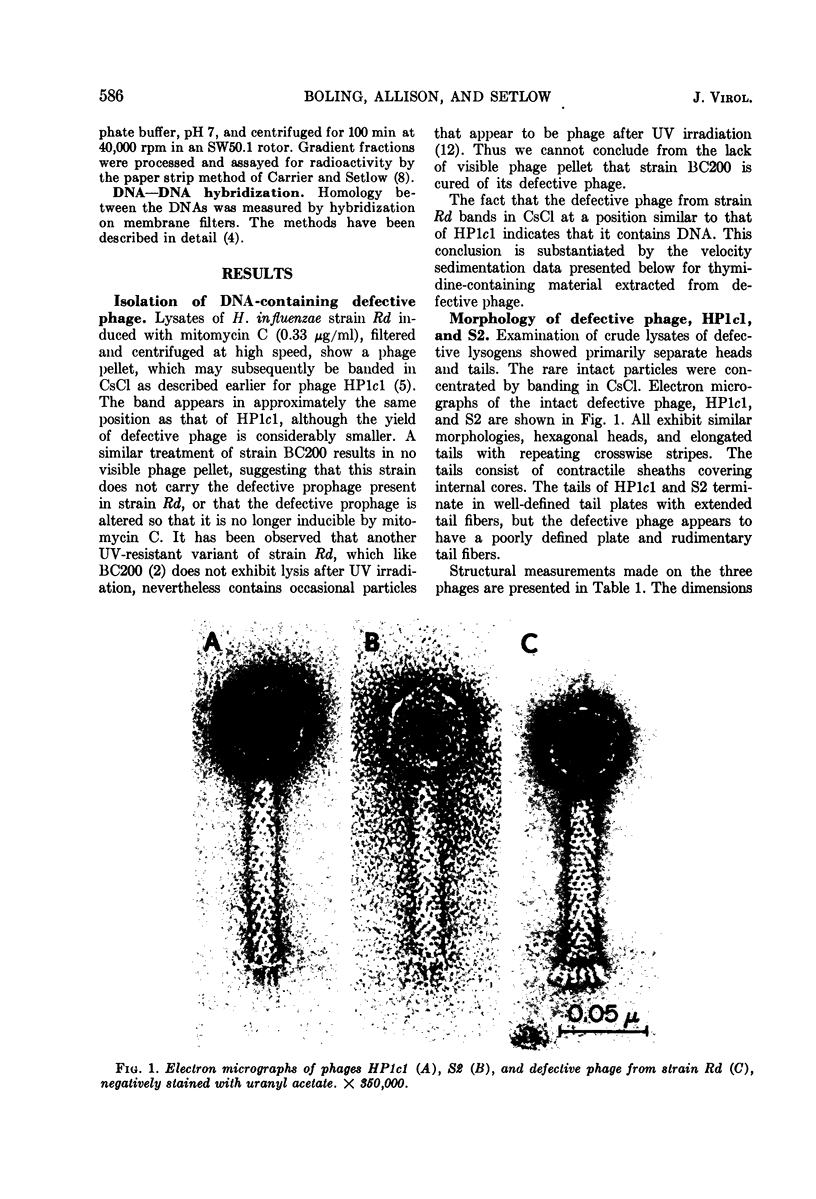
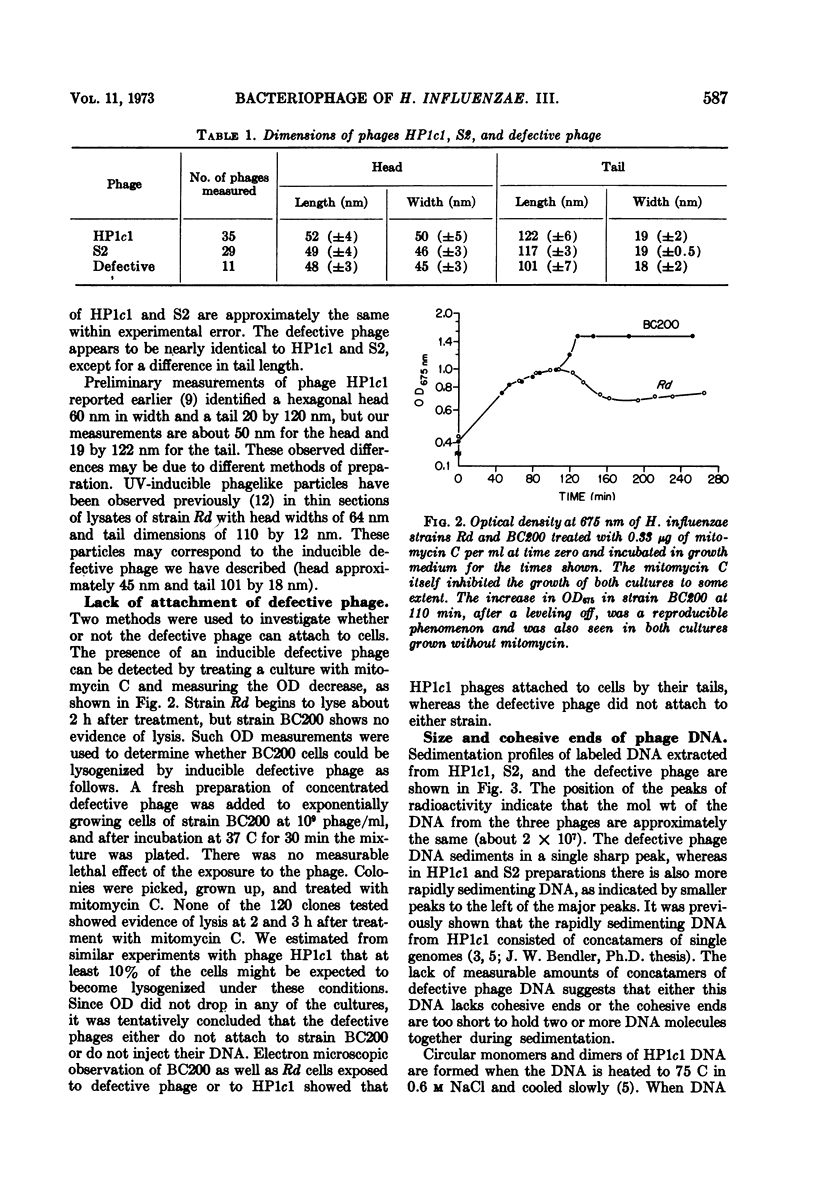
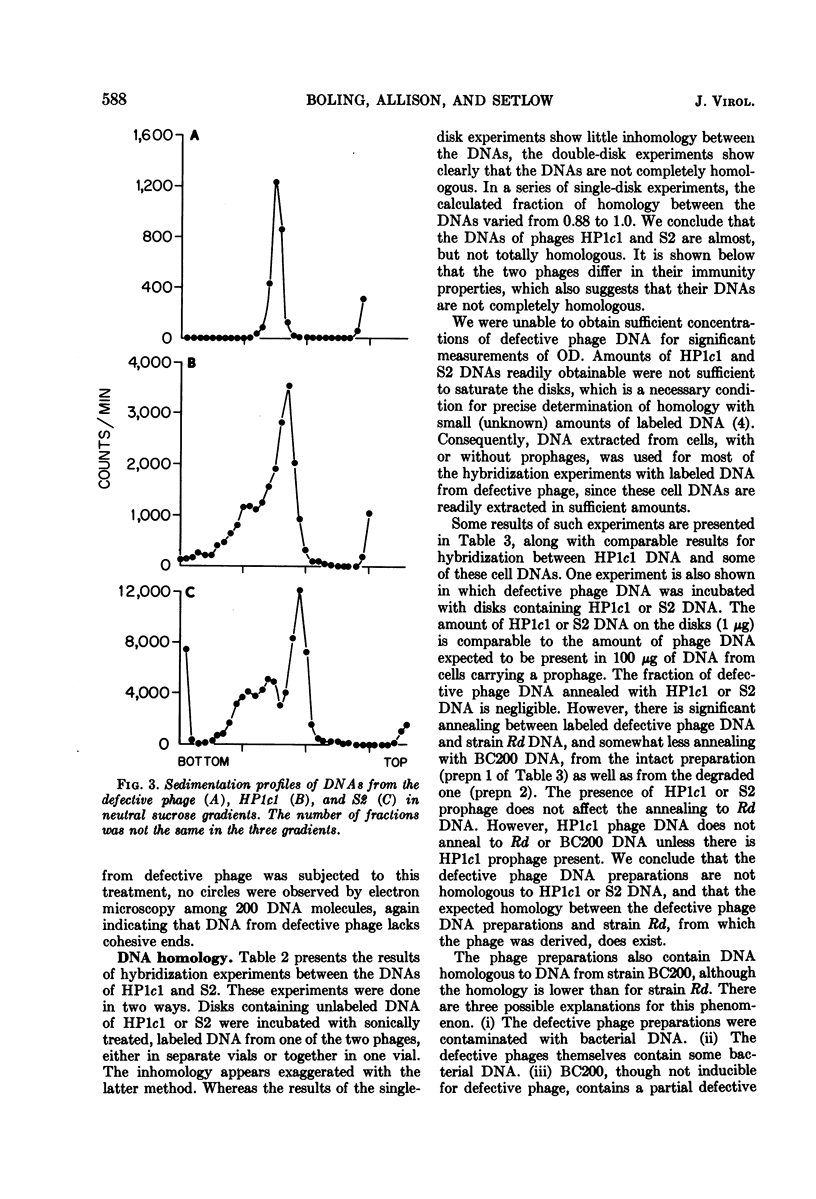
Abstract
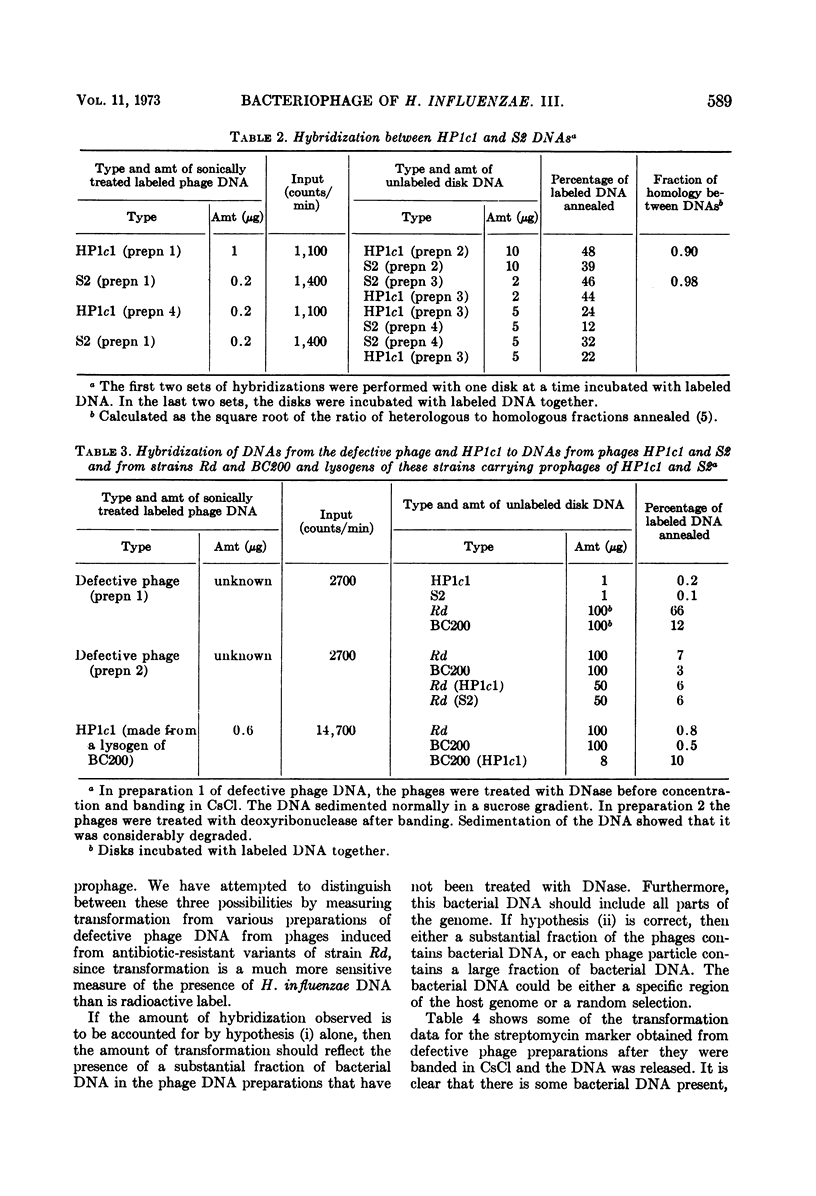
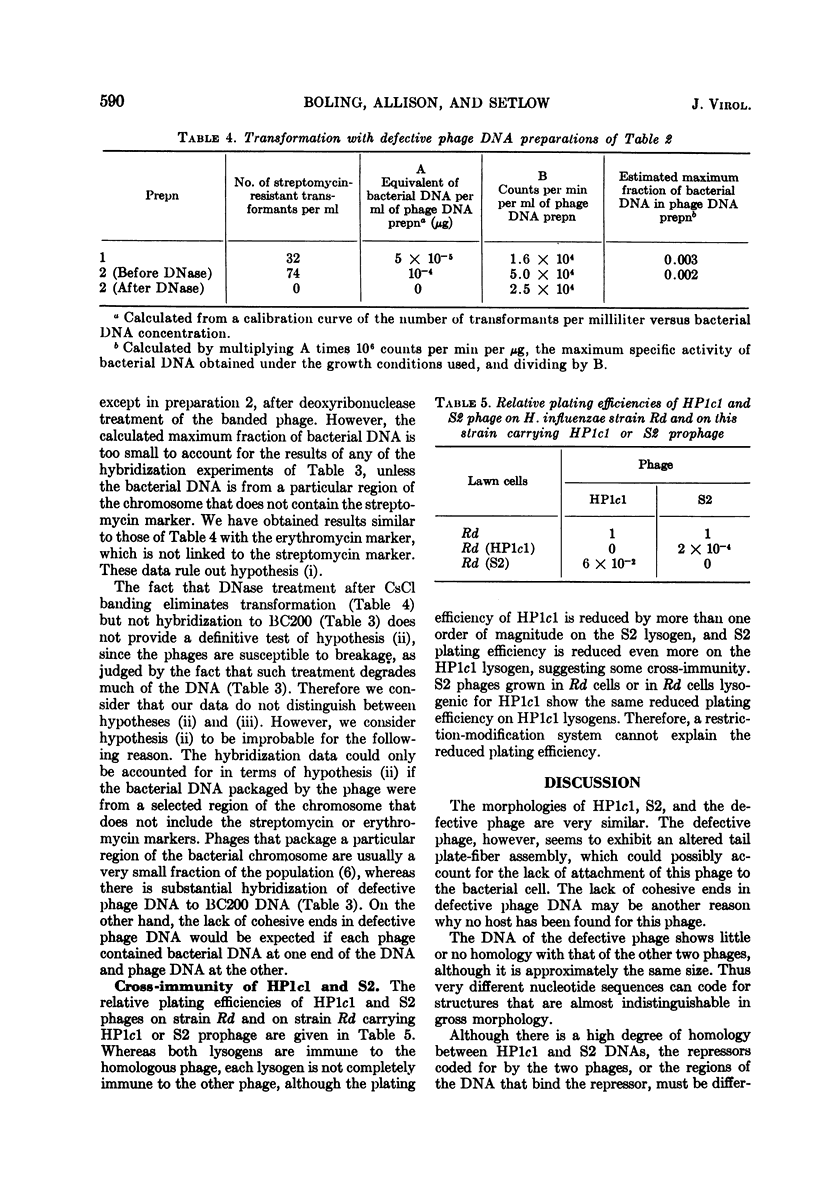
The phages HP1c1 and S2 and a defective phage of Haemophilus influenzae have been compared. The morphology of the phages and the mol wt of their DNAs are similar, although the defective phage appears to have a different tail plate region. Electron microscope observation indicates that the defective phage does not attach to the cell surface, and its DNA appears to lack cohesive ends. The homology of the DNAs of the phages has been measured by