Nemni R. Evaluating two group programmes of cognitive training in mild-to-moderate AD: is there any difference between a ‘global’ stimulation and a ‘cognitive-specific’ one? Aging Ment Health. 2006;10:211–218. doi: 10.1080/13607860500409492. [DOI] [PubMed] [Google Scholar]
- 37.Feldman H, Gauthier S, Hecker J, Vellas B, Xu Y, Ieni JR, Schwam EM. Efficacy and safety of donepezil in patients with more severe Alzheimer's disease: a subgroup analysis from a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2005;20:559–569. doi: 10.1002/gps.1325. [DOI] [PubMed] [Google Scholar]
- 38.Frankfort SV, Appels BA, de BA, Tulner LR, van Campen JP, Koks CH, Beijnen JH, Schmand BA. Identification of responders and reactive domains to rivastigmine in Alzheimer's disease. Pharmacoepidemiol Drug Saf. 2007;16:545–551. doi: 10.1002/pds.1345. [DOI] [PubMed] [Google Scholar]
- 39.Hampel H, Ewers M, Burger K, Annas P, Mortberg A, Bogstedt A, Frolich L, Schroder J, Schonknecht P, Riepe MW, Kraft I, Gasser T, Leyhe T, Moller HJ, Kurz A, Basun H. Lithium trial in Alzheimer's disease: a randomized, single-blind, placebo-controlled, multicenter 10-week study. J Clin Psychiatry. 2009;70:922–931. [PubMed] [Google Scholar]
- 40.Ito T, Meguro K, Akanuma K, Ishii H, Mori E. A randomized controlled trial of the group reminiscence approach in patients with vascular dementia. Dement Geriatr Cogn Disord. 2007;24:48–54. doi: 10.1159/000103631. [DOI] [PubMed] [Google Scholar]
- 41.Johannsen P, Salmon E, Hampel H, Xu Y, Richardson S, Qvitzau S, Schindler R. Assessing therapeutic efficacy in a progressive disease: a study of donepezil in Alzheimer's disease. CNS Drugs. 2006;20:311–325. doi: 10.2165/00023210-200620040-00005. [DOI] [PubMed] [Google Scholar]
- 42.Kadir A, Andreasen N, Almkvist O, Wall A, Forsberg A, Engler H, Hagman G, Larksater M, Winblad B, Zetterberg H, Blennow K, Langstrom B, Nordberg A. Effect of phenserine treatment on brain functional activity and amyloid in Alzheimer's disease. Ann Neurol. 2008;63:621–631. doi: 10.1002/ana.21345. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy J, Deberdt W, Siegal A, Micca J, Degenhardt E, Ahl J, Meyers A, Kaiser C, Baker RW. Olanzapine does not enhance cognition in non-agitated and non-psychotic patients with mild to moderate Alzheimer's dementia. Int J Geriatr Psychiatry. 2005;20:1020–1027. doi: 10.1002/gps.1397. [DOI] [PubMed] [Google Scholar]
- 44.Kurlan R, Cummings J, Raman R, Thal L. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology. 2007;68:1356–1363. doi: 10.1212/01.wnl.0000260060.60870.89. [DOI] [PubMed] [Google Scholar]
- 45.Kwak YS, Um SY, Son TG, Kim DJ. Effect of regular exercise on senile dementia patients. Int J Sports Med. 2008;29:471–474. doi: 10.1055/s-2007-964853. [DOI] [PubMed] [Google Scholar]
- 46.Kwok T, Lee J, Lam L, Woo J. Vitamin B(12) supplementation did not improve cognition but reduced delirium in demented patients with vitamin B(12) deficiency. Arch Gerontol Geriatr. 2008;46:273–282. doi: 10.1016/j.archger.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, Wilson J, Ritchie CW. Safety, efficacy, and biomarker findings of PBT2 in targeting A(beta) as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7:779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 48.Lee ST, Chu K, Sim JY, Heo JH, Kim M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:222–226. doi: 10.1097/WAD.0b013e31816c92e6. [DOI] [PubMed] [Google Scholar]
- 49.Lee SB, Park CS, Jeong JW, Choe JY, Hwang YJ, Park CA, Park JH, Lee DY, Jhoo JH, Kim KW. Effects of spaced retrieval training (SRT) on cognitive function in Alzheimer's disease (AD) patients. Arch Gerontol Geriatr. 2009;49:289–293. doi: 10.1016/j.archger.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Levin OS, Batukaeva LA, Smolentseva IG, Amosova NA. Efficacy and safety of memantine in Lewy body dementia. Neurosci Behav Physiol. 2009;39:597–604. doi: 10.1007/s11055-009-9167-x. [DOI] [PubMed] [Google Scholar]
- 51.Winblad B, Wimo A, Engedal K, Soininen H, Verhey F, Waldemar G, Wetterholm AL, Haglund A, Zhang R, Schindler R. 3-year study of donepezil therapy in Alzheimer's disease: effects of early and continuous therapy. Dement Geriatr Cogn Disord. 2006;21:353–363. doi: 10.1159/000091790. [DOI] [PubMed] [Google Scholar]
- 52.Mazza M, Capuano A, Bria P, Mazza S. Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer's dementia in a randomized placebo-controlled double-blind study. Eur J Neurol. 2006;13:981–985. doi: 10.1111/j.1468-1331.2006.01409.x. [DOI] [PubMed] [Google Scholar]
- 53.Mizukami K, Asada T, Kinoshita T, Tanaka K, Sonohara K, Nakai R, Yamaguchi K, Hanyu H, Kanaya K, Takao T, Okada M, Kudo S, Kotoku H, Iwakiri M, Kurita H, Miyamura T, Kawasaki Y, Omori K, Shiozaki K, Odawara T, Suzuki T, Yamada S, Nakamura Y, Toba K. A randomized cross-over study of a traditional Japanese medicine (kampo), yokukansan, in the treatment of the behavioural and psychological symptoms of dementia. Int J Neuropsychopharmacol. 2009;12:191–199. doi: 10.1017/S146114570800970X. [DOI] [PubMed] [Google Scholar]
- 54.Modrego PJ, Pina MA, Fayed N, Diaz M. Changes in metabolite ratios after treatment with rivastigmine in Alzheimer's disease: a nonrandomised controlled trial with magnetic resonance spectroscopy. CNS Drugs. 2006;20:867–877. doi: 10.2165/00023210-200620100-00006. [DOI] [PubMed] [Google Scholar]
- 55.Mohs RC, Shiovitz TM, Tariot PN, Porsteinsson AP, Baker KD, Feldman PD. Atomoxetine augmentation of cholinesterase inhibitor therapy in patients with Alzheimer disease: 6-month, randomized, double-blind, placebo-controlled, parallel-trial study. Am J Geriatr Psychiatry. 2009;17:752–759. doi: 10.1097/JGP.0b013e3181aad585. [DOI] [PubMed] [Google Scholar]
- 56.Moretti R, Torre P, Antonello RM, Cazzato G, Pizzolato G. Different responses to rivastigmine in subcortical vascular dementia and multi-infarct dementia. Am J Alzheimers Dis Other Demen. 2008;23:167–176. doi: 10.1177/1533317507312558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mowla A, Pani A. Comparison of topiramate and risperidone for the treatment of behavioral disturbances of patients with Alzheimer disease: a double-blind, randomized clinical trial. J Clin Psychopharmacol. 2010;30:40–43. doi: 10.1097/JCP.0b013e3181ca0c59. [DOI] [PubMed] [Google Scholar]
- 58.Onder G, Zanetti O, Giacobini E, Frisoni GB, Bartorelli L, Carbone G, Lambertucci P, Silveri MC, Bernabei R. Reality orientation therapy combined with cholinesterase inhibitors in Alzheimer's disease: randomised controlled trial. Br J Psychiatry. 2005;187:450–455. doi: 10.1192/bjp.187.5.450. [DOI] [PubMed] [Google Scholar]
- 59.Onor ML, Trevisiol M, Negro C, Signorini A, Saina M, Aguglia E. Impact of a multimodal rehabilitative intervention on demented patients and their caregivers. Am J Alzheimers Dis Other Demen. 2007;22:261–272. doi: 10.1177/1533317507302071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ootani M, Nara I, Kaneko F, Okamura H. Construction of a speed feedback therapy system to improve cognitive impairment in elderly people with dementia: a preliminary report. Dement Geriatr Cogn Disord. 2005;20:105–111. doi: 10.1159/000086611. [DOI] [PubMed] [Google Scholar]
- 61.Penner J, Rupsingh R, Smith M, Wells JL, Borrie MJ, Bartha R. Increased glutamate in the hippocampus after galantamine treatment for Alzheimer disease. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:104–110. doi: 10.1016/j.pnpbp.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 62.Plastino M, Fava A, Pirritano D, Cotronei P, Sacco N, Sperli T, Spano A, Gallo D, Mungari P, Consoli D, Bosco D. Effects of insulinic therapy on cognitive impairment in patients with Alzheimer disease and diabetes mellitus type-2. J Neurol Sci. 2010;288:112–116. doi: 10.1016/j.jns.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 63.Porsteinsson AP, Grossberg GT, Mintzer J, Olin JT. Memantine treatment in patients with mild to moderate Alzheimer's disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5:83–89. doi: 10.2174/156720508783884576. [DOI] [PubMed] [Google Scholar]