hydridization. DNA from the defective phage shows little or no homology with the other phage DNAs. HP1c1 and S2 DNAs show a high level of homology. Each of these phages can form plaques on lawns of the lysogen of the other phage but at reduced plating efficiencies, suggesting that the two phages have related but not identical immunity systems.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnhart B. J., Cox S. H. Radiation-sensitive and radiation-resistant mutants of Haemophilus influenzae. J Bacteriol. 1968 Jul;96(1):280–282. doi: 10.1128/jb.96.1.280-282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart D. J., Cox S. H. Recovery of Haemophilus influenzae from ultraviolet and x-ray damage. Photochem Photobiol. 1970 Mar;11(3):147–162. doi: 10.1111/j.1751-1097.1970.tb05983.x. [DOI] [PubMed] [Google Scholar]
- Bendler J. W., Goodgal S. H. Prophage S2 mutants in Haemophilus influenzae: a technique for their production and isolation. Science. 1968 Oct 25;162(3852):464–465. doi: 10.1126/science.162.3852.464. [DOI] [PubMed] [Google Scholar]
- Boling M. E. Homology between the deoxyribonucleic acids of Haemophilus influenzae and Haemophilus parainfluenzae. J Bacteriol. 1972 Nov;112(2):745–750. doi: 10.1128/jb.112.2.745-750.