- 64.Potkin SG, Alva G, Gunay I, Koumaras B, Chen M, Marski D. A pilot study evaluating the efficacy and safety of rivastigmine in patients with mixed dementia. Drugs Aging. 2006;23:241–249. doi: 10.2165/00002512-200623030-00006. [DOI] [PubMed] [Google Scholar]
- 65.Rainer M, Haushofer M, Pfolz H, Struhal C, Wick W. Quetiapine versus risperidone in elderly patients with behavioural and psychological symptoms of dementia: efficacy, safety and cognitive function. Eur Psychiatry. 2007;22:395–403. doi: 10.1016/j.eurpsy.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 66.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006;63:49–54. doi: 10.1001/archneur.63.1.49. [DOI] [PubMed] [Google Scholar]
- 67.Requena C, Maestu F, Campo P, Fernandez A, Ortiz T. Effects of cholinergic drugs and cognitive training on dementia: 2-year follow-up. Dement Geriatr Cogn Disord. 2006;22:339–345. doi: 10.1159/000095600. [DOI] [PubMed] [Google Scholar]
- 68.Roman GC, Wilkinson DG, Doody RS, Black SE, Salloway SP, Schindler RJ. Donepezil in vascular dementia: combined analysis of two large-scale clinical trials. Dement Geriatr Cogn Disord. 2005;20:338–344. doi: 10.1159/000088494. [DOI] [PubMed] [Google Scholar]
- 69.Savaskan E, Schnitzler C, Schroder C, Cajochen C, Muller-Spahn F, Wirz-Justice A. Treatment of behavioural, cognitive and circadian rest-activity cycle disturbances in Alzheimer's disease: haloperidol vs. quetiapine. Int J Neuropsychopharmacol. 2006;9:507–516. doi: 10.1017/S1461145705006036. [DOI] [PubMed] [Google Scholar]
- 70.Scheltens P, Kamphuis PJ, Verhey FR, Olde Rikkert MG, Wurtman RJ, Wilkinson D, Twisk JW, Kurz A. Efficacy of a medical food in mild Alzheimer's disease: a randomized, controlled trial. Alzheimers Dement. 2010;6:1–10. doi: 10.1016/j.jalz.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Soininen H, West C, Robbins J, Niculescu L. Long-term efficacy and safety of celecoxib in Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;23:8–21. doi: 10.1159/000096588. [DOI] [PubMed] [Google Scholar]
- 72.Sparks DL, Connor DJ, Sabbagh MN, Petersen RB, Lopez J, Browne P. Circulating cholesterol levels, apolipoprotein E genotype and dementia severity influence the benefit of atorvastatin treatment in Alzheimer's disease: results of the Alzheimer's Disease Cholesterol-Lowering Treatment (ADCLT) trial. Acta Neurol Scand Suppl. 2006;185:3–7. doi: 10.1111/j.1600-0404.2006.00690.x. [DOI] [PubMed] [Google Scholar]
- 73.Bullock R, Bergman H, Touchon J, Gambina G, He Y, Nagel J, Lane R. Effect of age on response to rivastigmine or donepezil in patients with Alzheimer's disease. Curr Med Res Opin. 2006;22:483–494. doi: 10.1185/030079906X89685. [DOI] [PubMed] [Google Scholar]
- 74.Winblad B, Kilander L, Eriksson S, Minthon L, Batsman S, Wetterholm AL, Jansson-Blixt C, Haglund A, Severe Alzheimer's Disease Study Group Donepezil in patients with severe Alzheimer's disease: double-blind, parallel-group, placebo-controlled study. Lancet. 2006;367:1057–1065. doi: 10.1016/S0140-6736(06)68350-5. [DOI] [PubMed] [Google Scholar]
- 75.Winstein CJ, Bentzen KR, Boyd L, Schneider LS. Does the cholinesterase inhibitor, donepezil, benefit both declarative and non-declarative processes in mild to moderate Alzheimer's disease? Curr Alzheimer Res. 2007;4:273–276. doi: 10.2174/156720507781077296. [DOI] [PubMed] [Google Scholar]
- 76.Yu J, Zhang X, Liu C, Meng Y, Han J. Effect of acupuncture treatment on vascular dementia. Neurol Res. 2006;28:97–103. doi: 10.1179/016164106X91951. [DOI] [PubMed] [Google Scholar]
- 77.Miu DKY, Szeto SL, Mak YF. A randomized controlled trial on the effect of exercise on physical, cognitive and affective function in dementia subjects. Asian J Gerontol Geriatr. 2008;3:8–16. [Google Scholar]
- 78.Yaguez L, Shaw KN, Morris R, Matthews D. The effects on cognitive functions of a movement-based intervention in patients with Alzheimer's type dementia: a pilot study. Int J Geriatr Psychiatry. 2011;26:173–181. doi: 10.1002/gps.2510. [DOI] [PubMed] [Google Scholar]
- 79.Venturelli M, Scarsini R, Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Demen. 2011;26:381–388. doi: 10.1177/1533317511418956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aronson S, Van Baelen B, Kavanagh S, Schwalen S. Optimal dosing of galantamine in patients with mild or moderate Alzheimer's disease: post hoc analysis of a randomized, double-blind, placebo-controlled trial. Drugs Aging. 2009;26:231–239. doi: 10.2165/00002512-200926030-00004. [DOI] [PubMed] [Google Scholar]
- 81.Auchus AP, Brashear HR, Salloway S, Korczyn AD, De Deyn PP, Gassmann-Mayer C. Galantamine treatment of vascular dementia: a randomized trial. Neurology. 2007;69:448–458. doi: 10.1212/01.wnl.0000266625.31615.f6. [DOI] [PubMed] [Google Scholar]
- 82.Bakchine S, Loft H. Memantine treatment in patients with mild to moderate Alzheimer's disease: results of a randomised, double-blind, placebo-controlled 6-month study. J Alzheimers Dis. 2007;11:471–479. doi: 10.3233/jad-2007-11409. [DOI] [PubMed] [Google Scholar]
- 83.Chappell AS, Gonzales C, Williams J, Witte MM, Mohs RC, Sperling R. AMPA potentiator treatment of cognitive deficits in Alzheimer disease. Neurology. 2007;68:1008–1012. doi: 10.1212/01.wnl.0000260240.46070.7c. [DOI] [PubMed] [Google Scholar]
- 84.Lu PH, Masterman DA, Mulnard R, Cotman C, Miller B, Yaffe K, Reback E, Porter V, Swerdloff R, Cummings JL. Effects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly men. Arch Neurol. 2006;63:177–185. doi: 10.1001/archneur.63.2.nct50002. [DOI] [PubMed] [Google Scholar]
- 85.Modrego PJ, Fayed N, Errea JM, Rios C, Pina MA, Sarasa M. Memantine versus donepezil in mild to moderate Alzheimer's disease: a randomized trial with magnetic resonance spectroscopy. Eur J Neurol. 2010;17:405–412. doi: 10.1111/j.1468-1331.2009.02816.x. [DOI] [PubMed] [Google Scholar]
- 86.Mori S, Mori E, Iseki E, Kosaka K. Efficacy and safety of donepezil in patients with dementia with Lewy bodies: preliminary findings from an open-label study. Psychiatry Clin Neurosci. 2006;60:190–195. doi: 10.1111/j.1440-1819.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- 87.Muresanu DF, Alvarez XA, Moessler H, Buia M, Stan A, Pintea D, Moldovan F, Popescu BO. A pilot study to evaluate the effects of cerebrolysin on cognition and qEEG in vascular dementia: cognitive improvement correlates with qEEG acceleration. J Neurol Sci. 2008;267:112–119. doi: 10.1016/j.jns.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 88.Paleacu D, Barak Y, Mirecky I, Mazeh D. Quetiapine treatment for behavioural and psychological symptoms of dementia in Alzheimer's disease patients: a 6-week, double-blind, placebo-controlled study. Int J Geriatr Psychiatry. 2008;23:393–400. doi: 10.1002/gps.1892. [DOI] [PubMed] [Google Scholar]
- 89.Peskind ER, Potkin SG, Pomara N, Ott BR, Graham SM, Olin JT, McDonald S. Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomized, controlled trial. Am J Geriatr Psychiatry. 2006;14:704–715. doi: 10.1097/01.JGP.0000224350.82719.83. [DOI] [PubMed] [Google Scholar]
- 90.Pomara N, Ott BR, Peskind E, Resnick EM. Memantine treatment of cognitive symptoms in mild to moderate Alzheimer disease: secondary analyses from a placebo-controlled randomized trial. Alzheimer Dis Assoc Disord. 2007;21:60–64. doi: 10.1097/WAD.0b013e318032cf29. [DOI] [PubMed] [Google Scholar]