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K., Allison D. P. Bacteriophage of Haemophilus influenzae. I. Differences between infection by whole phage, extracted phage DNA and prophage DNA extracted from lysogenic cells. J Mol Biol. 1972 Feb 14;63(3):335–348. doi: 10.1016/0022-2836(72)90431-7. [DOI] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. The attachment of the male-specific bacteriophage F1 to sensitive strains of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):126–132. doi: 10.1073/pnas.56.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Paper strip method for assaying gradient fractions containing radioactive macromolecules. Anal Biochem. 1971 Oct;43(2):427–432. doi: 10.1016/0003-2697(71)90272-7. [DOI] [PubMed] [Google Scholar]
- HARM W., RUPERT C. S. INFECTION OF TRANSFORMABLE CELLS OF HAEMOPHILUS INFLUENZAE BY BACTERIOPHAGE AND BACTERIOPHAGE DNA. Z Vererbungsl. 1963 Dec 30;94:336–348. doi: 10.1007/BF00897593. [DOI] [PubMed] [Google Scholar]
- Samuels J., Clarke J. K. New bacteriophage of Haemophilus influenzae. J Virol. 1969 Nov;4(5):797–798. doi: 10.1128/jvi.4.5.797-798.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachura I., Mckinley F. W., Leidy G., Alexander H. E. Incomplete bacteriophage-like particles in ultraviolet-irradiated haemophilus. J Bacteriol. 1969 May;98(2):818–820. doi: 10.1128/jb.98.2.818-820.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]