- 91.Raggi A, Iannaccone S, Marcone A, Ginex V, Ortelli P, Nonis A, Giusti MC, Cappa SF. The effects of a comprehensive rehabilitation program of Alzheimer's disease in a hospital setting. Behav Neurol. 2007;18:1–6. doi: 10.1155/2007/782959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, Mulnard R, Barakos J, Gregg KM, Liu E, Lieberburg I, Schenk D, Black R, Grundman M. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suh GH, Jung HY, Lee CU, Oh BH, Lee SK, Lee N, Kim J, Kee BS, Ko D, Kim YH, Ju YS, Hong I, Choi S. Effect of the apolipoprotein E epsilon4 allele on the efficacy and tolerability of galantamine in the treatment of Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;21:33–39. doi: 10.1159/000089217. [DOI] [PubMed] [Google Scholar]
- 94.Wilkinson D, Schindler R, Schwam E, Waldemar G, Jones RW, Gauthier S, Lopez OL, Cummings J, Xu Y, Feldman HH. Effectiveness of donepezil in reducing clinical worsening in patients with mild-to-moderate Alzheimer's disease. Dement Geriatr Cogn Disord. 2009;28:244–251. doi: 10.1159/000241877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Winblad B, Grossberg G, Frolich L, Farlow M, Zechner S, Nagel J, Lane R. IDEAL: a 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer disease. Neurology. 2007;69:S14–S22. doi: 10.1212/01.wnl.0000281847.17519.e0. [DOI] [PubMed] [Google Scholar]
- 96.McCarney R, Fisher P, Iliffe S, van Haselen R, Griffin M, van der Meulen J, Warner J. Ginkgo biloba for mild to moderate dementia in a community setting: a pragmatic, randomised, parallel-group, double-blind, placebo-controlled trial. Int J Geriatr Psychiatry. 2008;23:1222–1230. doi: 10.1002/gps.2055. [DOI] [PubMed] [Google Scholar]
- 97.Frolich L, Ashwood T, Nilsson J, Eckerwall G, Sirocco Investigators Effects of AZD3480 on cognition in patients with mild-to-moderate Alzheimer's disease: a phase IIb dose-finding study. J Alzheimers Dis. 2011;24:363–374. doi: 10.3233/JAD-2011-101554. [DOI] [PubMed] [Google Scholar]
- 98.Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- 99.Eggermont LH, Swaab DF, Hol EM, Scherder EJ. Walking the line: a randomised trial on the effects of a short term walking programme on cognition in dementia. J Neurol Neurosurg Psychiatry. 2009;80:802–804. doi: 10.1136/jnnp.2008.158444. [DOI] [PubMed] [Google Scholar]
- 100.Napryeyenko O, Sonnik G, Tartakovsky I. Efficacy and tolerability of ginkgo biloba extract EGb 761 by type of dementia: analyses of a randomised controlled trial. J Neurol Sci. 2009;283:224–229. doi: 10.1016/j.jns.2009.02.353. [DOI] [PubMed] [Google Scholar]
- 101.Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, Liu L, Ayalon L, He F, Loredo JS. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076–2081. doi: 10.1111/j.1532-5415.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scherder EJ, van Tol MJ, Swaab DF. High-frequency cranial electrostimulation (CES) in patients with probable Alzheimer's disease. Am J Phys Med Rehabil. 2006;85:614–618. doi: 10.1097/01.phm.0000223221.17301.50. [DOI] [PubMed] [Google Scholar]
- 103.Scherder EJ, Vuijk PJ, Swaab DF, Van Someren EJ. Estimating the effects of right median nerve stimulation on memory in Alzheimer's disease: a randomized controlled pilot study. Exp Aging Res. 2007;33:177–186. doi: 10.1080/03610730701238915. [DOI] [PubMed] [Google Scholar]
- 104.Eggermont LH, Knol DL, Hol EM, Swaab DF, Scherder EJ. Hand motor activity, cognition, mood, and the rest-activity rhythm in dementia A clustered RCT. Behav Brain Res. 2009;196:271–278. doi: 10.1016/j.bbr.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 105.Homma A, Imai Y, Tago H, Asada T, Shigeta M, Iwamoto T, Takita M, Arimoto I, Koma H, Ohbayashi T. Donepezil treatment of patients with severe Alzheimer's disease in a Japanese population: results from a 24-week, double-blind, placebo-controlled, randomized trial. Dement Geriatr Cogn Disord. 2008;25:399–407. doi: 10.1159/000122961. [DOI] [PubMed] [Google Scholar]
- 106.Schmitt FA, van Dyck CH, Wichems CH, Olin JT. Cognitive response to memantine in moderate to severe Alzheimer disease patients already receiving donepezil: an exploratory reanalysis. Alzheimer Dis Assoc Disord. 2006;20:255–262. doi: 10.1097/01.wad.0000213860.35355.d4. [DOI] [PubMed] [Google Scholar]
- 107.van Dyck CH, Schmitt FA, Olin JT. A responder analysis of memantine treatment in patients with Alzheimer disease maintained on donepezil. Am J Geriatr Psychiatry. 2006;14:428–437. doi: 10.1097/01.JGP.0000203151.17311.38. [DOI] [PubMed] [Google Scholar]
- 108.Stevens J, Killeen M. A randomised controlled trial testing the impact of exercise on cognitive symptoms and disability of residents with dementia. Contemp Nurse. 2006;21:32–40. doi: 10.5172/conu.2006.21.1.32. [DOI] [PubMed] [Google Scholar]
- 109.Rahman S, Robbins TW, Hodges JR, Mehta MA, Nestor PJ, Clark L, Sahakian BJ. Methylphenidate (‘ritalin’) can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia. Neuropsychopharmacology. 2006;31:651–658. doi: 10.1038/sj.npp.1300886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paskavitz JF, Gunstad JJ, Samuel JE. Clock drawing and frontal lobe behavioral effects of memantine in Alzheimer's disease: a rater-blinded study. Am J Alzheimers Dis Other Demen. 2006;21:454–459. doi: 10.1177/1533317506294474. [DOI] [PubMed] [Google Scholar]
- 111.Kemoun G, Thibaud M, Roumagne N, Carette P, Albinet C, Toussaint L, Paccalin M, Dugue B. Effects of a physical training programme on cognitive function and walking efficiency in elderly persons with dementia. Dement Geriatr Cogn Disord. 2010;29:109–114. doi: 10.1159/000272435. [DOI] [PubMed] [Google Scholar]
- 112.Anthony JC, LeResche L, Niaz U, von Korff MR, Folstein MF. Limits of the ‘Mini-Mental State’ as a screening test for dementia and delirium among hospital patients. Psychol Med. 1982;12:397–408. doi: 10.1017/s0033291700046730. [DOI] [PubMed] [Google Scholar]
- 113.Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental State’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 114.Dick JP, Guiloff RJ, Stewart A, Blackstock J, Bielawska C, Paul EA, Marsden CD. Mini-Mental State Examination in neurological patients. J Neurol Neurosurg Psychiatry. 1984;47:496–499. doi: 10.1136/jnnp.47.5.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Molloy DW, Alemayehu E, Roberts R. Reliability of a Standardized Mini-Mental State Examination compared with the traditional Mini-Mental State Examination. Am J Psychiatry. 1991;148:102–105. doi: 10.1176/ajp.148.1.102. [DOI] [PubMed] [Google Scholar]
- 116.Fillenbaum GG, Heyman A, Wilkinson WE, Haynes CS. Comparison of two screening tests in Alzheimer's disease. The correlation and reliability of the Mini-Mental State Examination and the Modified Blessed Test. Arch Neurol. 1987;44:924–927. doi: 10.1001/archneur.1987.00520210026014. [DOI] [PubMed] [Google Scholar]
- 117.Thal LJ, Grundman M, Golden R. Alzheimer's disease: a correlational analysis of the Blessed Information-Memory-Concentration Test and the Mini-Mental State Exam. Neurology. 1986;36:262–264. doi: 10.1212/wnl.36.2.262. [DOI] [PubMed] [Google Scholar]
- 118.Uhlmann RF, Larson EB, Buchner DM. Correlations of Mini-Mental State and Modified Dementia Rating Scale to measures of transitional health status in dementia. J Gerontol. 1987;42:33–36. doi: 10.1093/geronj/42.1.33. [DOI] [PubMed] [Google Scholar]
- 119.Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 120.McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50:377–383. doi: 10.1016/s0895-4356(97)00060-7. [DOI] [PubMed] [Google Scholar]
- 121.Holzer CE, Tischler GL, Leaf PJ, Myers JK. An epidemiologic assessment of cognitive impairment in a community population. Res Com Health. 1984;4:3–32. [Google Scholar]
- 122.Kay DW, Henderson AS, Scott R, Wilson J, Rickwood D, Grayson DA. Dementia and depression among the elderly living in the Hobart community: the effect of the diagnostic criteria on the prevalence rates. Psychol Med. 1985;15:771–788. doi: 10.1017/s0033291700005006. [DOI] [PubMed] [Google Scholar]
- 123.Foreman MD. Reliability and validity of mental status questionnaires in elderly hospitalized patients. Nurs Res. 1987;36:216–220. [PubMed] [Google Scholar]
- 124.Albert M, Cohen C. The test for severe impairment: an instrument for the assessment of patients with severe cognitive dysfunction. J Am Geriatr Soc. 1992;40:449–453. doi: 10.1111/j.1532-5415.1992.tb02009.x. [DOI] [PubMed] [Google Scholar]
- 125.Tombaugh TN, McDowell I, Kristjansson B, Hubley AM. Mini-Mental State Examination (MMSE) and the Modified MMSE (3MS): a psychometric comparison and normative data. Arch Clin Neuropsychol. 1996;8:48–59. [Google Scholar]
- 126.Mavioglu H, Gedizlioglu M, Akyel S, Aslaner T, Eser E. The validity and reliability of the Turkish version of Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog) in patients with mild and moderate Alzheimer's disease and normal subjects. Int J Geriatr Psychiatry. 2006;21:259–265. doi: 10.1002/gps.1457. [DOI] [PubMed] [Google Scholar]
- 127.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 128.Pena-Casanova J. Alzheimer's disease assessment scale – cognitive in clinical practice. Int Psychogeriatr. 1997;9(Suppl 1):105–114. [PubMed] [Google Scholar]
- 129.Weyer G, Erzigkeit H, Kanowski S, Ihl R, Hadler D. Alzheimer's disease assessment scale: reliability and validity in a multicenter clinical trial. Int Psychogeriatr. 1997;9:123–138. doi: 10.1017/s1041610297004298. [DOI] [PubMed] [Google Scholar]
- 130.Liu HC, Teng EL, Chuang YY, Lin KN, Fuh JL, Wang PN. The Alzheimer's disease assessment scale: findings from a low-education population. Dement Geriatr Cogn Disord. 2002;13:21–26. doi: 10.1159/000048629. [DOI] [PubMed] [Google Scholar]
- 131.Chu LW, Chiu KC, Hui SL, Yu GK, Tsui WJ, Lee PW. The reliability and validity of the Alzheimer's Disease Assessment Scale cognitive subscale (ADAS-cog) among the elderly Chinese in Hong Kong. Ann Acad Med Singapore. 2000;29:474–485. [PubMed] [Google Scholar]
- 132.Kim YS, Nibbelink DW, Overall JE. Factor structure and reliability of the Alzheimer's disease assessment scale in a multicenter trial with linopirdine. J Geriatr Psychiatry Neurol. 1994;7:74–83. doi: 10.1177/089198879400700202. [DOI] [PubMed] [Google Scholar]
- 133.Mohs RC. The Alzheimer's disease assessment scale. Int Psychogeriatr. 1996;8:195–203. doi: 10.1017/s1041610296002578. [DOI] [PubMed] [Google Scholar]
- 134.Tsolaki M, Fountoulakis K, Nakopoulou E, Kazis A, Mohs RC. Alzheimer's disease assessment scale: the validation of the scale in Greece in elderly demented patients and normal subjects. Dement Geriatr Cogn Disord. 1997;8:273–280. doi: 10.1159/000106644. [DOI] [PubMed] [Google Scholar]
- 135.Cano SJ, Posner HB, Moline ML, Hurt SW, Swartz J, Hsu T, Hobart JC. The ADAS-cog in Alzheimer's disease clinical trials: psychometric evaluation of the sum and its parts. J Neurol Neurosurg Psychiatry. 2010;81:1363–1368. doi: 10.1136/jnnp.2009.204008. [DOI] [PubMed] [Google Scholar]
- 136.Ahn IS, Kim JH, Ku HM, Saxton J, Kim DK. Reliability and validity of the severe impairment battery (SIB) in Korean dementia patients. J Korean Med Sci. 2006;21:506–517. doi: 10.3346/jkms.2006.21.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Suh GH, Kang CJ. Validation of the severe impairment battery for patients with Alzheimer's disease in Korea. Int J Geriatr Psychiatry. 2006;21:626–632. doi: 10.1002/gps.1537. [DOI] [PubMed] [Google Scholar]
- 138.Panisset M, Roudier M, Saxton J, Boller F. Severe impairment battery. A neuropsychological test for severely demented patients. Arch Neurol. 1994;51:41–45. doi: 10.1001/archneur.1994.00540130067012. [DOI] [PubMed] [Google Scholar]
- 139.Schmitt FA, Ashford W, Ernesto C, Saxton J, Schneider LS, Clark CM, Ferris SH, Mackell JA, Schafer K, Thal LJ. The severe impairment battery: concurrent validity and the assessment of longitudinal change in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S51–S56. [PubMed] [Google Scholar]
- 140.Bergh S, Selbaek G, Engedal K. Reliability and validity of the Norwegian version of the Severe Impairment Battery (SIB) Int J Geriatr Psychiatry. 2008;23:896–902. doi: 10.1002/gps.2001. [DOI] [PubMed] [Google Scholar]
- 141.Schmitt FA, Saxton JA, Xu Y, McRae T, Sun Y, Richardson S, Li H. A brief instrument to assess treatment response in the patient with advanced Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:377–383. doi: 10.1097/WAD.0b013e3181ac9cc1. [DOI] [PubMed] [Google Scholar]
- 142.Kim H, Chey J. Effects of education, literacy, and dementia on the Clock Drawing Test performance. J Int Neuropsychol Soc. 2010;16:1138–1146. doi: 10.1017/S1355617710000731. [DOI] [PubMed] [Google Scholar]
- 143.Shulman KI, Pushkar Gold D, Cohen CA, Zucchero CA. Clock-drawing and dementia in the community: a longitudinal study. Int J Geriatr Psychiatry. 1993;8:487–496. [Google Scholar]
- 144.Sunderland T, Hill JL, Mellow AM, Lawlor BA, Gundersheimer J, Newhouse PA, Grafman JH. Clock drawing in Alzheimer's disease. A novel measure of dementia severity. J Am Geriatr Soc. 1989;37:725–729. doi: 10.1111/j.1532-5415.1989.tb02233.x. [DOI] [PubMed] [Google Scholar]
- 145.Wolf-Klein GP, Silverstone FA, Levy AP, Brod MS. Screening for Alzheimer's disease by clock drawing. J Am Geriatr Soc. 1989;37:730–734. doi: 10.1111/j.1532-5415.1989.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 146.Schramm U, Berger G, Muller R, Kratzsch T, Peters J, Frolich L. Psychometric properties of Clock Drawing Test and MMSE or Short Performance Test (SKT) in dementia screening in a memory clinic population. Int J Geriatr Psychiatry. 2002;17:254–260. doi: 10.1002/gps.585. [DOI] [PubMed] [Google Scholar]
- 147.Manos PJ. The utility of the Ten-Point Clock Test as a screen for cognitive impairment in general hospital patients. Gen Hosp Psychiatry. 1997;19:439–444. doi: 10.1016/s0163-8343(97)00072-8. [DOI] [PubMed] [Google Scholar]
- 148.Chiu YC, Li CL, Lin KN, Chiu YF, Liu HC. Sensitivity and specificity of the Clock Drawing Test, incorporating Rouleau scoring system, as a screening instrument for questionable and mild dementia: scale development. Int J Nurs Stud. 2008;45:75–84. doi: 10.1016/j.ijnurstu.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 149.Seigerschmidt E, Mosch E, Siemen M, Forstl H, Bickel H. The Clock Drawing Test and questionable dementia: reliability and validity. Int J Geriatr Psychiatry. 2002;17:1048–1054. doi: 10.1002/gps.747. [DOI] [PubMed] [Google Scholar]
- 150.Netz Y, Axelrad S, Argov E. Group physical activity for demented older adults – feasibility and effectiveness. Clin Rehabil. 2007;21:977–986. doi: 10.1177/0269215507078318. [DOI] [PubMed] [Google Scholar]
- 151.Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, Riviere D, Vellas B. Exercise program for nursing home residents with Alzheimer's disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55:158–165. doi: 10.1111/j.1532-5415.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 152.Santana-Sosa E, Barriopedro MI, Lopez-Mojares LM, Perez M, Lucia A. Exercise training is beneficial for Alzheimer's patients. Int J Sports Med. 2008;29:845–850. doi: 10.1055/s-2008-1038432. [DOI] [PubMed] [Google Scholar]
- 153.Ries JD, Echternach JL, Nof L, Gagnon Blodgett M. Test-retest reliability and minimal detectable change scores for the timed ‘up & go’ test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys Ther. 2009;89:569–579. doi: 10.2522/ptj.20080258. [DOI] [PubMed] [Google Scholar]
- 154.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86:876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]